Waiting more than 24 hours for hip fracture surgery is associated with increased risk of adverse outcomes for sicker patients: a nationwide cohort study of 63,998 patients using the Swedish Hip Fracture Register
Katarina GREVE 1,2, Stina EK 3, Erzsébet BARTHA 1,2, Karin MODIG 3, and Margareta HEDSTRÖM 1,4
1 Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institutet, Stockholm; 2 Function Perioperative Medicine and Intensive Care (PMI), Karolinska University Hospital, Stockholm; 3 Institute of Environmental Medicine (IMM), Karolinska Institutet, Stockholm; 4 Trauma and Reparative Medicine Theme (TRM), Karolinska University Hospital, Stockholm, Sweden
Background and purpose — Waiting time to surgery is a modifiable risk factor in hip fracture surgery. However, there is no consensus regarding the acceptable duration of waiting time. We used the Swedish Hip Fracture Register RIKSHÖFT and 3 administrative registers to explore the association between time to surgery and adverse outcomes after discharge.
Patients and methods — 63,998 patients ≥ 65 years, admitted to a hospital between January 1, 2012, and August 31, 2017 were included. Time to surgery was divided into < 12, 12–24, and > 24 hours. Diagnoses investigated were atrial fibrillation/flutter (AF), congestive heart failure (CHF), pneumonia, and “acute ischemia” (a combination of stroke/intracranial bleeding, myocardial infarction, and acute kidney injury). Crude and adjusted survival analyses were performed. Time spent in hospital following the initial hospitalization was described for the 3 groups.
Results — Waiting > 24 hours was associated with an increased risk of AF (HR 1.4, 95%CI 1.2–1.6), CHF (HR 1.3, CI 1.1–1.4) and “acute ischemia” (HR 1.2, CI 1.01–1.3). However, stratifying for ASA grade revealed that these associations were present only in patients with ASA 3–4. There was no association between waiting time and pneumonia after the initial hospitalization (HR 1.1, CI 0.97–1.2), but one was found with pneumonia during hospital stay OR 1.2 (CI 1.1–1.4). Time in hospital after the initial hospitalization was similar over the waiting time groups.
Conclusion — The associations between waiting > 24 hours for hip fracture surgery and AF, CHF, and acute ischemia suggest that shorter waiting time may reduce adverse outcomes for the sicker patients.
Citation: Acta Orthopaedica 2023; 94: 87–96. DOI: https://doi.org/10.2340/17453674.2023.9595.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, provided proper attribution to the original work.
Submitted: 2022-06-01. Accepted: 2023-01-14. Published: 2023-02-27.
Correspondence: katarina.greve@ki.se
This study was conceived by KG, SE, EB, KM, and MH. SE and KG performed the analyses. All authors contributed to the interpretation of the data. KG wrote the initial draft. All authors contributed to revising the manuscript.
The authors would like to thank RIKSHÖFT for providing data to this study.
Handling co-editors: Cecilia Rogmark and Robin Christensen
Acta thanks Matthew Costa and Jan-Erik Gjertsen for help with peer review of this study.
Hip fracture is a common and potentially devastating injury that entails a 1-year all-cause mortality of about 25% (1). Waiting time to surgery has been studied as a potential modifiable risk factor of complications and death (2-5), with the suggested mechanism that prolonged waiting exposes the patient to a state of hypercoagulability, catabolism, and stress (6), caused by the injury itself and the immobilization and fasting thereafter (7). Waiting > 24 hours for surgery has also been associated with increased intraoperative instability (8), perhaps partly due to compromised fluid balance.
There is no consensus regarding what duration of waiting time to hip fracture surgery can be considered acceptable. We have previously studied the association between waiting more vs. less than 24 hours to hip fracture surgery and death within 4 months in a large Swedish cohort (9). We did not find an association between waiting longer than 24 hours and an increased risk of mortality among the healthier patients (American Society of Anesthesiologists Physical Status Classification System [ASA] (10) 1 and 2). Among the sicker patients (ASA 3 and 4), there was an association between waiting > 24 hours and death within 4 months, and it was stronger for women than for men. However, evaluating mortality only is insufficient; postoperative morbidity is of importance for quality of life after hip fracture (11).
Therefore, the main objective of this study was to explore the association between waiting time to surgery and adverse outcomes; specifically, atrial fibrillation/flutter, congestive heart failure, pneumonia, and acute ischemia, up to 120 days after initial hospital department discharge. An additional objective was to describe time spent in hospital, after initial hospital department discharge, for patients operated on for hip fracture, based on waiting time to surgery.
Patients and methods
Study design
This is a nationwide cohort study of the exposure waiting time to surgery, as registered in the Swedish Hip Fracture Register RIKSHÖFT (SHR) (12). Time to surgery was influenced by nationwide guidelines, which at the time prescribed surgery within 24 hours (13). The outcome “postoperative morbidity” was estimated from diagnoses from the administrative healthcare registers Swedish National Patient Register (NPR) (14) and Swedish Cause of Death Register (CDR) (14). The outcome data is presented for the whole cohort as well as stratified for sex and ASA category and adjusted for potential confounders in 3 different models. The outcome “time readmitted” is constructed from data in NPR. The results are reported as recommended by the The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) Statement (15).
Setting and participants
SHR is a national clinical quality register on patients with hip fracture. It contains prospective data such as fracture type, time to surgery, surgical method, and ASA classification on the individuals therein, routinely collected in the patients’ care processes, and had > 80% completeness between 2008 and 2017 (16). NPR holds information on all inpatient care and outpatient specialized care (i.e., no primary care data) in Sweden and has shown high levels of validity for many diagnoses (17). For this study, we used inpatient data only. CDR is updated every year and includes information on the cause of death of every person who has died in Sweden (14,18). CDR has been reported as having a high concordance with medical records for cardiovascular causes of death, but generally more uncertainty for older people compared with younger (19). The National Prescribed Drug Register (NPD) holds information on all dispensed prescribed drugs in Sweden, including for nursing home residents (14). Linkage between these registers is facilitated by the personal identity numbers assigned to every person residing within the country.
Inclusion criteria were individuals ≥ 65 years of age with hip fracture admitted to a hospital between January 1, 2012, and August 31, 2017, and treated surgically. Exclusion criteria were time to surgery < 2 hours (assumed as erroneous), or > 7 days, ASA score ≥ 5, pathological fracture, death during the initial hospitalization, and missing data regarding date of surgery, surgical method, or ASA score. If an individual appeared in SHR more than once during the study period, only the first fracture was considered.
Variables
The exposure was waiting time to surgery (time, in hours, between hospital arrival and start of surgery). The continuous variable was transformed into a categorical variable with 3 potential values, < 12 hours, 12–24 hours, and > 24 hours. Predefined cutoffs for time to surgery have been described as arbitrary (5,20). However, surgery within 24 hours was the national recommendation during the study period as it has been suggested as a point of inflection for death and complications (5). As further reduction in time to surgery has been studied (6), those operated on within 12 hours were studied in a separate group.
The outcomes for specific diagnoses were defined as ICD-10 codes registered in NPR or CDR. We selected diagnoses that are common postoperatively and can reasonably be attributed to the wait for hip fracture surgery: atrial fibrillation/flutter (AF): I48, congestive heart failure (CHF): I50, and bacterial pneumonia or pneumonia with an unknown etiology (combined into the variable “pneumonia”): J13–15, J18. Finally, we combined ischemic stroke/intracranial bleeding: I61–64, myocardial infarction (MI): I21–22, and acute kidney failure: N17 into a variable termed “acute ischemia”. This variable was constructed to reflect the organs most vulnerable to perioperative changes in blood pressure (21). Longer waiting time to hip fracture surgery has previously been shown to be associated with intraoperative hypotension (8). However, hypotension alone cannot fully explain perioperative organ injury, and has been suggested to be a biomarker rather than a direct mediator (22).
The diagnoses in NPR are registered at the end of a hospital department stay and it is not possible to determine whether the event occurred before or after the fracture. Due to this, all patients with the outcome diagnoses registered during the index hospitalization were excluded from the analysis of that particular outcome, creating 1 full study population and 4 slightly different sub-populations (Figure 1). If a patient was transferred directly from one department to another, diagnoses originating from the second department could be counted as “outcome diagnoses.” 12,238 patients were excluded for the analysis of AF, 7,279 patients were excluded for the analysis of CHF, 3,078 patients were excluded for the analysis of pneumonia, and 1,914 patients were excluded for the analysis of acute ischemia. Those who died during the index hospitalization were excluded as they were not at-risk for the outcomes. However, because pneumonia has also been reported as one of the most common postoperative complications during the hospitalization for hip fracture (20,23), we additionally performed a regression analysis on the pneumonia cases that occurred during the index hospitalization only, although the potential for reverse causation remains.
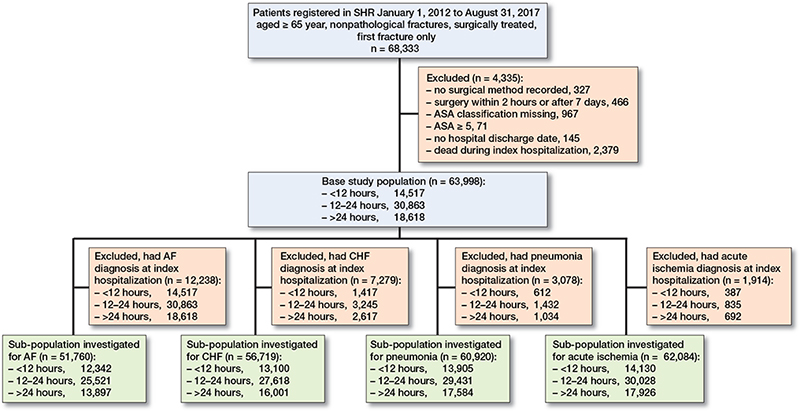
Figure 1. Flowchart of the base study population and the sub-populations.
The outcome “time spent in hospital after the initial hospitalization” was defined as total nights admitted between the initial hospital department discharge and 120 days, divided into 6 categories: none, < 1 week, 1–2 weeks, 2–3 weeks, 3–4 weeks, and > 4 weeks. Thus, if a patient was transferred to a new department in direct conjuncture with the initial hospitalization, this new hospital stay counted towards “time spent in hospital after the initial hospitalization.” This outcome was selected to reflect differences in overall postoperative morbidity rather than differences in readmission rates. The time categories were chosen for simplicity of interpretation.
ASA classification was used as a measure of comorbidity, and a potential confounder, and was divided into 2 groups (ASA1–2) and (ASA 3–4). A previous diagnosis of each of the outcomes was also considered a potential confounder. NPR was searched for previous registrations of all the outcome diagnoses, except pneumonia, within the last year before the hip fracture. For pneumonia, NPR was searched for registrations within 30 days before the fracture. Diagnoses of dementia (G30–31, F00–03) registered in the NPR in the last 5 years before fracture, were combined with the variable “known dementia” in SHR, creating the composite variable “previous diagnosis of dementia.” Type of surgery was divided into 2 groups, group 1 (intramedullary nail, hemiarthroplasty, and total hip replacement, which are all intramedullary procedures), group 2 (internal fixation other than intramedullary procedures). Antithrombotic therapy was considered a potential confounder. Patients who had filled a prescription for anticoagulants and/or platelet inhibitors, other than aspirin, within 120 days before hospital arrival, were considered to be on antithrombotic therapy. The drugs included are listed in Table 1 (see Appendix).
Statistics
Descriptive baseline characteristics were presented for the whole population and stratified by waiting time to surgery and presented as means, medians, or percentages. Cox proportional hazards models were used to analyze the association between waiting time and occurrence of the outcome variables from initial hospital department discharge until 120 days thereafter. For pneumonia that occurred during the hospitalization, logistic regression was used. A significance level of 95% was chosen and waiting time < 12 hours served as reference group. 3 different models were performed, with Model 1 being crude; Model 2 adjusted for age, sex, and ASA category; and Model 3 adjusted for age, sex, ASA category, type of surgery, previous diagnosis of dementia, previous occurrence of the studied outcome diagnosis, and antithrombotic therapy. The analyses were performed in the whole study sample as well as stratified by sex and ASA category with modified Models 2 and 3.
Differences between the waiting time categories in number of days spent at the hospital after initial discharge up until 120 days were presented with graphs stratified by ASA category.
Statistical analyses were conducted using STATA 16 (2019; StataCorp, College Station, TX, USA), and GraphPad Prism version 9.3.1 (GraphPad Software, CA; https://www.graphpad.com/).
Ethics, registration, data sharing plan, funding, and potential conflicts of interest
The study was approved by the regional Ethics Committee of Stockholm, Dnr 2011/1036-31 and 2018/84-32, and was funded by grants provided by the Kamprad Family Foundation for Entrepreneurship, Research and Charity (grant number 20190135), Region Stockholm (ALF project), the Promobilia Foundation, and by Karolinska University Hospital. The funding sources played no role in the investigation. The raw data used in this study is considered sensitive personal information that is protected by Swedish law and cannot therefore be shared without ethical approval. The authors report no conflicts of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.9595
Results
Descriptive data
63,998 patients were included in the study (Figure 1). Baseline characteristics are given in Table 2. 69% were women, and the mean age was 83 years (SD 8). The overall median waiting time was 20 hours. 23% were operated on within 12 hours, 48% between 12 and 24 hours and 29% after 24 hours. For those operated on after 24 hours, the median waiting time was 32 hours. Overall 30-day mortality was 5% and 120-day mortality was 13%. A majority, 71% of the patients, were admitted from their own home, 19% had a diagnosis of dementia at baseline, and 16% were on antithrombotic therapy.
| Factor | < 12 hours | 12–24 hours | > 24 hours | Total |
| Patients (row %) | 14,517 (23) | 30,863 (48) | 18,618 (29) | 63,998 (100) |
| Women | 10,146 (70) | 21,533 (70) | 12,441 (67) | 44,120 (69) |
| Mean age (SD) | 83 (8) | 83 (8) | 83 (8) | 83 (8) |
| ASA 1–2 | 6,595 (45) | 12,779 (41) | 6,599 (35) | 25,973 (41) |
| ASA 3–4 | 7,922 (55) | 18,084 (59) | 12,019 (65) | 38,025 (59) |
| Cervical hip fracture | ||||
| non-displaced, Garden 1+2 | 2,029 (14) | 3,862 (13) | 2,312 (12) | 8,203 (13) |
| displaced, Garden 3+4 | 4,476 (31) | 11,982 (39) | 7,992 (43) | 24,450 (38) |
| Non-cervical hip fracture | 8,012 (55) | 15,019 (49) | 8,314 (45) | 31,345 (49) |
| Surgical method 1 a | 8,100 (56) | 19,211 (62) | 11,791 (63) | 39,102 (61) |
| Surgical method 2 b | 6,417 (44) | 11,652 (38) | 6,827 (37) | 24,896 (39) |
| Hours to surgery, median (IQR) | 8 (6–10) | 19 (16–21) | 32 (27–43) | 20 (13–25) |
| 30-day survival (after fracture) | 13,810 (95) | 29,462 (95) | 17,627 (95) | 60,899 (95) |
| 4-month survival (after fracture) | 12,597 (87) | 27,000 (87) | 15,931 (86) | 55,528 (87) |
| Admitted from c | ||||
| own home | 10,034 (69) | 22,233 (72) | 13,412 (72) | 45,679 (71) |
| institutional care | 3,944 (27) | 7,591 (25) | 4,429 (24) | 15,964 (25) |
| other | 480 (3) | 856 (3) | 700 (4) | 2,036 (3) |
| On antithrombotic therapy | 1,454 (10) | 4,341 (14) | 4,564 (25) | 10,359 (16) |
| Previously known dementia | 3,001 (21) | 5,887 (19) | 3,272 (18) | 12,160 (19) |
| Previous diagnosis within 1 year of stroke/intracranial bleeding | 365 (3) | 805 (3) | 654 (4) | 1,824 (3) |
| atrial fibrillation/flutter) | 1,028 (7) | 2,586 (8) | 2,403 (13) | 6,017 (9) |
| congestive heart failure | 774 (5) | 1,789 (6) | 1,569 (8) | 4,132 (6) |
| myocardial infarction | 159 (1) | 368 (1) | 336 (2) | 863 (1) |
| acute kidney failure | 81 (1) | 187 (1) | 143 (1) | 411 (1) |
| stroke/intracranial bleeding or | ||||
| myocardial infarction or acute | ||||
| kidney failure | 584 (4) | 1,313 (4) | 1,089 (6) | 2,986 (5) |
| Previous diagnosis of pneumonia | ||||
| (last 30 days) | 98 (1) | 229 (1) | 175 (1) | 502 (1) |
| a Surgical method 1 = intramedullary nail, hemiarthroplasty, or total hip replacement. | ||||
| b Surgical method 2 = all others, non-intramedullary methods. | ||||
| c Missing patients in the “admitted from” category = 319 (0.5%). | ||||
Time spent in hospital after initial hospitalization
There were no major differences in time spent in hospital after the initial hospitalization depending on time to surgery. Approximately 60% of the ASA 1–2 patients and 50% of the ASA 3–4 patients spent no additional time in hospital after discharge from the index hospital department (Figure 2).
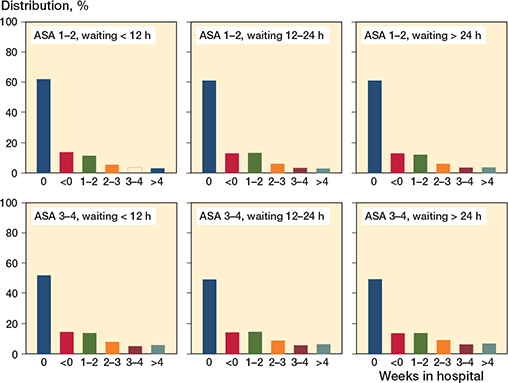
Figure 2. Weeks spent in hospital within 120 days after initial hospital department discharge following hip fracture surgery, stratified by waiting time to surgery category (< 12 hours, 12–24 hours, > 24 hours) and ASA category (1–2 and 3–4 respectively).
Survival analyses and logistic regression
Overall, there was a trend towards longer waiting times being associated with the outcomes investigated (Figure 3), with a similar pattern, albeit with varying effect magnitudes, for all the diagnoses. The differences between those operated on within 12 hours and between 12 and 24 hours were non-significant, but for no outcome was the hazard lower for later surgery compared with earlier.
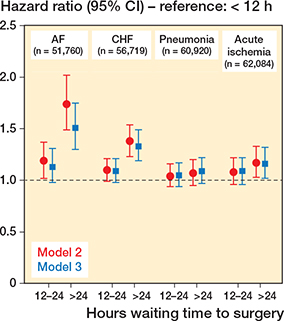
Figure 3. HRs for postoperative morbidity within 120 days of initial hospital department discharge after hip fracture surgery, depending on waiting time to surgery (waiting < 12 hours as reference). Model 2: analyses adjusted for age, sex, and ASA category. Model 3: adjusted for age, sex, ASA category, type of surgery, previous diagnosis of dementia, previous hospitalization for the diagnosis investigated, antithrombotic therapy.
Hazard ratios and absolute risks for the 4 diagnoses investigated are presented in Table 3. 1,387 patients (3%) had AF within the follow-up period. Survival analyses demonstrated an increased risk of AF in the group operated on after 24 hours compared with those operated on within 12 hours in the fully adjusted model, HR 1.4 (CI 1.2–1.6). Stratifying by sex revealed that the association between waiting > 24 h and AF was similar for women and men, HR 1.4 (CI 1.1–1.7) and HR 1.3 (CI 1.03-1.7), respectively (Table 4, see Appendix). Stratifying by ASA revealed that the associations remained statistically significant for the sicker patients only: HR 1.4 (CI 1.2–1.7) for the ASA 3–4 patients vs. 1.3 (CI 0.98–1.8) for the ASA 1–2 patients (Table 4, see Appendix).
| Hours t surgery | Cases | Events (%) | Model 1 | Model 2 | HRs (95% CI) Model 3 |
| AF | 51,760 | 1,387 (3) | |||
| < 12 | 12,342 | 250 (2) | Ref. | Ref. | Ref. |
| 12–24 | 25,521 | 627 (2) | 1.2 (1.1–1.4) a | 1.2 (1.02–1.4) a | 1.1 (0.95–1.3) |
| > 24 | 13,897 | 510 (4) | 1.8 (1.6–2.1) a | 1.7 (1.5–2.0) a | 1.4 (1.2–1.6) a |
| CHF | 56,719 | 2,484 (4) | |||
| < 12 | 13,100 | 483 (4) | Ref. | Ref. | Ref. |
| 12–24 | 27,618 | 1,145 (4) | 1.1 (1.01–1.3) a | 1.1 (0.99–1.2) | 1.1 (0.97–1.2) |
| > 24 | 16,001 | 856 (5) | 1.5 (1.3–1.6) a | 1.4 (1.2–1.5) a | 1.3 (1.1–1.4) a |
| Pneumonia | 60,920 | 2,226 (4) | |||
| < 12 | 13,905 | 473 (3) | Ref. | Ref. | Ref. |
| 12–24 | 29,431 | 1,069 (4) | 1.1 (0.96–1.2) | 1.0 (0.94–1.2) | 1.1 (0.94–1.2) |
| > 24 | 17,584 | 684 (4) | 1.2 (1.02–1.3) a | 1.1 (0.95–1.2) | 1.1 (0.97–1.2) |
| Acute ischemia | 62,084 | 1,856 (3) | |||
| < 12 | 14,130 | 377 (3) | Ref. | Ref. | Ref. |
| 12–24 | 30,028 | 888 (3) | 1.1 (0.98–1.3) | 1.1 (0.96–1.2) | 1.1 (0.96–1.2) |
| > 24 | 17,926 | 591 (3) | 1.3 (1.1–1.4) a | 1.2 (1.03–1.3) a | 1.2 (1.01–1.3) a |
| Model 1: crude. | |||||
| Model 2: adjusted for age, sex and ASA category. | |||||
| Model 3: adjusted for age, sex, ASA category, type of surgery, previous diagnosis of dementia, previous diagnosis of the outcome variable, and antithrombotic therapy. | |||||
| a Statistically significant. | |||||
2,484 patients (4%) had CHF within the follow-up period. There was an increased risk of being diagnosed with CHF in the group operated on after 24 hours compared with those operated on within 12 hours in the fully adjusted model, HR 1.3 (CI 1.1–1.4). After stratification by sex, the association remained among women only, HR 1.3 (CI 1.1–1.5) vs. 1.2 (0.99–1.4) for men (Table 5, see Appendix). Similarly to AF, stratifying by ASA grade revealed that an association remained for the sicker patients only, HR 1.3 (CI 1.1–1.4) for ASA 3–4 patients vs. 1.2 (CI 0.95–1.6) for ASA 1–2 patients (Table 5, see Appendix).
2,226 patients (4%) were registered as having pneumonia within 120 days after discharge. There was an increased risk of pneumonia with longer waiting time in the crude model, but no statistical significance after adjustment, HR 1.1 (CI 0.97–1.2). Similarly, there were no associations between waiting time and pneumonia post index hospitalization in the stratified analyses (Table 6, see Appendix). However, in the analysis of pneumonia cases during the index hospitalization, there was an increased risk of pneumonia among those operated on > 24 hours in the fully adjusted model, OR 1.2 (CI 1.1–1.4) (Table 7, see Appendix).
For “acute ischemia,” 1,856 patients (3%) were registered with either of the diagnoses that constituted this combined diagnosis during the follow-up period. There was an increased risk of “acute ischemia” for those operated on after 24 hours compared with those operated on within 12 hours in the fully adjusted model, HR 1.2 (CI 1.01–1.3). In contrast to the main analysis, no association between waiting time and acute ischemia remained in the stratified analyses (Table 8, see Appendix), suggesting insufficient statistical power.
Discussion
In contrast with much of the previous research on waiting time to surgery, which has often focused on mortality (24), this study contributes information on other adverse outcomes. We found that waiting > 24 hours is associated with increased risk of postoperative morbidity, primarily among sicker patients. This is in line with a previous study by us where we found that longer waiting time was associated with an increased risk of mortality among patients with ASA 3–4 but not among those with ASA 1–2 (9).
There are few randomized controlled trials (RCTs) on this subject. The HIP-ATTACK study (6) compared an accelerated surgery group (median waiting time 6 hours), with a control group (median waiting time 24 hours), with no difference in 90-day mortality or major complications. It did, however, detect decreased instances of delirium and urinary tract infection in the accelerated group. Pincus et al. (5) explored the association between waiting time to surgery with death and medical complications at 30 days in a large, population-based Canadian cohort, using time as a continuous variable in a model of risk-adjusted, restricted cubic splines. They found 24 hours to be an inflection point for death as well as for complications.
In our study, those exposed to the longest waiting time (> 24 hours) still had a comparatively short waiting time (median 32 hours in the base population). This means, though there may be a causal link between longer waiting time and postoperative morbidity, that the associations presented here are likely weaker than they would have been, had those patients waited even longer for surgery. The comparatively short waiting time of the patients in the control group of the HIP-ATTACK trial (6) could perhaps explain the lack of difference regarding mortality and complications there as well. Other important factors to consider are that prolonged waiting time to surgery is associated with longer overall length of hospital stay (20) and, importantly, longer time in immobility, care dependency, and pain due to a non-stabilized fracture. This means that increasing wait times could have implications far beyond the scope of this study.
Our study failed to demonstrate an association between longer waiting time and pneumonia diagnosed after discharge from the index hospital department, which could be due to most cases occurring in the early postoperative period (23).
Regarding time spent in hospital after the initial hospitalization, we were unable to demonstrate any major differences between the waiting time groups. As far as we are aware, time spent as an inpatient following hip fracture surgery has not previously been investigated in a Swedish context and could be a topic for future research.
Strengths and limitations
This nationwide study has high coverage, ensuring high generalizability for similar populations, and the analyses were adjusted for many of the major known confounding factors. A major strength is that the analyses were adjusted for antithrombotic therapy as this is an important determinant for delayed hip fracture surgery (25). Adjusting for antithrombotic therapy can also, indirectly, entail adjustment for unmeasured comorbidity, especially related to the specific heart diseases included in this study.
The greatest limitation of this study is that the diagnoses registered at the point of discharge from the index hospitalization could not be used, which has likely led to overall weaker associations. However, excluding patients with the outcome diagnosis during the index hospitalization was a necessary step to reduce the risk of reverse causality.
Moreover, we only had access to diagnose codes registered in conjunction with hospital visits and not in specialized outpatient care (or in primary care or nursing homes). This implies that this study likely misses cases of the outcomes. Yet this is not problematic unless these missed cases are different in their waiting time compared with the cases we observed in NPR. The cases included may be on the more severe end of the spectrum, which could even increase the clinical relevance.
We acknowledge that some of the more common and/or feared complications of hip fracture and hip fracture surgery have not been investigated in this study. Urinary tract infection, delirium, and pressure sores were not investigated due to a suspicion that these have a high tendency to be under-reported in hospital discharge notes and thus in NPR. Thromboembolic events, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), were not investigated due to their comparatively low prevalence (23). There is also reason to believe that cases of fatal PE may be misclassified due to a declining number of autopsies in Sweden (26).
Like all observational studies, this study cannot prove causal connections. There may also be a risk of residual confounding. One unaddressed potential confounder is the reason for surgical delay (administrative vs. medical). Despite attempts to minimize the risk of reverse causation, it cannot be eliminated completely, which warrants careful interpretation of the results. Adverse events associated with longer waiting time to surgery may partly be due to factors such as untreated pain, uncontrolled fluid balance, and prolonged fasting, which could decrease the risks of waiting for surgery if addressed appropriately. Differences in preoperative management could thus complicate comparisons of the effect of waiting time across studies.
Uniform criteria for postoperative complications across national registers and participating hospitals could be the best estimates of adverse outcomes and could support research in perioperative medicine. The specificity and sensitivity of diagnosis codes from administrative registers to detect adverse outcomes postoperatively need to be addressed in future trials.
Time spent admitted in a hospital can be difficult to compare across healthcare systems, as it depends on what options there are for assisted living outside hospital, but the internal validity remains unaffected.
Conclusions
Waiting > 24 hours to hip fracture surgery was associated with an increased risk of AF, CHF, and acute ischemia, primarily among sicker patients. These results suggest that shorter waiting time to surgery may reduce adverse outcomes in patients with ASA 3 and 4. This should be considered when creating new guidelines for the management of patients with hip fracture. Moreover, our findings highlight the importance of considering the health status of the patients when conducting research on prognostic factors.
- Meyer A C, Ek S, Drefahl S, Ahlbom A, Hedstrom M, Modig K. Trends in hip fracture incidence, recurrence, and survival by education and comorbidity: a Swedish register-based study. Epidemiology 2021; 32(3): 425-33. doi: 10.1097/EDE.0000000000001321
- Belmont P J, Jr., Garcia E J, Romano D, Bader J O, Nelson K J, Schoenfeld A J. Risk factors for complications and in-hospital mortality following hip fractures: a study using the National Trauma Data Bank. Arch Orthop Trauma Surg 2014; 134: 597-604. doi: 10.1007/s00402-014-1959-y.
- Alvi H M, Thompson R M, Krishnan V, Kwasny M J, Beal M D, Manning D W. Time-to-surgery for definitive fixation of hip fractures: a look at outcomes based upon delay. Am J Orthop 2018; 47. doi: 10.12788/ajo.2018.0071.
- Ryan D J, Yoshihara H, Yoneoka D, Egol K A, Zuckerman J D. Delay in hip fracture surgery: an analysis of patient-specific and hospital-specific risk factors. J Orthop Trauma 2015; 29: 343-8. doi: 10.1097/BOT.0000000000000313.
- Pincus D, Ravi B, Wasserstein D, Huang A, Paterson J M, Nathens A B, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA 2017; 318: 1994–2003. doi: 10.1001/jama.2017.17606.
- HIP ATTACK investigators. Accelerated surgery versus standard care in hip fracture (HIP ATTACK): an international, randomised, controlled trial. Lancet 2020; 395: 698-708. doi: 10.1016/s0140-6736(20)30058-1.
- Hedström M, Ljungqvist O, Cederholm T. Metabolism and catabolism in hip fracture patients: nutritional and anabolic intervention—a review. Acta Orthop 2006; 77: 741-7. doi: 10.1080/17453670610012926.
- Leer-Salvesen S, Engesaeter L B, Dybvik E, Furnes O, Kristensen T B, Gjertsen J E. Does time from fracture to surgery affect mortality and intraoperative medical complications for hip fracture patients? An observational study of 73 557 patients reported to the Norwegian Hip Fracture Register. Bone Joint J 2019; 101-B: 1129-37. doi: 10.1302/0301-620X.101B9.BJJ-2019-0295.R1.
- Greve K, Modig K, Talback M, Bartha E, Hedström M. No association between waiting time to surgery and mortality for healthier patients with hip fracture: a nationwide Swedish cohort of 59,675 patients. Acta Orthop 2020; 91: 396-400. doi: 10.1080/17453674.2020.1754645.
- Anesthesiologists. ASo. ASA Physical Status Classification System [web page] (updated 13-12-2020; cited 2021). Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system.
- Griffiths F, Mason V, Boardman F, Dennick K, Haywood K, Achten J, et al. Evaluating recovery following hip fracture: a qualitative interview study of what is important to patients. BMJ Open 2015; 5: e005406. doi: 10.1136/bmjopen-2014-005406.
- Swedish National Hip Fracture Registry (RIKSHÖFT) 2022. Available from: https://www.rikshoft.se/english.
- [Socialstyrelsen]. Socialstyrelsens riktlinjer för vård och behandling av höftfraktur; 2003. ISBN 91-7201-758-9.
- Laugesen K, Ludvigsson J F, Schmidt M, Gissler M, Valdimarsdottir U A, Lunde A, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol 2021; 13: 533-54. doi: 10.2147/CLEP.S314959.
- Benchimol E I, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement. PLoS Med 2015; 12: e1001885. doi: 10.1371/journal.pmed.1001885.
- Meyer A C, Hedstrom M, Modig K. The Swedish Hip Fracture Register and National Patient Register were valuable for research on hip fractures: comparison of two registers. J Clin Epidemiol 2020; 125: 91-9. doi: 10.1016/j.jclinepi.2020.06.003.
- Ludvigsson J F, Andersson E, Ekbom A, Feychting M, Kim J L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. doi: 10.1186/1471-2458-11-450.
- [Socialstyrelsen]. Dödsorsaksregistret [Cause of Death Register] (updated 2019-10-18). Available from: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/dodsorsaksregistret/.
- Brooke H L, Talback M, Hornblad J, Johansson LA, Ludvigsson J F, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol 2017; 32: 765-73. doi: 10.1007/s10654-017-0316-1.
- Kelly-Pettersson P, Samuelsson B, Muren O, Unbeck M, Gordon M, Stark A, et al. Waiting time to surgery is correlated with an increased risk of serious adverse events during hospital stay in patients with hipfracture: a cohort study. Int J Nurs Stud 2017; 69: 91-7. doi: 10.1016/j.ijnurstu.2017.02.003.
- Sessler D I, Bloomstone J A, Aronson S, Berry C, Gan T J, Kellum J A, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth 2019; 122: 563-74. doi: 10.1016/j.bja.2019.01.013.
- Ackland G L, Abbott T E F. Hypotension as a marker or mediator of perioperative organ injury: a narrative review. Br J Anaesth 2022; 128: 915-30. doi: 10.1016/j.bja.2022.01.012.
- Goh E L, Lerner R G, Achten J, Parsons N, Griffin X L, Costa P M L. Complications following hip fracture: results from the World Hip Trauma Evaluation cohort study. Injury 2020; 51: 1331-6. doi: 10.1016/j.injury.2020.03.031.
- Welford P, Jones C S, Davies G, Kunutsor S K, Costa M L, Sayers A, et al. The association between surgical fixation of hip fractures within 24 hours and mortality: a systematic review and meta-analysis. Bone Joint J 2021; 103-b: 1176-86. doi: 10.1302/0301-620x.103b7.Bjj-2020-2582.R1.
- You D, Xu Y, Ponich B, Ronksley P, Skeith L, Korley R, et al. Effect of oral anticoagulant use on surgical delay and mortality in hip fracture. Bone Joint J 2021; 103-b: 222-33. doi: 10.1302/0301-620x.103b2.Bjj-2020-0583.R2.
- Friberg N, Ljungberg O, Berglund E, Berglund D, Ljungberg R, Alafuzoff I, et al. Cause of death and significant disease found at autopsy. Virchows Arch 2019; 475: 781-8. doi: 10.1007/s00428-019-02672-z.
Appendix
| Hours to surgery | Cases | Events (%) | Model 1 | HRs (95% CI) Model 2 | Model 3 |
| Women a | 36,577 | 823 (2) | |||
| < 12 | 8,814 | 154 (2) | Ref | Ref | Ref |
| 12–24 | 18,201 | 374 (2) | 1.2 (0.98–1.4) | 1.2 (0.96–1.4) | 1.1 (0.89–1.3) |
| > 24 | 9,562 | 295 (3) | 1.8 (1.5–2.2) c | 1.7 (1.4–2.1) c | 1.4 (1.1–1.7) c |
| Men a | 15,183 | 564 (4) | |||
| < 12 | 3,528 | 96 (3) | Ref | Ref | Ref |
| 12–24 | 7,320 | 253 (3) | 1.3 (1.01–1.6) c | 1.2 (0.97–1.5) | 1.1 (0.9–1.4) |
| > 24 | 4,335 | 215 (5) | 1.9 (1.5–2.4) c | 1.7 (1.4–2.2) c | 1.3 (1.03–1.7) c |
| ASA 1–2 b | 23,526 | 322 (1) | |||
| < 12 | 6,108 | 75 (1) | Ref | Ref | Ref |
| 12–24 | 11,685 | 148 (1) | 1.0 (0.78–1.4) | 1.1 (0.80–1.4) | 1.0 (0.77–1.4) |
| > 24 | 5,733 | 99 (2) | 1.4 (1.04–1.9) c | 1.5 (1.1–2.0) c | 1.3 (0.98–1.8) |
| ASA 3–4 b | 28,234 | 1,065 (4) | |||
| < 12 | 6,234 | 175 (3) | Ref | Ref | Ref |
| 12–24 | 13,836 | 479 (3) | 1.2 (1.04–1.5) c | 1.2 (1.1–1.5) c | 1.1 (0.95–1.3) |
| > 24 | 8,164 | 411 (5) | 1.8 (1.5–2.2) c | 1.8 (1.5–2.2) c | 1.4 (1.2–1.7) c |
| a Model 1: crude; Model 2: adjusted for age and ASA category; Model 3: adjusted for age, ASA category, type of surgery, previous diagnosis of dementia, previous diagnosis of atrial fibrillation, and antithrombotic therapy. | |||||
| b Model 1: crude; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, type of surgery, previous diagnosis of dementia, previous diagnosis of atrial fibrillation, and antithrombotic therapy. | |||||
| c Statistically significant. | |||||
| Hours to surgery | Cases | Events (%) | Model 1 | HRs (95% CI) Model 2 | Model 3 |
| Women a | 39,526 | 1,522 (4) | |||
| < 12 | 9,227 | 295 (3) | Ref | Ref | Ref |
| 12–24 | 19,476 | 704 (4) | 1.1 (0.99–1.3) | 1.1 (0.97–1.3) | 1.1 (0.95–1.3) |
| > 24 | 10,823) | 523 (5) | 1.5 (1.3–1.8)c | 1.5 (1.3–1.7)c | 1.3 (1.1–1.5)c |
| Men a | 17,193 | 962 (6) | |||
| < 12 | 3,873 | 188 (5) | Ref | Ref | Ref |
| 12–24 | 8,142 | 441 (5) | 1.1 (0.94–1.3) | 1.1 (0.90–1.3) | 1.1 (0.89–1.5) |
| > 24 | 5,178 | 333 (6) | 1.3 (1.12–1.6)c | 1.3 (1.1–1.5)c | 1.2 (0.99–1.4) |
| ASA 1–2 b | 25,048 | 494 (2) | |||
| < 12 | 6,380 | 114 (2) | Ref | Ref | Ref |
| 12–24 | 12,348 | 242 (2) | 1.1 (0.89–1.4) | 1.1 (0.91–1.4) | 1.1 (0.91–1.4) |
| > 24 | 6,320 | 138 (2) | 1.2 (0.95–1.6) | 1.3 (1.02–1.7)c | 1.2 (0.95–1.6) |
| ASA 3–4 b | 31,671 | 1,990 (6) | |||
| < 12 | 6,720 | 369 (5) | Ref | Ref | Ref |
| 12–24 | 15,270 | 903 (6) | 1.1 (0.95–1.2) | 1.1 (0.96–1.2) | 1.1 (0.94–1.2) |
| > 24 | 9,681 | 718 (7) | 1.4 (1.2–1.6)c | 1.4 (1.2–1.6)c | 1.3 (1.1–1.4)c |
| a Model 1: crude; Model 2: adjusted for agea and ASA category; Model 3: adjusted for age, ASA category, type of surgery, previous diagnosis of dementia, previous diagnosis of congestive heart failure, and antithrombotic therapy. | |||||
| b Model 1: crude; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, type of surgery, previous diagnosis of dementia, previous diagnosis of congestive heart failure, and antithrombotic therapy. | |||||
| c Statistically significant. | |||||
| Hours to surgery | Cases | Events (%) | Model 1 | HRs (95% CI) Model 2 | Model 3 |
| Women a | 39,526 | 1,522 (4) | |||
| < 12 | 9,227 | 295 (3) | Ref | Ref | Ref |
| 12–24 | 19,476 | 704 (4) | 1.1 (0.99–1.3) | 1.1 (0.97–1.3) | 1.1 (0.95–1.3) |
| > 24 | 10,823) | 523 (5) | 1.5 (1.3–1.8)c | 1.5 (1.3–1.7)c | 1.3 (1.1–1.5)c |
| Men a | 17,193 | 962 (6) | |||
| < 12 | 3,873 | 188 (5) | Ref | Ref | Ref |
| 12–24 | 8,142 | 441 (5) | 1.1 (0.94–1.3) | 1.1 (0.90–1.3) | 1.1 (0.89–1.5) |
| > 24 | 5,178 | 333 (6) | 1.3 (1.12–1.6)c | 1.3 (1.1–1.5)c | 1.2 (0.99–1.4) |
| ASA 1–2 b | 25,048 | 494 (2) | |||
| < 12 | 6,380 | 114 (2) | Ref | Ref | Ref |
| 12–24 | 12,348 | 242 (2) | 1.1 (0.89–1.4) | 1.1 (0.91–1.4) | 1.1 (0.91–1.4) |
| > 24 | 6,320 | 138 (2) | 1.2 (0.95–1.6) | 1.3 (1.02–1.7)c | 1.2 (0.95–1.6) |
| ASA 3–4 b | 31,671 | 1,990 (6) | |||
| < 12 | 6,720 | 369 (5) | Ref | Ref | Ref |
| 12–24 | 15,270 | 903 (6) | 1.1 (0.95–1.2) | 1.1 (0.96–1.2) | 1.1 (0.94–1.2) |
| > 24 | 9,681 | 718 (7) | 1.4 (1.2–1.6)c | 1.4 (1.2–1.6)c | 1.3 (1.1–1.4)c |
| a Model 1: crude; Model 2: adjusted for age and ASA category; Model 3: adjusted for age, ASA category, type of surgery, previous diagnosis of dementia, previous diagnosis of pneumonia, and antithrombotic therapy. | |||||
| b Model 1: crude; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, type of surgery, previous diagnosis of dementia, previous diagnosis of pneumonia, and antithrombotic therapy. | |||||
| c Statistically significant. | |||||
| Hours to surgery | Cases | Events (%) | Model 1 | ORs (95% CI) Model 2 | Model 3 |
| All | 63,998 | 3,078 (5) | |||
| < 12 | 14,517 | 612 (4) | Ref | Ref | Ref |
| 12–24 | 30,863 | 1,432 (5) | 1.1 (1.0–1.2) | 1.1 (0.98–1.2) | 1.1 (0.97–1.2) |
| > 24 | 18,618 | 1,034 (6) | 1.3 (1.2–1.5) a | 1.3 (1.1–1.4) a | 1.2 (1.1–1.4 a |
| Model 1: crude; Model 2: adjusted for age, sex, and ASA category; Model 3: adjusted for age, sex, ASA category, type of surgery, previous diagnosis of dementia, previous diagnosis of pneumonia, and antithrombotic therapy. | |||||
| a Statistically significant. | |||||
| Hours to surgery | Cases | Events (%) | Model 1 | HRs (95% CI) Model 2 | Model 3 |
| Women a | 42,917 | 1,104 (3) | |||
| < 12 | 9,898 | 236 (2) | Ref | Ref | Ref |
| 12–24 | 21,016 | 514 (2) | 1.0 (0.88–1.2) | 1.0 (0.87–1.2) | 1.0 (0.86–1.2) |
| > 24 | 12,003 | 354 (3) | 1.3 (1.1–1.5) c | 1.2 (1.02–1.4) c | 1.2 (0.99–1.4) |
| Men a | 19,167 | 752 (4) | |||
| < 12 | 4,232 | 141 (3) | Ref | Ref | Ref |
| 12–24 | 9,012 | 374 (4) | 1.2 (1.02–1.5) c | 1.2 (0.99–1.5) | 1.2 (1.0–1.5) |
| > 24 | 5,923 | 237 (4) | 1.2 (0.98–1.5) | 1.1 (0.93–1.4) | 1.1 (0.92–1.4) |
| ASA 1–2 b | 25,526 | 470 (2) | |||
| < 12 | 6,476 | 111 (2) | Ref | Ref | Ref |
| 12–24 | 12,565 | 225 (2) | 1.0 (0.83–1.3) | 1.1 (0.84–1.3) | 1.1 (0.84–1.3) |
| > 24 | 6,485 | 134 (2) | 1.2 (0.94–1.6) | 1.2 (0.96–1.6) | 1.2 (0.95–1.6) |
| ASA 3–4 b | 36,558 | 1,386 (4) | |||
| < 12 | 7,654 | 266 (3) | Ref | Ref | Ref |
| 12–24 | 17,463 | 663 (4) | 1.1 (0.94–1.3) | 1.1 (0.95–1.3) | 1.1 (0.95–1.3) |
| > 24 | 11,441 | 457 (4) | 1.2 (0.99–1.3) | 1.2 (0.99–1.3) | 1.1 (0.97–1.3) |
| a Model 1: crude; Model 2: adjusted for age and ASA category; Model 3: adjusted for age, ASA category, type of surgery, previous diagnosis of dementia, previous diagnosis of acute ischemia (stroke/intracranial bleeding or myocardial infarction or acute kidney failure), and antithrombotic therapy. | |||||
| b Model 1: crude; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, type of surgery, previous diagnosis of dementia, previous diagnosis of acute ischemia (stroke/intracranial bleeding or myocardial infarction or acute kidney failure), and antithrombotic therapy. | |||||
| c Statistically significant. | |||||