Effects of extended oral antibiotic prophylaxis on surgical site infections after instrumented spinal fusion: a cohort study of 901 patients with a minimum follow-up of 1 year
Miguel MENENDEZ GARCIA 1, Iñaki OTERMIN MAYA 2, Julian LIBRERO LOPEZ 3, Jorge GUTIERREZ DUBOIS 2, Diego MANRIQUE CUEVAS 1, Jose Ignacio ALAEZ CRUZ 2, Leyre AZCONA SALVATIERRA 1, Isabel AYECHU DIAZ 1, and Angel M HIDALGO OVEJERO 1
1 Spine Surgery Unit, Department of Trauma and Orthopaedics, Hospital Universitario de Navarra, Pamplona (Navarra), 2 Internal Medicine Department, Hospital Universitario de Navarra, Pamplona (Navarra), 3 Research Methodology Unit, Navarrabiomed, Pamplona (Navarra), Spain
Background and purpose — We aimed to determine whether an extended oral antibiotic prophylaxis protocol may reduce the rate of surgical site infection (SSI) in patients undergoing instrumented spinal fusion.
Patients and methods — This retrospective cohort study comprise 901 consecutive patients subjected to spinal fusion between September 2011 and December 2018 with a minimum 1-year follow-up. 368 patients operated on between September 2011 and August 2014 were administered standard intravenous prophylaxis. 533 patients operated on between September 2014 and December 2018 were administered an extended protocol with 500 mg of oral cefuroxime axetil every 12 hours (clindamycin or levofloxacin in allergic individuals) until the removal of sutures. SSI was defined following the Centers for Disease Control and Prevention criteria. The association between risk factors and the incidence of SSI was evaluated by odds ratio (OR) with a multiple logistic regression model.
Results — The bivariate analysis showed a statistically significant association between SSI and the type of prophylaxis used (“extended”’ = 1.7% vs. “standard” = 6.2%, p= 0.001), with a lower proportion of superficial SSIs with the extended regimen (0.8% vs. 4.1%, p = 0.001). The multiple logistic regression model showed an OR = 0.25 (95% confidence interval [CI] 0.10–0.53) for extended prophylaxis and an OR = 3.5 (CI 1.3–8.1) for non-beta-lactams antibiotics.
Conclusion — Extended antibiotic prophylaxis seems to be associated with a reduction in the incidence of superficial SSI in instrumented spine surgery.
Citation: Acta Orthopaedica 2023; 94: 80–86. DOI: https://doi.org/10.2340/17453674.2023.9409.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-07-17. Accepted: 2023-01-18. Published: 2023-02-21.
Correspondence: miguel.menendez.garcia@navarra.es
MMG, IOM, JGD, and JIAC contributed to the data collection. All authors contributed to the study design and interpretation of the results. JLL contributed to the statistical analyses. MMG wrote the initial manuscript and all authors revised and approved the manuscript.
Handling co-editors: Eivind Witsø and Philippe Wagner
Acta thanks Scott Boden, Federico Canavese, and Rihard Trebse for help with peer review of this study.
The last few decades have seen an increase in spinal fusion surgery across the world combined with a reduction in noninstrumented procedures (1,2). Instrumented spinal fusion is associated with a risk of surgical site infection (SSI) 2 or even 3 times higher than that of non-instrumented procedures, with rates ranging between 1.4% and 13%, depending on the type of surgery (3). Therefore, its prevention is a priority for any spine surgeon. In this respect, systemic antimicrobial prophylaxis (SAP) has been shown to be an effective tool (4).
Regarding the duration of SAP, the general consensus favors single-dose antibiotic regimens or no longer than 24 hours postoperatively (5). For spine surgery, the North American Spine Society (NASS) guidelines recommend a single preoperative dose and as many intraoperative doses as may be required for uncomplicated procedures, although they recommend considering alternative prophylactic regimens for patients with comorbidities or undergoing complex surgery (6). But, despite these recommendations, some surgeons continue to administer antibiotics in the postoperative days.
Extended SAP could result in acute renal failure or pseudomembranous colitis and aggravate the problem of antibiotic resistance, which has been estimated to cause 10 million deaths and $100 billion in increased costs by 2050 (7,8).
The primary aim of our study is to determine whether the extended oral antibiotic prophylaxis (EOAP) protocol reduced the SSI rate among patients undergoing instrumented spinal fusion. The secondary aim included identifying other risk factors for SSI, and relating the prophylactic regimen to length of stay, readmissions in the first month, and death.
Patients and methods
Patients
A retrospective review was carried out of a cohort of 901 patients subjected to instrumented spine surgery between September 2011 and December 2018 at surgical ward D of the Navarre Hospital Complex (Pamplona, Spain). All the patients were included in the hospital’s database by means of a prospective and consecutive sampling process; patients were not randomized according to the type of prophylaxis received. STROBE guidelines were followed to report this study.
Antibiotic prophylaxis protocols
Patients operated on between September 2011 and August 2014 (standard SAP [S-SAP] group) received an intravenous prophylaxis protocol consisting of administration of 2 g IV cefazolin 30 minutes preoperatively, any required additional doses during the procedure, and 1 g IV cefazolin every 8 hours until removal of the surgical drainage (24 hours after the procedure when drainage was < 200 mL or, otherwise, within the first 48 hours). Required additional dosages were given every 3 hours or when a blood loss of 1,500 mL was estimated. Patients with allergy to beta-lactams received alternative antibiotics with 600 mg IV clindamycin 30 minutes before the procedure, with any required intraoperative doses, and a subsequent 300 mg IV clindamycin every 8 hours until removal of the surgical drain.
Patients operated on between September 2014 and December 2018 (extended oral SAP group [EOAP]) received the protocol described above with the addition of 500 mg oral cefuroxime axetil every 12 hours until removal of the sutures from the surgical wound. Those patients in the EOAP group with allergy to beta-lactams were given the perioperative regimen with 600 mg IV clindamycin extended with 300 mg oral every 8 hours, or 750 mg IV levofloxacin extended with 750 mg oral every 24 hours.
There was no intentional variation between the groups with respect to surgical technique, surgical team, preoperative disinfection protocol, surgical site preparation, or postoperative wound care. Every patient was followed up for at least 1 year.
SSI definition, variables analyzed and exclusions
SSI subtypes (superficial, deep, and organ-space [O-S]) were defined according to the surveillance definitions for specific types of infections established by the Centers for Disease Control and Prevention (CDC) (9): (i) purulent drainage from a superficial incision (skin and subcutaneous tissue), deep layers (subfascial muscles and soft tissues), or organ-space (O-S) layers (intervertebral disc, epidural space, or implant infection); (ii) organism identification by culture from an aseptically obtained fluid or tissue; (iii) incision that spontaneously dehisces or is deliberately opened by a surgeon or physician, and the patient has at least 1 of the following: localized pain or tenderness, localized inflammation (heat, erythema, and swelling), or fever (> 38°C); and (iv) evidence of abscess on images or surgical revision. Implant-related infection was confirmed following the criteria of the International Consensus Meeting on Surgical Site and Periprosthetic Joint Infection (10).
Apart from the type of prophylaxis used (S-SAP vs. EOAP) and the type of SSI (superficial, deep, or O-S), the following patient-dependent variables were gathered: age, sex, anesthetic risk according to the American Society of Anesthesiologists (ASA score), diabetes mellitus (DM), body mass index (BMI) and obesity (BMI > 30), immunosuppression, smoking, and use of non-beta-lactam antibiotics. The surgery-dependent variables analyzed were: diagnosis (deformities of any kind, degenerative conditions [excluding deformities] and trauma), duration of surgery (DoS) in minutes and greater than 75th percentile (DoS > p75), type of bone graft used (allograft, autograft, or demineralized bone matrix [DBM]), number of levels, short fusion (1–2 levels), region (cervical, thoracic, thoracolumbar, lumbar), use of sacral or iliac screws, reoperation, and durotomy. Finally, the following postoperative variables were analyzed: duration of EOAP in days, length of hospital stay in days, readmission within 30 days from discharge, and death.
Exclusion criteria were spine surgery without implants, having been admitted to the intensive care unit (ICU) after surgery, having received topical vancomycin, having received an antibiotic prophylaxis other than S-SAP or EOAP mentioned above, and having an active infection either in the spine or in another region. Those patients with multiple traumata requiring surgery at other levels with additional prophylaxis, those aged under 14 years, and those with an American Anesthesiology Association (ASA) score of IV or higher were not included in the database as they were operated on in a different surgical ward.
Statistics
Descriptive analyses were conducted using frequency distributions for categorical variables and central tendency measures for quantitative variables. Non-adjusted comparisons of categorical variables were performed using Pearson’s chisquare test when ≤ 20% of expected cell counts were < 5, or Fisher’s non-parametric exact test if > 20% of expected cell counts were < 5 (immunosuppression, iliac screws, durotomy, readmission, and death). Continuous variables were analyzed by means of Student’s t-test (duration of EOAP, DoS in minutes, BMI, and number of levels) or the Mann–Whitney U-test (length of stay).
A multiple logistic regression model was designed to obtain adjusted odds ratios (OR) with 95% confidence intervals (CIs) as estimations of the effect of the SAP regimens on SSI. Moreover, the adjusted number needed to treat (NNT) was calculated from the adjusted OR and the mean risk of unexposed persons estimated by means of logistic regression (OR approach) (11), using an online calculator (12). Given the observational nature of the study, the variables included in the model were selected based on an analysis of the cause–effect relations between them using directed acyclic graphs (DAG) and the DAGitty. net software to facilitate estimation of cause–effect relations, comparison of hypotheses, and statistical analysis (13,14). Following application of this method, the variables identified as confounders were use of non-beta-lactam antibiotics and BMI as both were related to the exposure and the outcome variables. Model fit was assessed by calibration with the Hosmer–Lemeshow test, and discrimination with Statistic C.
The statistical analyses were carried out with the R statistical software, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at an alpha value of 0.05.
Ethics, funding, data sharing, and potential conflicts of interest
This study was approved by the Navarra Research Ethics Committee (approval reference PI_2019/128). The patient consent for data collection and surgery was obtained and protected in the registry. This study did not receive any form of grants or funding. Due to privacy regulation, the original data cannot be shared. Anonymized summary tables can be shared on reasonable request. The authors declare no conflict of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.9409
Results
Baseline data
After exclusions, data from a total of 901 patients was collected with 553 in the EOAP group and 368 in the S-SAP group (Figure 1). Missing values existed in ASA score (n = 17), diabetes (n = 16), smoking (n = 10), and obesity (n = 2). As these values represented < 5% of the cases, a complete record analysis was used.
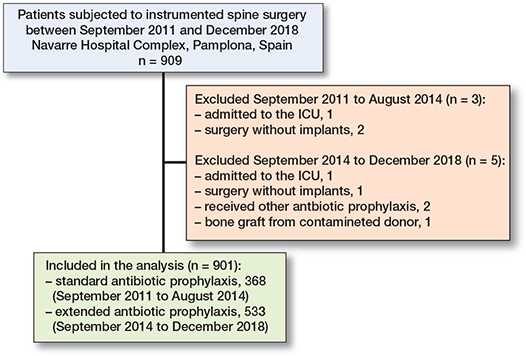
Figure 1. Flowchart of patients undergoing instrumented spine surgeries who were excluded or included in the study analysis.
Significant differences between the groups were found with respect to some surgery-related characteristics (Table 1, see Appendix). Patients in the EOAP group had undergone more deformity-related procedures than those in the S-SAP group (16% vs. 12%, p = 0.02). In relation to this, those patients in the EOAP group had the highest number of iliac screws (8% vs. 2%, p = 0.001), more non-infection-related reoperations (35% vs. 24%, p = 0.001), and received allograft more frequently (41% vs. 2%, p < 0.001).
Regarding the length of the procedure, surgery exceeded the 75th percentile of time (set at 250 minutes in our sample) in 30% of patients in the S-SAP group versus 23% of those in the EOAP group. However, the mean DoS difference between the groups was only 9 minutes (p = 0.04).
Regarding the postoperative variables, the duration of extended antibiotic prophylaxis was 7.9 days (SD 3.0). In addition, patients in the EOAP group had a lower proportion of readmissions in the first month (0.2% vs. 2.2%, p = 0.004) (Table 4).
Primary outcome
Patients in the EOAP group developed a significantly lower overall proportion of SSI than those in the S-SAP group (1.7% vs. 6.2%, p = 0.001). The differences were found mainly in patients with superficial SSI (0.8% vs. 4.1%, p = 0.001) rather than those with deep or organ-space infections (0.9% vs. 2.2%, p = 0.2) (Table 2).
| SSI | Total n = 901 | EOAP n = 533 | S-SAP n = 368 | p-value |
| Overall | 32 (3.6) | 9 (1.7) | 23 (6.2) | 0.001 |
| Superficial | 19 (2.1) | 4 (0.8) | 15 (4.1) | 0.001 |
| Deep/organ-space | 13 (1.4) | 5 (0.9) | 8 (2.2) | 0.2 |
| For abbreviations, see Table 1. | ||||
Secondary outcomes
The number and proportion of patients with SSI according to the type of prophylaxis received (standard vs. extended), and whether beta-lactams were administered, is given in Table 3. We found an association between the use of non-beta-lactam antibiotics and the presence of SSI. In the group of 32 patients diagnosed with SSI, 7 received non-beta-lactam antibiotics, compared with the group of 869 patients without SSI of whom only 66 received this type of drugs (p = 0.01) (Table 2).
| SSI | EOAP n = 533 | S-SAP n = 368 | |||
| Cefazoline IV + cefuroxime oral 500 mg/12 h n = 491 | Clindamycine IV + clindamycine oral 300 mg/8 h n = 20 | Levofloxacin IV + levofloxacin oral 750 mg/24 h n = 19 | Cefazoline IV n = 337 | Clindamycine IV n = 31 | |
| Superficial | 3 (0.6) | 0 | 1 | 11 (3.3) | 4 |
| Deep/organ-space | 3 (0.6) | 1 | 1 | 8 (2.4) | 0 |
| IV = intravenous. For other abbreviations, see Table 1. | |||||
The presence of SSI was associated with longer hospital stays and a higher proportion of readmissions during the first month postoperatively (Table 4).
| Factor | Total n = 901 | EOAP n = 533 | S-SAP n = 368 | No SSI n = 869 | SSI n = 32 |
| Days of EOAP, mean (SD) | 7.9 (3.0) | 7.9 (3.0) | N/A | 7.8 (3.0) | 10.9 (4.6) |
| Days of hospital stay, median (IQR) a | 10 (3) | 10 (3) | 10 (4) | 10 (3) | 22 (20) |
| Readmissions at 30 days a, b | 9 (1.0) | 1 (0.2) | 8 (2.2) | 6 (0.7) | 3 |
| Death | 2 (0.2) | 0 | 2 (0.5) | 2 (0.2) | 0 |
| a Statistically significant difference between patients who suffered SSI and those who did not (p-value < 0.001). | |||||
| b Statistically significant difference between EOAP and S-SAP groups (p-value = 0.004). | |||||
| IQR = interquartile range. For other abbreviations, see Table 1. | |||||
The analysis of the cause–effect association between type of prophylaxis, SSI, and the minimum set of confounders to be controlled was based on a DAG (Figure 2). After adjusting for confounders, BMI and use of non-beta-lactam antibiotics (Figure 3), the model showed that EOAP protocol was associated with a lower SSI risk with an OR = 0.25 (CI 0.10–0.53). In the EAOP group, the adjusted NNT to avoid a case of SSI was 22 patients (CI 19–39). The use of non-beta-lactams was an independent risk factor associated with SSI, with an OR = 3.5 (CI 1.3 – 8.1) (Table 5).
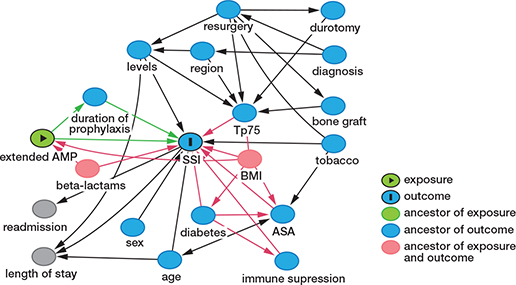
Figure 2. Directed acyclic graph (DAG) describing the potential cause–effect association between the extended antimicrobial prophylaxis (AMP), the surgical site infection (SSI), and the rest of the variables in the model. Confounders and biased relationships are shown in red.
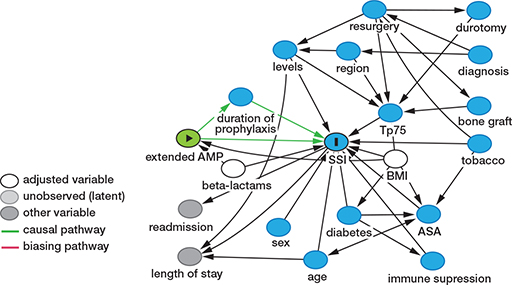
Figure 3. Directed acyclic graph (DAG) describing the total effect of the extended antimicrobial prophylaxis (AMP) on the surgical site infection (SSI) after adjusting for confounders (shown in white).
Discussion
We found a significant association between extending SAP into the postoperative days and a lower proportion of SSI due to reduction in superficial but not deep infections.
Our results contrast with current evidence as very few authors have found extended prophylactic regimens to be beneficial in spine surgery. Maciejczak et al., for example, found a significant 3.6% reduction in the SSI rate in patients undergoing instrumented spine surgery who were administered extended intravenous SAP for 72 hours (15). However, their results do not coincide with those of other authors (16), who did not find any differences with a similar regimen.
In a randomized study, Hellbusch et al. compared a group of patients who received a single dose of IV cefazolin with a group that received IV cefazolin for 3 days supplemented with an oral dose of oral cefalexin every 6 hours for 7 days. Although the SSI rate fell from 4.3% to 1.7% with the extended prophylactic regimen, the difference did not reach statistical significance (17). Although the regimen proposed by Hellbusch et al. was similar to that investigated in our study, those authors only evaluated 233 patients. Our study analyzed a larger number of patients and obtained a statistically significant difference.
Another study, by Warren et al., studied the proportion of patients undergoing spinal fusion who were kept on oral SAP following hospital discharge (18). This study found that the most commonly administered antibiotics following discharge were ciprofloxacin, trimethoprim/sulfamethoxazole, and cefalexin. These authors did not find any significant differences in the SSI rate between the patients who received standard and extended prophylaxis.
In our study, there were no patient- or surgery-related characteristics that led to a change in the SAP regimen administered, thus avoiding selection biases. Despite this, given that administration of the drug was not adjusted for bodyweight, the same antibiotic dose could have had different effects in patients with different BMIs. In addition, those patients allergic to beta-lactams had to receive alternative antibiotics. These were therefore the confounders for which the multivariate model was adjusted.
In terms of risk factors, our study confirmed the findings of previous studies with regard to the fact that the prophylactic effect of beta-lactams is superior to that of other drugs (19). In relation to this, we should also mention that, in our health area, the overall percentage of gram-positive and gramnegative bacterial strains that are sensitive to clindamycin and levofloxacin ranges from 60% to 80% (20).
All other classical risk factors (21), both patient- and surgery-dependent, did not appear to be related to SSI. Diabetes mellitus, although on the verge of statistical significance, did not show any association either, which could be attributable to the perioperative patient optimization usually performed in our hospital, as all those who are going to receive spinal instrumentation are previously evaluated in consultation with the Internal Medicine service.
Strengths
It must be noted that the groups were comparable in terms of patient characteristics, although surgeries in the EOAP group were more complex (more deformity surgeries, more reoperations, iliac screws…), which would seem to reinforce the association observed between a lower SSI risk and the extended prophylaxis regimen. However, surgeries in the EOAP group had a mean duration of 9 minutes less, which seems insignificant, but could reflect a greater experience or focus of the surgical team that could be relevant to infection. On the other hand, both groups were also comparable in terms of postoperative surgical wound care, and all followed the same sterile gauze dressing policy on the inpatient ward.
Another strength of our study is the large size of the sample analyzed, although, without a previous sample size calculation, 901 patients are a sufficient sample to detect a reduction, between comparable groups, of approximately 4% (from 6.2% to 1.7%) in the incidence of SSI. Nevertheless, as our results are contrary to the latest published evidence (22), it is paramount to consider the study’s potential limitations.
Limitations
The most evident of these is the observational retrospective design and the failure to randomize patients according to the type of antimicrobial prophylaxis (AMP) regimen administered. In addition to the above, the number of patients with SSI was low, which limited the number of covariates that could be included in the multivariate model. Moreover, although a DAG may help decide what variables to include in the analysis, it is still an oversimplification of reality (23), which hampers the accuracy of the predictions made by the model as the causes of SSI tend to be multifactorial. In fact, even if small imbalances in the variables classically related to infection were not significant, they could have affected the SSI rate of the study. In addition, some variables that have not been taken into consideration may have influenced the SSI rate, such as the surgeons’ technical skill, the number of staff members available, the exact time at which the first antibiotic dose was administered (5) or the implementation of care bundles, which have been shown to significantly reduce the SSI rate as well as the costs associated with spine surgery (24).
It should be noted that the infection rate in the S-SAP group (6.2%) was higher than the pooled rate published in recent meta-analyses (4.4%) (3), so it is possible that strategies such as ours may be more effective when the baseline infection rate is high. However, we believe it is necessary to apply infection control strategies such as controlling the patient’s modifiable risk factors, ensuring administration of the antibiotic within 1 hour before surgery, implementing hygiene measures and preoperative bathing with chlorhexidine gluconate and hemostasis, controlling operating room traffic, and maintaining normothermia and oxygen therapy among other options (25,26) before introducing any changes to the SAP regimen. Acting simultaneously on multiple risk factors may indeed be effective in reducing the SSI rate and preventing complications associated with antibiotic overuse such as acute renal failure, pseudomembranous colitis, microbial resistance (8), and increased health care costs (7).
Conclusion
In conclusion, the SSI rate obtained in our study for patients who received an extended oral prophylactic regimen during the early postoperative period was lower than that obtained in those who received the usual intravenous regimen. This difference was mainly due to a lower proportion of superficial SSI but not deep infection. To expand the existing evidence base, further, larger-scale studies are needed, which should randomize patients according to the type of prophylaxis received.
- Reisener M J, Pumberger M, Shue J, Girardi F P, Hughes A P. Trends in lumbar spinal fusion: a literature review. J Spine Surg 2020; 6(4): 752-61. doi: 10.21037/jss-20-492.
- Sheikh S R, Thompson N R, Benzel E, Steinmetz M, Mroz T, Tomic D, et al. Can we justify it? Trends in the utilization of spinal fusions and associated reimbursement. Neurosurgery 2020; 86(2): E193-202. doi: 10.1093/neuros/nyz400.
- Zhou J, Wang R, Huo X, Xiong W, Kang L, Xue Y. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2020; 45(3): 208-16. doi: 10.1097/BRS.0000000000003218.
- Barker F G. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery 2002; 51(2): 391-400; discussion 400-1.
- Bratzler D W, Dellinger E P, Olsen K M, Perl T M, Auwaerter P G, Bolon M K, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013; 14(1): 73-156. doi: 10.2146/ajhp120568.
- Shaffer W O, Baisden J L, Fernand R, Matz P G, North American Spine Society. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J 2013; 13(10): 1387-92. doi: 10.1016/j.spinee.2013.06.030.
- O’Neill J. Tackling drug resistant infections globally: Final report and recommendations. The Review on Antimicrobial Resistance; 2016 [Internet]. [cited May 27, 2021]. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
- Branch-Elliman W, O’Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019; 154(7): 590-8. doi: 10.1001/jamasurg.2019.0569.
- Horan T C, Andrus M, Dudeck M A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36(5): 309-32. doi: 10.1016/j.ajic.2008.03.002.
- Parvizi J, Tan T L, Goswami K, Higuera C, Della Valle C, Chen A F, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018; 33(5): 1309-1314.e2. doi: 10.1016/j.arth.2018.02.078.
- Bender R, Kuss O, Hildebrandt M, Gehrmann U. Estimating adjusted NNT measures in logistic regression analysis. Stat Med 2007; 26(30): 5586-95. doi: 10.1002/sim.3061.
- Visual Rx—Dr Chris Cates’ EBM Website [Internet]. [cited Oct 11, 2022]. Available from: http://www.nntonline.net/visualrx/
- Textor J, van der Zander B, Gilthorpe M S, Liskiewicz M, Ellison G T. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol 2016; 45(6): 1887-94. doi: 10.1093/ije/dyw341.
- Tennant P W G, Murray E J, Arnold K F, Berrie L, Fox M P, Gadd S C, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol 2021; 50(2): 620-32. doi: 10.1093/ije/dyaa213.
- Maciejczak A, Wolan-Nieroda A, Wałaszek M, Kołpa M, Wolak Z. Antibiotic prophylaxis in spine surgery: a comparison of single-dose and 72-hour protocols. J Hosp Infect 2019; 103(3): 303-10. doi: 10.1016/j.jhin.2019.04.017.
- Marimuthu C, Abraham V T, Ravichandran M, Achimuthu R. Antimicrobial prophylaxis in instrumented spinal fusion surgery: a comparative analysis of 24-hour and 72-hour dosages. Asian Spine J 2016; 10(6): 1018-22. doi: 10.4184/asj.2016.10.6.1018.
- Hellbusch L C, Helzer-Julin M, Doran S E, Leibrock L G, Long D J, Puccioni M J, et al. Single-dose vs multiple-dose antibiotic prophylaxis in instrumented lumbar fusion--a prospective study. Surg Neurol 2008; 70(6): 622-7; discussion 627. doi: 10.1016/j.surneu.2007.08.017.
- Warren D K, Nickel K B, Han J H, Tolomeo P, Hostler C J, Foy K, et al. Postdischarge antibiotic use for prophylaxis following spinal fusion. Infect Control Hosp Epidemiol 2020; 41(7): 789-98. doi: 10.1017/ice.2020.117.
- Wyles C C, Hevesi M, Osmon D R, Park M A, Habermann E B, Lewallen D G, et al. John Charnley Award: Increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: the value of allergy testing for antibiotic prophylaxis. Bone Joint J 2019; 101-B(6_Supple_B): 9-15. doi: 10.1302/0301-620X.101B6.BJJ-2018-1407.R1.
- Guía antimicrobiana HUN (adultos).pdf [Internet]. [cited Dec 21, 2022]. Available from: https://gcextsalud.navarra.es/Salud03/CHN/Estructura/JTACC/ComInfecc/Gua%20antimicrobiana%20tratamiento%20y%20profilaxis/Gu%C3%ADa%20antimicrobiana%20HUN%20(adultos).pdf
- Pull ter Gunne A F, Hosman A J F, Cohen D B, Schuetz M, Habil D, van Laarhoven C J H M, et al. A methodological systematic review on surgical site infections following spinal surgery, Part 1: risk factors. Spine (Phila Pa 1976) 2012; 37(24): 2017-33. doi: 10.1097/BRS.0b013e31825bfca8.
- Orenday-Barraza J M, Cavagnaro M J, Avila M J, Strouse I M, Farhadi D S, Dowell A, et al. Is the routine use of systemic antibiotics after spine surgery warranted? A systematic review and meta-analysis. Eur Spine J 2022; 31(10): 2481-92. doi: 10.1007/s00586-022-07294-9.
- Pollmann C T, Dahl F A, Røtterud J H M, Gjertsen J E, Årøen A. Surgical site infection after hip fracture—mortality and risk factors: an observational cohort study of 1,709 patients. Acta Orthop 2020; 91(3): 347-52. doi: 10.1080/17453674.2020.1717841.
- Agarwal N, Agarwal P, Querry A, Mazurkiewicz A, Tempel Z J, Friedlander R M, et al. Implementation of an infection prevention bundle and increased physician awareness improves surgical outcomes and reduces costs associated with spine surgery. J Neurosurg Spine 2018; 29(1): 108-14. doi: 10.3171/2017.11.SPINE17436.
- Bagga R S, Shetty A P, Sharma V, Vijayanand K S S, Kanna R M, Rajasekaran S. Does preventive care bundle have an impact on surgical site infections following spine surgery? An analysis of 9607 patients. Spine Deform 2020; 8(4): 677-84. doi: 10.1007/s43390-020-00099-0.
- Nasser R, Kosty J A, Shah S, Wang J, Cheng J. Risk factors and prevention of surgical site infections following spinal procedures. Global Spine J 2018; 8(4 Suppl.): 44S-48S. doi: 10.1177/2192568218806275.
Appendix
| Factor | Total n = 901 | EOAP n = 533 | S-SAP n = 368 | No SSI n = 869 | SSI n = 32 | p-value |
| Age, mean (SD) | 54.5 (14) | 54.6 (14) | 54.3 (14) | 54.5 (14) | 55.2 (12) | |
| Women | 457 (51) | 275 (52) | 182 (49) | 440 (51) | 17 | |
| ASA score | ||||||
| I | 279 (31) | 154 (29) | 125 (34) | 273 (31) | 6 | |
| II | 468 (52) | 273 (51) | 195 (53) | 448 (52) | 20 | |
| > II | 137 (15) | 92 (17) | 45 (12) | 131 (15) | 6 | |
| Missing | 17 (1.9) | 14 (2.6) | 3 (0.8) | 17 (2.0) | 0 | |
| Diabetes | 112 (12) | 60 (11) | 52 (14) | 104 (12) | 8 | |
| Missing | 16 (1.7) | 14 (2.6) | 2 (0.5) | 16 (1.8) | 0 | |
| BMI, mean (SD) | 27.9 (4.7) | 28.0 (4.9) | 27.7 (4.6) | 27.8 (4.7) | 28.6 (4.5) | |
| Obesity | 306 (34) | 192 (36) | 114 (31) | 294 (34) | 12 | |
| Missing | 2 (0.2) | 1 (0.1) | 1 (0.2) | 2 (0.2) | 0 | |
| Immunodepression | 14 (1.5) | 10 (1.8) | 4 (1.0) | 13 (1.5) | 1 | |
| Smoking | 351 (39) | 205 (38) | 146 (40) | 338 (39) | 13 | |
| Missing | 10 (1.1) | 5 (0.9) | 5 (1.3) | 9 (1.0) | 1 | |
| Non-beta-lactam antibiotics | 73 (8.8) | 42 (7.9) | 31 (8.4) | 66 (7.6) | 7 | 0.01 a |
| Diagnosis | 0.02 b | |||||
| Deformity | 129 (14) | 85 (16) | 44 (12) | 127 (15) | 2 | |
| Degenerative | 765 (85) | 447 (84) | 318 (86) | 735 (85) | 30 | |
| Trauma | 7 (0.8) | 1 (0.2) | 6 (1.6) | 7 (0.8) | 0 | |
| DoS minutes, mean (SD) | 218 (69) | 215 (74) | 224(61) | 218 (69) | 221 (78) | 0.04 b |
| DoS > Tp75 | 236 (26) | 124 (23) | 112 (30) | 226 (26) | 10 | 0.02 b |
| Missing | 12 (1.3) | 6 (1.1) | 6 (1.6) | 11 (1.2) | 1 | |
| Graft | < | 0.001 b | ||||
| Allograft | 225 (25) | 218 (41) | 7 (1.9) | 221 (25) | 4 | |
| Autograft | 241 (27) | 97 (18) | 144 (39) | 232 (27) | 9 | |
| DBM | 434 (48) | 218 (41) | 217 (59) | 416 (48) | 19 | |
| No. levels, mean (SD) | 2.7 (2.7) | 2.9 (3.0) | 2.5 (2.0) | 2.7 (2.7) | 2.8 (2.6) | |
| Short fixation | 620 (69) | 372 (70) | 248 (67) | 601 (69) | 19 | |
| Region | ||||||
| Cervical | 50 (5.5) | 26 (4.9) | 24 (6.5) | 50 (5.8) | 0 | |
| Thoracic | 10 (1.1) | 6 (1.1) | 4 (1.0) | 10 (1.1) | 0 | |
| Toracolumbar | 80 (8.9) | 58 (11) | 22 (6.0) | 79 (9.1) | 1 | |
| Lumbar | 761 (84) | 443 (83) | 318 (86) | 730 (84) | 31 | |
| Sacral screws | 468 (52) | 281 (53) | 187 (51) | 452 (52) | 16 | |
| Iliac screws | 52 (5.7) | 43 (8.0) | 9 (2.4) | 50 (5.7) | 2 < | 0.001 b |
| Reoperation | 271 (30) | 184 (35) | 87 (24) | 263 (30) | 8 < | 0.001 b |
| Durotomy | 58 (6.4) | 41 (7.7) | 17 (4.6) | 56 (6.4) | 2 | |
| a Statistically significant difference between patients who suffered SSI and those who did not. | ||||||
| b Statistically significant difference between EOAP and S-SAP groups. | ||||||
| Since missing data represented < 5% of the cases, a complete record analysis was used. EAOP = extended oral antibiotic prophylaxis group, S-SAP = standard systemic antibiotic prophylaxis, SSI = surgical site infection, SD = standard deviation, ASA = American Society of Anesthesiologists, BMI = body mass index, DoS = duration of surgery, DoS> Tp75 = duration of surgery greater than 75th percentile. | ||||||