Efficacy of epinephrine in local infiltration analgesia on pain relief and opioid consumption following total knee arthroplasty: a randomized controlled trial
Keerati CHAREANCHOLVANICH, Suphawat TANTITHAWORNWAT, Pakpoom RUANGSOMBOON, Rapeepat NARKBUNNAM, Swist CHATMAITRI, and Chaturong PORNRATTANAMANEEWONG
Department of Orthopaedic Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Background and purpose — Local infiltration analgesia (LIA) is one of the effective regimens to reduce pain after total knee arthroplasty (TKA). Epinephrine is a commonly used sympathetic adjunct in LIA. It is expected to enhance the intensity and extend the duration of LIA. The primary aim of the study was to evaluate the efficacy of epinephrine on postoperative pain control after primary TKA.
Patients and methods — A total of 80 patients who underwent primary TKA were randomized into an epinephrine (EN) and a control (C) group. Postoperative visual analogue pain score (VAPS) and morphine consumption were recorded every 6 hours until 48 hours after operation. The VAPS 6–48 hours were compared using repeated measure statistics. The range of motion (ROM) on discharge and complications were also compared between these 2 groups.
Results — The study showed that although VAPS differed statistically between the 2 groups at 12 hours (C higher) and 48 hours (C lower) postoperatively (p = 0.04 and 0.02, respectively), repeated measures analysis revealed that there were no significant differences in 6–48 hours VAPS (p = 0.6). Total morphine consumption in the EN and C groups was 3.4 (SD 3.7) and 4.2 (SD 4.4) mg, respectively (p = 0.4). ROM on discharge was also similar between the groups. No complications were detected in this study.
Conclusion — Our study showed that additional epinephrine in LIA had a statistically significant reduction in VAPS at 12 hours and morphine usage during 6–12 hours when compared with the control group. However, the magnitude of difference did not reach minimal clinically importance difference (MCID) value for TKA.
Citation: Acta Orthopaedica 2023; 94: 97–101. DOI: https://doi.org/10.2340/17453674.2023.8482.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-09. Accepted: 2023-01-09. Published: 2023-02-28.
Correspondence: toonchaturong@gmail.com
KC carried out the data collection and drafted the manuscript. ST, PR, and RN participated in the data collection and interpreted the data. SC performed statistical analysis and edited the manuscript. CP conceived the study design, interpreted the data, checked the statistical analysis, and drafted the manuscript. All authors read and approved the final manuscript.
The authors gratefully acknowledge Miss Nichakorn Khomawut for assistance with data collection and statistical analysis.
Handling co-editors: Li Felländer-Tsai and Jonas Ranstam
Acta thanks Johan Creutzfeldt and Rikard Wedin for help with peer review of this study.
Total knee arthroplasty (TKA) is a successful orthopedic procedure that can improve quality of life for patients with endstage knee osteoarthritis. Postoperative pain may affect patient satisfaction and delays recovery and is controlled by a multimodal pain control regimen (1). Local infiltration analgesia (LIA) or periarticular analgesic injection are useful adjuncts to this regimen. This technique is effective up to 24 hours after the operation (2). Incorporating a variety of ingredients in the periarticular cocktail is still debated.
Fundamentally, the ingredients of LIA include anesthetic base medication and added medications such as a nonsteroidal anti-inflammatory drug, corticosteroid, and sympathetic nervous system modulator (3). Epinephrine is the most commonly used sympathetic adjunct in LIA. The mechanism of epinephrine is a non-specific alpha- and beta-adrenergic agonist, which results in peripheral vasoconstriction. Therefore, it may help to decrease the absorption of other medications, enhance the intensity, and prolong the duration of LIA (4). Nevertheless, caution should be taken when utilizing peripheral epinephrine administration due to concern over skin necrosis and wound complication (3).
Because there is little data concerning the efficacy of epinephrine in LIA, we conducted a randomized controlled trial (RCT) to establish its efficacy in terms of pain reduction after primary TKA. The primary aim was to evaluate whether or not the presence of epinephrine in LIA could reduce early postoperative pain during the first 48 hours, and secondary aims were to evaluate the impact of epinephrine on morphine consumption and knee range of motion.
We hypothesized that adding epinephrine to the LIA regimen could further reduce postoperative pain and lower the morphine consumption after TKA.
Patients and methods
The study was reported according to CONSORT statement. Eligibility criteria for this study were patients aged between 40 and 80 years who were diagnosed with primary osteoarthritis of the knee scheduled to undergo unilateral TKA. The exclusion criteria were (i) severe deformity that required stem or metal augmentation, (ii) previous knee surgery, (iii) allergy to the drug used in the study, (iv) chronic kidney disease (estimated glomerular filtration rate < 60 mL/min), (v) liver cirrhosis, and (vi) refusal to participate. The patients were blinded to the intervention and randomly assigned into 2 groups: epinephrine (EN) and control (C) groups. A block-of-4 randomized sequence was generated using www.randomization.com and concealed by opaque envelopes. The process was done by a research coordinator who was not involved in the study. The flow of patients in this study is summarized in Figure 1.
3 experienced arthroplasty surgeons (RN, KC, and CP) performed all the procedures with the same surgical techniques. All operations were carried out under spinal anesthesia and adductor canal block. A tourniquet pressure of 300 mmHg was inflated before skin incision and deflated after skin closure. A mini-medial parapatellar approach was used (5). The neutral mechanical axis of the lower limb was restored. After trial components removal, a scrub nurse, who did not take part in the study, opened the concealed envelope and assigned the patients to the allocated treatment.
LIA, including 20 mL 0.5% bupivacaine, 30 mg ketorolac, and 0.6 mg epinephrine (1:1000) was used in the EN group while in the C group similar ingredients without epinephrine were used. LIA in each group was diluted with normal saline until the total volume was 100 mL. The surgeon was blinded to the intervention and injected LIA into 4 areas (25 mL per area); (i) posterior, posteromedial and posterolateral capsules, (ii) medial gutter, (iii) lateral gutter, and (iv) quadriceps muscles, retinacular tissue, pes anserinus, suprapatellar and infrapatellar fat pads (6). Cemented Nexgen LPS-Flex fixed bearing prostheses (Zimmer, Warsaw, IN, USA) were implanted without patellar resurfacing. A vacuum drain was placed intra-articularly and retained for 48 hours after surgery. The same rehabilitative program was started on the 1st postoperative day. For postoperative pain management until 48 hours after surgery, all patients received intravenous parecoxib (40 mg) every 12 hours and oral acetaminophen with codeine (300/15 mg) every 6 hours. Intravenous morphine (2 mg) every 2 hours was given as a rescue drug. All patients and outcome assessors were blinded to the intervention. Postoperative visual analog pain score (VAPS, ranging from 0 [no pain] to 10 [extreme pain]) and morphine consumption were recorded every 6 hours until 48 hours after TKA. Before discharge, postoperative range of motion (ROM) and complications including infection, skin necrosis, and wound complication were also recorded.
Sample size calculation
The primary outcome was VAPS during the first 48 hours. Based on our pilot study of 10 patients, the EN group had a mean difference of 1.5 points less in VAPS at 48 hours postoperatively than the C group with a standard deviation of 2.0 points; a sample size of 38 knees per group was calculated to have a power of 90% to detect a significant difference (alpha = 0.05). For anticipating possible losses of 5%, 40 knees were enrolled in each intervention.
Statistics
Our data was analyzed using SPSS program version 18.0 (SPSS Inc., Chicago, Illinois). Continuous data was presented as mean (standard deviation [SD]). Categorical data was presented as numbers and percentages. VAPS every 6 hours until 48 hours was compared using repeated measures statistics. VAPS at each time point, morphine consumption, and ROM before discharge were also compared using the independent t-test. Statistical significance was set as a p-value < 0.05.
Ethics, registration, funding, data sharing, and disclosures
This study was approved by our institutional review board (study identification number 696/2560) and registered with ClinicalTrials.gov (NCT03549221). Every process of the study was performed in agreement with the principles of the Helsinki Declaration. Written informed consent was obtained from all participants. This was an unfunded study. The data that supports the results of this study is available from the corresponding author upon reasonable request. All authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.8482
Results
40 patients in each group completed the study (Figure 1). There was no difference in baseline data, but the majority of our patients were females (Table 1). There were no significant differences in VAPS at any time points except VAPS at 12 (Control higher) and 48 hours (Control lower) postoperatively (Table 2). However, repeated measures statistics revealed no significant difference in VAPS every 6 hours until 48 hours between the 2 groups (p = 0.6) (Figure 2). Maximal VAPS within 24 hours and within 48 hours in both groups were also comparable. In terms of morphine consumption, there were no significant differences at any time points except at 6–12 hours postoperatively. Nevertheless, total morphine consumption was similar for both groups (Table 3). At discharge, there was no significant difference in postoperative ROM between the 2 groups (Table 3). No complication was observed in this study.
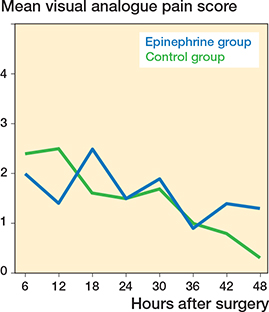
Figure 2. Mean of postoperative visual analogue pain score (VAPS) during the first 48 hours in both groups
Discussion
The present study aimed to evaluate the effect of epinephrine as part of the LIA regimen on pain score and morphine consumption after TKA. Our study showed that additional epinephrine in LIA had a statistically significant effect on VAPS at 12 and 48 hours and morphine usage during 6–12 hours when compared with the control group. However, the magnitude of difference did not reach the minimal clinically importance difference (MCID) for TKA, which was 2.3 points and not even minimal detectable change (MDC), which was 1.6 points (7).
After thorough review, we found only 2 studies focused on this topic. Schotanus et al. (8) conducted an RCT to compare the effectiveness of LIA between using ropivacaine with and without epinephrine. The authors also summarized that VAPS during the first 48 hours, rescue medication, and functional outcomes were not significantly different between the groups. In another recent RCT, Kong et al. (9) also found that the use of epinephrine in LIA using ropivacaine after TKA did not have any effect on postoperative pain control, cumulative fentanyl usage, and ROM. Their results were in accordance with our study.
Considering the anesthetic base medication in LIA, both above-mentioned studies used ropivacaine. In an animal study, Kopacz et al. (10) found that cutaneous blood flow after injection of ropivacaine was significantly lower than after injection of bupivacaine. This vasoconstrictive effect of ropivacaine might explain why the combination of ropivacaine and epinephrine did not affect the blood flow (9). To the best of our knowledge, our study was the first that used bupivacaine and compared its efficacy with and without epinephrine in TKA. Although ropivacaine represented a useful alternative to bupivacaine due to less cardiotoxicity and less central nervous system toxicity, this drug was not available in every institute. In terms of the vascular effect, intradermal bupivacaine injection caused vasodilatation in a human study (11). Thus, the addition of epinephrine that decreases this vasodilatation effect should increase the maximum dose and the duration of bupivacaine by delayed systemic absorption (12,13). This effect of epinephrine has also been demonstrated in intrathecal and locoregional local anesthetics (14). However, the efficacy of this combination could not be demonstrated in our study.
Some studies were concerned about the complications of LIA with epinephrine, including wound leakage and skin necrosis (3,8). These complications were not observed in our study. Nevertheless, Yoo et al. (15) observed another complication that should be considered when using epinephrinecontaining LIA. Hypertensive response after deflating of the tourniquet during TKA was more commonly found in their patients who received LIA with epinephrine. Thus, it should be administered cautiously, especially in patients with cardiovascular comorbidities.
The concentration of epinephrine was another issue that must be considered during the injection because it might affect the clinical outcome regarding the vasoconstriction effect. In our study, 0.6 mg epinephrine (1:1000) was diluted with other ingredients up to a total volume of 100 mL. Therefore, the final concentration of epinephrine in our study was 1:166,667, which is well in the effective range according to the study by Liu et al. (13).
There were several limitations to this study. First, we did not measure the blood level of bupivacaine. Thus, it was not proven that epinephrine could extend the local anesthetic effect by maintaining it localized to the area of injection. Second, the effect of epinephrine on pain alleviation might not be large enough when compared with our pain management protocol. Third, we included only TKA without patellar resurfacing. Therefore, the pre-existing patellar pathology in some patients might affect the evaluation of VAPS. Additionally, during the periarticular analgesic injection, the unintended intra-articular injection of bupivacaine could damage the patellar chondrocytes (16). This effect might influence our results.
The strength of the study was that this was the first study to evaluate the efficacy of epinephrine regarding pain and opioid consumption in which bupivacaine was part of an LIA regimen.
Conclusion
Our study showed that additional epinephrine in LIA had a statistically significant reduction in VAPS at 12 hours and morphine usage during 6–12 hours when compared with the control group. However, the magnitude of difference did not reach MCID value for TKA (7). Moreover, repeated measures analysis found no difference in pain score between the 2 groups. We also found no difference in total morphine consumption. Therefore, we suggest that epinephrine might be unnecessary for LIA during primary TKA.
- Akyol O, Karayurt O, Salmond S. Experiences of pain and satisfaction with pain management in patients undergoing total knee replacement. Orthop Nurs 2009; 28(2): 79-85.
- Seangleulur A, Vanasbodeekul P, Prapaitrakool S, Worathongchai S, Anothaisintawee T, McEvoy M, et al. The efficacy of local infiltration analgesia in the early postoperative period after total knee arthroplasty: a systematic review and meta-analysis. Eur J Anaesthesiol 2016; 33(11): 816-31.
- Ross J A, Greenwood A C, Sasser P, Jiranek W A. Periarticular injections in knee and hip arthroplasty: where and what to inject. J Arthroplasty 2017; 32(9S): S77-80.
- Förster J G, Rosenberg P H. Clinically useful adjuvants in regional anaesthesia. Curr Opin Anaesthesiol 2003; 16(5): 477-86.
- Chareancholvanich K, Pornrattanamaneewong C. Does the length of incision in the quadriceps affect the recovery of strength after total knee replacement? A prospective randomised clinical trial. Bone Joint J 2014; 96-B(7): 902-6.
- Pinsornsak P, Nangnual S, Boontanapibul K. Multimodal infiltration of local anaesthetic in total knee arthroplasty: is posterior capsular infiltration worth the risk? A prospective, double-blind, randomised controlled trial. Bone Joint J 2017; 99-B(4): 483-8.
- Danoff J R, Goel R, Sutton R, Maltenfort M G, Austin M S. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplasty 2018; 33(7s): S71-S5.e2.
- Schotanus M G M, Bemelmans Y F L, van der Kuy P H M, Jansen J, Kort N P. No advantage of adrenaline in the local infiltration analgesia mixture during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2017; 25(9): 2778-83.
- Kong D Y, Oh J H, Choi W R, Ko Y-I, Choi C H. The impact of epinephrine in the periarticular injection cocktail using ropivacaine for total knee arthroplasty: a prospective, randomized, double-blind comparison study. J Arthroplasty 2020; 35(9): 2439-43.
- Kopacz D J, Carpenter R L, Mackey D C. Effect of ropivacaine on cutaneous capillary blood flow in pigs. Anesthesiology 1989; 71(1): 69-74.
- Newton D J, Burke D, Khan F, McLeod G A, Belch J J, McKenzie M, et al. Skin blood flow changes in response to intradermal injection of bupivacaine and levobupivacaine, assessed by laser Doppler imaging. Reg Anesth Pain Med 2000; 25(6): 626-31.
- Newton D J, McLeod G A, Khan F, Belch J J. Vasoactive characteristics of bupivacaine and levobupivacaine with and without adjuvant epinephrine in peripheral human skin. Br J Anaesth 2005; 94(5): 662-7.
- Liu S, Carpenter R L, Chiu A A, McGill T J, Mantell S A. Epinephrine prolongs duration of subcutaneous infiltration of local anesthesia in a dose-related manner: correlation with magnitude of vasoconstriction. Reg Anesth 1995; 20(5): 378-84.
- Tschopp C, Tramèr M R, Schneider A, Zaarour M, Elia N. Benefit and harm of adding epinephrine to a local anesthetic for neuraxial and locoregional anesthesia: a meta-analysis of randomized controlled trials with trial sequential analyses. Anesth Analg 2018; 127(1): 228-39.
- Yoo S, Chung J-Y, Ro D H, Han H-S, Lee M C, Kim J-T. The hemodynamic effect of epinephrine-containing local infiltration analgesia after tourniquet deflation during total knee arthroplasty: a retrospective observational study. J Arthroplasty 2020; 35(1): 76-81.
- Kreuz P C, Steinwachs M, Angele P. Single-dose local anesthetics exhibit a type-, dose-, and time-dependent chondrotoxic effect on chondrocytes and cartilage: a systematic review of the current literature. Knee Surg Sports Traumatol Arthrosc 2018; 26(3): 819-30.