Association between duration of anticoagulant thromboprophylaxis and revision rate in primary total hip arthroplasty: a Danish and Norwegian nationwide cohort study
Dennis VINTHER 1,2, Aurelie MAILHAC 1, Ina Trolle ANDERSEN 1, Søren OVERGAARD 3,4, Stein Atle LIE 5,6, Anne Marie FENSTAD 5, Jan-Erik GJERTSEN 5,7, Ove FURNES 5,7, and Alma B PEDERSEN 1,2
1 Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus; 2 Department of Clinical Medicine, Aarhus University, Aarhus; 3 Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg, and The Danish Hip Arthroplasty Register; 4 Department of Clinical Medicine, Faculty of Health and Medical Sciences Register, Copenhagen, Denmark; 5 The Norwegian Arthroplasty Register, Department of Orthopaedic Surgery, Haukeland University Hospital, Bergen; 6 Department of Clinical Dentistry, University of Bergen, Bergen; 7 Department of Clinical Medicine, University of Bergen, Bergen, Norway
Abstract
Background and purpose — There are concerns that bleeding following primary total hip arthroplasty (THA) contributes to prolonged wound drainage and prosthetic joint infection (PJI). We examined whether short (1–5 days), medium (6–14 days), and extended (≥ 15 days) duration of thromboprophylaxis is associated with the 5-year revision rate after THA due to osteoarthritis.
Patients and methods — We performed a cohort study based on data from hip arthroplasty and administrative registries in Denmark and Norway (2008–2014). The outcome was revision surgery due to PJI, aseptic loosening or any cause, and patient mortality. Adjusted cause-specific hazard ratios (HRs) were analyzed with Cox regression analyses.
Results — Among 50,482 THA patients, 8,333 received short, 17,009 received medium, and 25,140 received extended thromboprophylaxis. The HRs for revision due to PJI within 5 years were 1.0 (95%CI 0.7–1.3) and 1.1 (CI 0.9–1.3) for short and extended vs. medium treatment, whereas HR for extended vs. medium prophylaxis was 1.5 (CI 1.2–2.0) within 3 months. The HRs for revision due to aseptic loosening within 5 years were 1.0 (CI 0.7–1.4) and 1.1 (CI 0.9–1.4) for short and extended vs. medium treatment. The HRs for any revision within 5 years were 0.9 (CI 0.8–1.1) and 0.9 (CI 0.8–1.0) for short and extended vs. medium treatment. Extended vs. medium prophylaxis was associated with a decreased 0–3 month mortality. The absolute differences at 5 years were ≤ 1%.
Conclusion — Our data suggests no association between duration of anticoagulant thromboprophylaxis and revision rate within 5 years of primary THA. The extended thromboprophylaxis might be associated with early increased revision rate due to PJI but also with lower mortality; however, the clinical relevance of this finding requires further research.
Citation: Acta Orthopaedica 2022; 93: 930–937. DOI https://doi.org/10.2340/17453674.2022.6243.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-05-10. Accepted: 2022-12-08. Published: 2022-12-27.
Correspondence: abp@clin.au.dk
All authors contributed substantially to the design of the study, interpretation of data, critical revision of the manuscript, and final approval of the submitted version. ABP, ITA, and AM contributed to the statistical analyses. DV and ABP drafted the manuscript and accept full responsibility for the work, the conduct of the study, and access to the data.
Handling co-editors: Bart Swierstra and Philippe Wagner.
Acta thanks Harmen Ettema and Ian Harris for help with peer review of this study.
All patients undergoing total hip arthroplasty (THA) are recommended anticoagulant thromboprophylaxis to prevent venous thromboembolism (VTE) (1-3). Recommendations on the duration of thromboprophylaxis are ambiguous. Depending on the guideline used, THA patients are recommended to use thromboembolic prophylaxis for 6–35 days postoperatively, and less than 5 days if fast-track THA surgery is performed (1-5).
Periprosthetic joint infection (PJI) is responsible for 24% of all revisions after THA corresponding to a revision rate of 1–2% of all primary THAs (6,7). The risk of infection has been shown to increase with the use of anticoagulant thromboprophylaxis due to postoperative bleeding and wound drainage (8-10). Both rivaroxaban and warfarin used as anticoagulant thromboprophylaxis have shown increased risk of surgical site infection, and early PJI has been associated with the use of rivaroxaban (10,11). Warfarin is reported to be associated with a higher risk of deep infection and revision compared with aspirin, but not compared with low-molecular-weight heparin (LMWH) (12). No difference has been observed in revision rates due to aseptic loosening when comparing aspirin with warfarin (13). The overall mortality after THA seems to be higher with the use of enoxaparin compared with rivaroxaban, but similar when comparing enoxaparin with dabigatran (14). Short-term anticoagulant thromboprophylaxis after THA has been associated with increased 90-day mortality (15), but no studies have investigated the long-term mortality. The use of non-vitamin K antagonist oral anticoagulants (NOAC) has been associated with slightly lower revision rate due to infection compared with LMWH, but higher revision rates due to aseptic loosening and all-cause revision (16).
We have previously investigated whether various durations of anticoagulant thromboprophylaxis are associated with 90-day risk of VTE or major bleeding after THA, showing no clinically relevant difference (15). Another study on major orthopedic surgery patients has reported conflicting results regarding major and minor bleeding compared with our previous study (15,17). No studies have investigated whether the duration of anticoagulant thromboprophylaxis affects revision due to PJI and aseptic loosening. This is important as the clinical decisions regarding the utility of thromboprophylaxis require the simultaneous consideration of both efficacy and safety.
We examined whether short (1–5 days), medium (6–14 days), and extended (≥ 15 days) duration of thromboprophylaxis was associated with the 5-year revision rate due to PJI in patients undergoing elective THA in Denmark and Norway. In addition, we examined risk of revision due to aseptic loosening and due to any cause. In view of general patient safety, long-term mortality was also studied.
Patients and methods
Study design and setting
This multinational, population-level cohort study was conducted using prospectively collected data, available from the Nordic Arthroplasty Register Association (NARA) database. All Danish, Norwegian, Swedish, and Finnish citizens are assigned a unique civil registration number at birth. This allows unambiguous linkage between hip registries and other medical databases in each country, as well as the tracking of deceased or emigrated patients. The study is reported according to the RECORD guidelines.
Study population
Due to individual-level data on anticoagulant thromboprophylaxis, only patients operated on in Denmark and Norway were identified through the NARA database and included in this study.
Inclusion criteria were primary THAs due to primary osteoarthritis (OA) between January 1, 2010 and December 31, 2014 from Denmark (n = 34,625) and between January 1, 2008 and December 31, 2013 from Norway (n = 34,801). This cohort is the same as was used in our previous study on shortterm risks with thromboprophylaxis (15).
As in the previous study, after exclusions, 23,940 patients from Denmark and 26,542 from Norway, a total of 50,482 with 1st-time unilateral THA due to primary OA, were available for the complete-case analysis. For details on patient exclusions see Pedersen et al. (15).
The data in the Danish Hip Arthroplasty Registry and Norwegian Arthroplasty Register is documented to have a completeness higher than 95% for primary arthroplasties and around 85–93% for revisions (18,19). In addition, the accuracy of OA diagnosis in the Danish Hip Arthroplasty Registry and Norwegian Arthroplasty Register is more than 95% (19,20). Further, the completeness and accuracy of antithrombotic treatment in the Danish Hip Arthroplasty Registry is 99% and 78%, respectively (21). The accuracy of duration of thromboprophylaxis treatment has not been evaluated but given the high accuracy of other variables included in Danish and Norwegian arthroplasty registries we have no reason to suspect that opposite is applicable to our exposure.
Duration of anticoagulant thromboprophylaxis
The duration of anticoagulant thromboprophylaxis for each individual patient is registered in the NARA database. The duration is based on the number of days the patient was planned to receive thromboprophylaxis after THA by surgeon, including the planned days of treatment after discharge. The planned duration is based on the recommendations of the clinical practice guidelines for thromboprophylaxis in Denmark and Norway (4,5). Duration of thromboprophylaxis in relation to THA surgery was categorized as short term (1–5 days), medium (6–14 days), or extended (≥ 15 days). The following anticoagulant agents were included: parenteral LMWH (including enoxaparin, dalteparin, and tinzaparin), fondaparinux, and NOACs (dabigatran and rivaroxaban), administered both pre- and postoperatively. Dosage of treatment was not available for this study.
Outcome
The primary outcome was revision due to PJI. The secondary outcomes were THA revision due to aseptic loosening, revision due to any cause, and patient death. Information on revisions was obtained from the NARA database. The causes of revision are registered in NARA based on clinical signs before and during the surgery, and diagnostic work-up. Microbiology results after surgery are not registered in the NARA database (18). The registration of causes of revision in NARA database will not be changed even if positive cultures later change the cause of revision.
The Danish Civil Registration System and Statistics Norway were used to obtain information on death for each patient in the national registries before transferring the data to NARA.
Variables and covariates
Information on comorbidity was collected from the Danish National Patient Registry (DNRP) and the Norwegian Patient Registry (NPR), before transferring data to the NARA database (22,23). All primary and secondary discharge diagnoses for all hospitalizations and hospital outpatient visits occurring 1 year before primary THA were identified for all included THA patients. The Charlson Comorbidity Index (CCI) was then computed for each patient at the time of surgery and patients were classified according to 1 of the 3 comorbidity levels: a score of 0 (low); a score of 1–2 (medium); and a score of ≥ 3 (high).
Information on age (in the categories: 10–59, 60–69, 70–79, and ≥ 80 years), sex, fixation type (cemented, uncemented, and hybrid fixation), as well as the start of thromboprophylaxis (pre- or postoperative) and length were collected from the NARA database. Furthermore, the Danish National Database of Reimbursed Prescriptions and the Norwegian Prescription Database were used to collect information on the use of acetylsalicylic acid (Anatomical Therapeutic Chemical [ATC] code: N02BA01), low-dose and high-dose vitamin-K antagonists (VKA) (ATC code: B01AA), and platelet inhibitors (clopidogrel, prasugrel, and ticagrelor [ATC code: B01AC04, B01AC22, B01AC24]) 2 years prior to primary THA.
Statistics
Cumulative incidence function was used to compute the 5-year risk of revision due to PJI. Revision due to aseptic loosening and revision due to other causes were included as a competing risk event in analyses of revision due to PJI. Similarly, revision due to aseptic loosening was calculated, with revision due to PJI and revision due to other causes as competing risk. Death was not added as a competing risk for revision based on published recommendations (24). Kaplan–Meier (KM) was used to calculate the risk for any cause of revision and for death. Cox regression was used for the analysis of cause-specific hazard ratios (HRs) adjusted for potential confounding factors with 95% confidence intervals (CIs).
The following variables were included as potential confounders: age, sex, CCI, use of anticoagulant prior to THA, type of fixation, duration of surgery, start of thromboprophylaxis, and type of anticoagulant thromboprophylaxis in relation to THA. Furthermore, stratification was done on the same variables to examine the potential effect modification of these variables on outcome. In addition, countrylevel stratification was performed. By inspecting Schoenfeld residuals, the Cox proportional hazards models were found to be reasonable to use. We also evaluated the proportional hazards assumptions by calculating HRs for the follow-up time intervals: 0–3 months, 3–6 months, 0.5–1 year, 1–2 years, and 2–5 years (Table 1, see Supplementary data).
Ethics, funding, data sharing, and disclosures
The Norwegian Arthroplasty Register has permission from the Norwegian Data Inspectorate to collect patient data based on written consent from patients (ref. 24.1.2017: 16/1622-3/CDG) and for this study from the Regional Ethical Committee of western Norway (ref. 2015/880/REK Vest). The Danish Data Protection Agency has approved the study (j. nr. 1-16-02-54-17). The study was supported by a grant from Aarhus University Research Foundation. No competing interests were declared. Data sharing is not possible due to existing legislation (18-21).
Results
Description of the study population
Approximately 15% of THA patients received thromboprophylaxis for a short period, 31% for medium, and 45% for an extended period (Table 2). Data regarding the duration of thromboprophylaxis was missing in 9% of the patients and these were excluded from the complete-case analysis. These patients’ characteristics (age, sex, CCI) were similar to the characteristics of patients included in the study population (data not shown).
| Baseline characteristics | Short | Medium | Extended | Total |
| Total number | 8,333 | 17,009 | 25,140 | 50,482 |
| Age group | ||||
| 10–59 | 1,487 (18) | 2,790 (16) | 3,811 (15) | 8,088 (16) |
| 60–69 | 2,819 (34) | 5,646 (33) | 8,665 (35) | 17,130 (34) |
| 70–79 | 2,890 (35) | 6,129 (36) | 8,971 (38) | 17,990 (36) |
| ≥ 80 | 1,137 (14) | 2,444 (14) | 3,693 (15) | 7,274 (14) |
| Female sex | 4,586 (55) | 10,326 (61) | 15,975 (64) | 30,887 (61) |
| Charlson Comorbidity Index a | ||||
| Low | 7,354 (88) | 14,201 (83) | 20,586 (82) | 42,141 (83) |
| Medium | 864 (10) | 2,486 (15) | 3,975 (16) | 7,325 (15) |
| High | 115 (2) | 322 (2) | 579 (2) | 1,016 (2) |
| Used before surgery | ||||
| Acetylsalicylic acid | 1,739 (21) | 3,901 (23) | 5,938 (24) | 11,578 (23) |
| Antiplatelet drugs b | 226 (3) | 374 (2) | 413 (2) | 1,103 (2) |
| Anticoagulants c | 634 (8) | 1,245 (7) | 1,170 (5) | 3,049 (6) |
| Type of fixation d | ||||
| Missing data | 16 (0) | 178 (1) | 343 (1) | 537 (1) |
| Cemented | 481 (6) | 5,164 (30) | 8,971 (36) | 14,616 (29) |
| Uncemented | 5,934 (71) | 8,625 (51) | 9,123 (36) | 23,682 (47) |
| Hybrid A | 1,691 (20) | 855 (5) | 774 (3) | 3,320 (7) |
| Hybrid B | 211 (3) | 2,187 (13) | 5,929 (24) | 8,327 (16) |
| Anticoagulant thromboprophylaxis at THA | ||||
| Start | ||||
| Missing data | 51 (1) | 555 (3) | 1,866 (7) | 2,472 (5) |
| Preoperative | 2,677 (32) | 4,892 (29) | 6,334 (25) | 13,903 (27) |
| Postoperative | 5,605 (67) | 11,562 (68) | 16,940 (67) | 34,107 (68) |
| Type | ||||
| Dabigatran | 86 (1) | 830 (5) | 3,277 (13) | 4,193 (8) |
| Dalteparin | 1,962 (24) | 8,177 (48) | 13,299 (53) | 23,438 (48) |
| Enoxaparin | 1,259 (15) | 4,859 (29) | 4,460 (18) | 10,578 (21) |
| Fondaparinux | 865 (10) | 465 (3) | 53 (1) | 1,383 (3) |
| Rivaroxaban | 3,663 (44) | 1,040 (6) | 3,904 (16) | 8,607 (17) |
| Tinzaparin | 476 (5.7) | 1,568 (9) | 16 (1) | 2,060 (4) |
| Other | 22 (1) | 70 (1) | 131 (1) | 223 (1) |
| a After tabulating CCI by exposure the number of patients in some exposure groups was < 5, and thus details cannot be given due to ethics constrain. These patients were for the table purpose moved into “low” CCI group. b Clopidogrel, prasugrel, ticagrelor. c Warfarin, marcoumar, dagigratran, apixaban, rivaroxaban. d Hybrid A = cemented stem, Hybrid B = uncemented stem. |
||||
Patients in the short-duration group were slightly younger, included fewer females, had lower CCI scores, and had lower acetylsalicylic acid use, but were more often prescribed VKA and platelet inhibitors than patients in the mediumduration group. Patients in the extended-duration group were also slightly younger, included more females, had higher CCI scores, and fewer had VKA and platelet inhibitors prescribed than patients in the medium-duration group. A larger proportion of patients in the short-duration group received NOACs, whereas LWMH was more often prescribed in the medium- and extended-duration group. A larger proportion in the extended-duration group received dabigatran compared with the short- and medium-duration group. A more detailed description of characteristics can be found in our previous study (15).
Revision due to PJI
The cumulative incidences for revision due to PJI are presented in Table 3 and Figure 1. The adjusted HRs for revision due to PJI within 5 years were 1.0 (CI 0.7–1.3) for short and 1.1 (CI 0.9–1.3) for extended thromboprophylaxis vs. medium thromboprophylaxis (Table 3).
| Outcome | |||||
| Duration of thromboprophylaxis | Events | Number at risk | Crude HR | Adjusted HR a | Cumulative incidence |
| Revision due to PJI | |||||
| Short | 68 | 8,333 | 0.8 (0.6–1.0) | 1.0 (0.7–1.3) | 0.9 (0.7–1.1) |
| Medium | 180 | 17,009 | Reference | Reference | 1.1 (0.9–1.3) |
| Extended | 281 | 25,140 | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.2 (1.0–1.3) |
| Revision due to aseptic loosening | |||||
| Short | 50 | 8,333 | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 0.7 (0.5–0.9) |
| Medium | 116 | 17,009 | Reference | Reference | 0.8 (0.7–1.0) |
| Extended | 225 | 25,140 | 1.3 (1.1–1.7) | 1.1 (0.9–1.4) | 1.1 (0.9–1.2) |
| All-cause revision | |||||
| Short | 317 | 8,333 | 1.0 (0.9–1.2) | 1.0 (0.7–1.3) | 4.3 (3.8–4.9) b |
| Medium | 677 | 17,009 | Reference | Reference | 4.4 (4.0–4.8) b |
| Extended | 924 | 25,140 | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 4.0 (3.8–4.4) b |
| Death | |||||
| Short | 506 | 8,333 | 1.0 (0.9–1.1) | 0.9 (0.8–1.1) | 8.8 (8.0–9.6) b |
| Medium | 1,253 | 17,009 | Reference | Reference | 9.0 (8.5–9.5) b |
| Extended | 1,620 | 25,140 | 0.9 (0.8–1.0) | 0.9 (0.8–0.9) | 8.0 (7.7–8.4) b |
| a Adjusted HR was adjusted for: age, sex, Charlson Comorbidity Index, type of fixation, regular use of acetylsalicylic acid, clopidogrel/prasugrel/ticagrelor, warfarin/marcumar/dabigatran/apixaban/rivaroxaban before THA, start of anticoagulant thromboprophylaxis in relation to THA, type of anticoagulant thromboprophylaxis drug used in relation to THA (LMWH-group (dalteparin, enoxaparin, tinzaparin)/NOAC-group (dabigatran, rivaroxaban)/Other-group (other, fondaparinux)). b 1–KM survival (95% CI). |
|||||
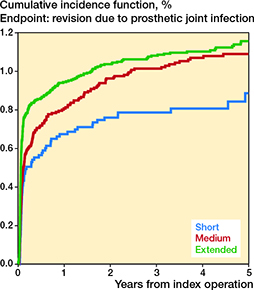
Figure 1. Cumulative incidences for revision due to periprosthetic joint infection in total hip arthroplasty patients within 5 years of surgery with other causes of revision as competing risk.
However, the adjusted HRs for revision due to PJI within 3 months were 1.0 (CI 0.7–1.5) and 1.5 (CI 1.2–2.0) for short and extended vs. medium thromboprophylaxis. The risk of revision due to PJI was not increased beyond the first 3 months (Table 1, see Supplementary data).
Revision due to aseptic loosening
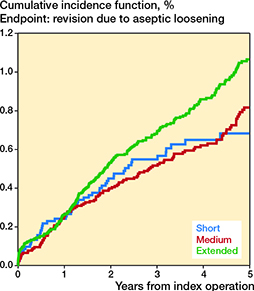
The cumulative incidences for revision due to aseptic loosening are presented in Table 3 and Figure 2. The HRs within 0–5 years were 1.0 (CI 0.7–1.4) for the short-duration and 1.1 (CI 0.9–1.4) for the extended-duration when compared with the medium-duration group (Table 3). The follow-up time-intervals’ specific HR estimates were similar to those for 0–5 years (Table 1, see Supplementary data).
 %
%
Figure 2. Cumulative incidences for revision due to aseptic loosening in total hip arthroplasty patients within 5 years of surgery with other causes of revision as competing risk.
All-cause revision
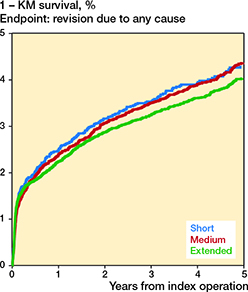
The 1-KM estimates for all-cause revision are presented in Table 3 and Figure 3. The HRs within 5 years were 0.9 (CI 0.8–1.1) for the short-duration and 0.9 (CI 0.8–1.0) for the extended-duration when compared with the medium-duration treatment group (Table 3). The follow-up time-intervals’ specific HR estimates were similar to those of 0–5 years, except that HR was 0.5 (CI 0.3–0.8) in the time interval 3–6 months for extended vs. medium duration (Table 1, see Supplementary data).
 %
%
Figure 3. Cumulative revision rate (1 – KM revision-free survival) for revision due to all causes in total hip arthroplasty patients within 5 years of surgery.
Death
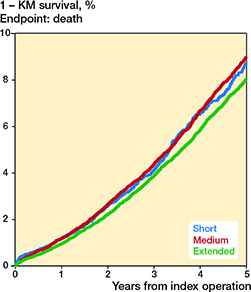
The 1–KM estimates for death are presented in Table 3 and Figure 4. The HRs for death within 5 years were 0.9 (CI 0.8–1.0) for the shortduration and 0.9 (CI 0.8–0.9) for the extended-duration when compared with the medium-duration treatment group (Table 3). The adjusted HRs for death within 3 months were 1.0 (CI 0.6–1.5) and 0.7 (CI 0.5–1.0) for short and extended vs. medium thromboprophylaxis. Some, but not all, subsequent follow-up time-intervals’ specific HR estimates for death indicate a trend towards lower mortality for extended vs. medium thromboprophylaxis (Table 1, see Supplementary data).
 %
%
Figure 4. Cumulative mortality rate (1 – KM survival) in total hip arthroplasty patients within 5 years of surgery.
At all endpoints, the absolute differences in cumulative incidences were less than 1% after 5 years.
Stratified analyses
The country-specific HRs (Table 4, see Supplementary data) did not differ from the overall HRs. The same applies to analyses stratified on age, sex, CCI, fixation, and start of thromboprophylaxis (data not shown). Statistical precision of HRs from the stratified analyses was low, expressed by wide CI and a low number of outcomes.
Discussion
We found no difference in revision rate due to PJI, aseptic loosening, all-cause revision, or death within 5 years, with respect to the duration of thromboprophylaxis among THA patients. There was an indication that extended prophylaxis was associated with increased risk of revision due to PJI but lower mortality within 3 months compared with medium prophylaxis, which requires further research.
The risk of surgically relevant complications such as revision surgery due to duration of thromboprophylaxis has not been evaluated previously. Several studies have suggested that specific anticoagulants, such as rivaroxaban and LWMH, have an impact on development of infection after hip or knee arthroplasty (9,10). In these studies, rivaroxaban was administered for more than 14 days or for 28–35 days in accordance with the NICE guidelines. Bleeding and hematoma formation can result in prolonged wound drainage, which may predispose to infections. A Cochrane systematic review on randomized control trials concluded that extended duration of anticoagulants was associated with increased risk of minor bleeding after hip or knee arthroplasty (25). This observation was further supported by studies on medical patients with or without cancer, showing that extended duration of thromboprophylaxis with apixaban, enoxaparin, and rivaroxaban increases the risk of major bleeding (26). In line with this, the extended-duration group in our study had a larger proportion of NOAC users than the medium-duration group, but not all studies have reported increased risk of bleeding with extended anticoagulant treatment (15). However, in our previous study surgical bleeding and hematoma formation around the joint were not studied, in terms of either risk of reoperation or revision arthroplasty due to infection. Our present finding of an association between extended duration of thromboprophylaxis and the early risk of revision due to PJI is consistent with previous reports that bleeding might lead to prolonged wound drainage and development of infections that require revision (8-10).
Previous studies in unselected patients from routine clinical practice showed no difference in the 90-day risk of death with respect to duration of thromboprophylaxis (15,27); point estimates were below 1, but not statistically significant. Our study indicates that compared with patients receiving prophylaxis for 6–14 days, extended duration was a predictor of slightly lower early mortality. The difference between the studies is that in our study we have adjusted for type of anticoagulant drug used in relation to THA, which was not the case in previous studies. Lower mortality in extended-prophylaxis groups could also be due to residual confounding and selection bias. Nevertheless, the benefits of treatment (lower mortality for extended duration of treatment) should be weighed against safety of treatment (increased PJI risk for extended treatment) and surgery performed in fast-track settings (28).
With differences in revision and mortality rate of less than 1% after 5 years, clinicians should continue to follow current national and local guidelines, but with increased focus on early devastating complications such as PJI.
Strengths and weaknesses
A study based on databases is only as accurate as the data submitted to the specific databases. Our study consists of quality data with high completeness from well-established databases (18-21). The relatively low incidence of PJI requires large numbers of patients/events to obtain appropriate statistical power, making the use of large databases optimal. However, the definition of infection might vary between surgeons, hospitals, and countries, and is often based on pre- and intraoperative findings. In addition, the results of tissue sampling with intraoperative cultures are usually not available at the time when registration of revision cause is reported to arthroplasty registries. This will lead to misclassification of PJI diagnosis. Gundtoft et al. found that the “true” incidence of PJI after primary THA might be as much as 40% higher when looking at several registries as opposed to the national registries alone (18). Thus, misclassification of revision due to PJI exists, but it is most likely not related to exposure in our study due to prospective registration of data.
The selection bias in our study is low because all THA patients treated were included, regardless of department, specific patient characteristics, or willingness to participate in the study. It is unlikely that the missing data on duration of treatment occurred systematically as data on duration of treatment and subsequent outcomes of interest was prospectively collected. Even though patients have received anticoagulant drugs (pills) from the hospital or through prescription, we cannot be sure about the patients’ compliance with treatment. Theoretically, if patients’ compliance with planned treatment is low, which would be most alarming for patients in the extended-treatment group, then this change could have affected our results in any direction. However, 1 previous study has shown that adherence to treatment prescribed by general practitioners in Denmark is more than 90% (29). Further, even though the patient potentially could switch from one to another exposure group because planned duration of treatment has changed for various reasons, we have analyzed our data following the intention-to-treat principle, similar to the principle used in randomized trials. Thus, the patient stayed in a specific exposure group planned at the time of surgery.
As we assume that orthopedic surgeons are familiar with and follow the general guidelines on anticoagulation treatment of medical patients, THA patients are likely to receive different duration of treatment depending on their individual risk. Thus, high-risk patients with a history of VTE or cancer will more likely receive extended prophylaxis. However, we do not have specific information as to whether this is true or not for patients with a history of VTE or cancer, but we have partly adjusted for this with CCI. Off-label use of NOACs could be explained by increasing use of fast-track THA surgery in both countries and a national guideline recommending NOACs only during hospitalization, which is less than 5 days (28).
Although we adjusted for several potential confounders, other factors might have an impact on our results. Newer studies suggest that socioeconomic status might affect revision after THA (30). Data was not available on smoking, which is shown to increase the overall revision rate, risk of aseptic loosening, and PJI (31). However, it is questionable whether these factors are true confounders, because they also relate to selected exposure. Lastly, we did not consider the body mass index (BMI) of patients, despite BMI being a significant risk factor for PJI (32). It is possible that BMI is a confounder if the dose and duration of thromboprophylaxis is modified in patients with high BMI.
In conclusion, in this large cohort study including 50,482 THA patients from Denmark and Norway, there was no association between the duration of pharmacological thromboprophylaxis and a clinically relevant rate of revision due to PJI, aseptic loosening, revision of any cause, or death within 5 years of THA. However, there is an indication that extended thromboprophylaxis is associated with increased revision rate due to PJI but lower mortality within 3 months of THA. The absolute differences in cumulative incidences after 5 years were all < 1%.
- Falck-Ytter Y, Francis C W, Johanson N A, Curley C, Dahl O E, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl.): e278S-e325S. doi: 10.1378/chest.11-2404.
- NICE (National Institute for Health and Care Excellence) 2018. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. Available from: https://www.nice.org.uk/guidance/ng89
- AAOS (American Academy of Orthopaedic Surgeons) 2011. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. Evidence-based guideline and evidence report. Available from: https://www.aaos.org/globalassets/quality-and-practice-resources/vte/vte_full_guideline_10.31.16.pdf
- Granan L-P. Prevention of VTE in orthopedic surgery patients: a Norwegian adaptation of the 9th ed. of the ACCP Antithrombotic Therapy and Prevention of Thrombosis Evidence-based Clinical Practice Guidelines. Norsk Selskap for Trombose og Hemostase; 04.08.2015. Available from: https://files.magicapp.org/guideline/6df24e98-47d6-4ee5-bc9e-7a3f8c41357d/0_2/pdf/published_guideline_401-0_2.pdf
- Medicinrådet (DK). December 12, 2018. Behandlingsvejledning inkl. lægemiddelrekommandation for tromboseprofylakse til ortopædkirurgiske patienter. Available from: https://medicinraadet.dk/media/asditulg/behandlingsvejl-inkl-laegemiddelrek-ortopaedkirurgiske-patienter-vers_adlegacy.pdf
- Gundtoft P H, Pedersen A B, Schønheyder H C, Møller J K, Overgaard S. One-year incidence of prosthetic joint infection in total hip arthroplasty: a cohort study with linkage of the Danish Hip Arthroplasty Register and Danish Microbiology Databases. Osteoarthritis Cartilage 2017; 25(5): 685-93. doi: 10.1016/j.joca.2016.12.010.
- Springer B D, Cahue S, Etkin C D, Lewallen D G, McGrory B J. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today 2017; 3(2): 137-40. doi: 10.1016/j.artd.2017.05.003.
- Patel V P, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare P E. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(1): 33-8. doi: 10.2106/jbjs.F.00163.
- Wang Z, Anderson F A Jr, Ward M, Bhattacharyya T. Surgical site infections and other postoperative complications following prophylactic anticoagulation in total joint arthroplasty. PLoS One 2014; 9(4): e91755. doi: 10.1371/journal.pone.0091755.
- Brimmo O, Glenn M, Klika A K, Murray T G, Molloy R M, Higuera C A. Rivaroxaban use for thrombosis prophylaxis is associated with early periprosthetic joint infection. J Arthroplasty 2016; 31(6): 1295-8. doi: 10.1016/j.arth.2015.12.027.
- Almustafa M A, Ewen A M, Deakin A H, Picard F, Clarke J V, Mahmood F F. Risk factors for surgical site infection following lower limb arthroplasty: a retrospective cohort analysis of 3932 lower limb arthroplasty procedures in a high volume arthroplasty unit. J Arthroplasty 2018; 33(6): 1861-7. doi: 10.1016/j.arth.2018.01.037.
- Hughes L D, Lum J, Mahfoud Z, Malik R A, Anand A, Charalambous C P. Comparison of surgical site infection risk between warfarin, LMWH, and aspirin for venous thromboprophylaxis in TKA or THA: a systematic review and meta-analysis. JBJS Rev 2020; 8(12): e20.00021. doi: 10.2106/jbjs.Rvw.20.00021.
- Goswami K, Tan T L, Rondon A J, Shohat N, Yayac M, Schlitt P K, et al. Aspirin thromboprophylaxis confers no increased risk for aseptic loosening following cementless primary hip arthroplasty. J Arthroplasty 2019; 34(12): 2978-82. doi: 10.1016/j.arth.2019.07.013.
- Huisman M V, Quinlan D J, Dahl O E, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3(6): 652-60. doi: 10.1161/circoutcomes.110.957712.
- Pedersen A B, Andersen I T, Overgaard S, Fenstad A M, Lie S A, Gjertsen J E, et al. Optimal duration of anticoagulant thromboprophylaxis in total hip arthroplasty: new evidence in 55,540 patients with osteoarthritis from the Nordic Arthroplasty Register Association (NARA) group. Acta Orthop 2019; 90(4): 298-305. doi: 10.1080/17453674.2019.1611215.
- Benson T E, Andersen I T, Overgaard S, Fenstad A M, Lie S A, Gjertsen J E, et al. Association of perioperative thromboprophylaxis on revision rate due to infection and aseptic loosening in primary total hip arthroplasty: new evidence from the Nordic Arthroplasty Registry Association (NARA). Acta Orthop 2022; 93: 417-23. doi: 10.2340/17453674.2022.2461.
- Sobieraj D M, Lee S, Coleman C I, Tongbram V, Chen W, Colby J, et al. Prolonged versus standard-duration venous thromboprophylaxis in major orthopedic surgery: a systematic review. Ann Intern Med 2012; 156(10): 720-7. doi: 10.7326/0003-4819-156-10-201205150-00423.
- Gundtoft P H, Overgaard S, Schønheyder H C, Møller J K, Kjærsgaard-Andersen P, Pedersen A B. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop 2015; 86(3): 326-34. doi: 10.3109/17453674.2015.1011983.
- Nasjonal Kompetansetjene for leddproteser oh hoftebrudd REPORT. Nasjonalt Servicemiljø for Medisinske Kvalitetsregistre. Norway: Department of Ortopaedic Surgery, Haukeland University Hospital 2021. Available from: https://helse-bergen.no/nasjonal-kompetansetjeneste-for-leddproteser-og-hoftebrudd
- Pedersen A, Johnsen S, Overgaard S, Søballe K, Sørensen H T, Lucht U. Registration in the Danish Hip Arthroplasty Registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications. Acta Orthop Scand 2004; 75(4): 434-41. doi: 10.1080/00016470410001213-1.
- Schelde A B, Petersen J, Jensen T B, Gromov K, Overgaard S, Olesen J B, et al. Validation of registration of pharmacological treatment in the Danish Hip and Knee Arthroplasty Registers. Basic Clin Pharmacol Toxicol 2021; 128(3): 455-62. doi: 10.1111/bcpt.13518.
- Schmidt M, Schmidt S A, Sandegaard J L, Ehrenstein V, Pedersen L, Sørensen H T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449-90. doi: 10.2147/clep.S91125.
- Bakken I J, Nyland K, Halsteinli V, Kvam U H, Skjeldestad F E. Norsk pasientregister: Administrativ database med mange forskningsmuligheter 2004: 65-90. Available from: https://www.ntnu.no/ojs/index.php/nor-epid/article/view/277
- Lie S A, Fenstad A M, Lygre S H L, Kroken G, Dybvik E, Gjertsen J-E, et al. Kaplan–Meier and Cox regression are preferable for the analysis of time to revision of joint arthroplasty: thirty-one years of follow-up for cemented and uncemented THAs inserted from 1987 to 2000 in the Norwegian Arthroplasty Register. JBJS Open Access 2022; 7(1): e21.00108. doi: 10.2106/jbjs.Oa.21.00108.
- Forster R, Stewart M. Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair. Cochrane Database Syst Rev 2016; 3: Cd004179. doi: 10.1002/14651858.CD004179.pub2.
- Liew A Y, Piran S, Eikelboom J W, Douketis J D. Extended-duration versus short-duration pharmacological thromboprophylaxis in acutely ill hospitalized medical patients: a systematic review and meta-analysis of randomized controlled trials. J Thromb Thrombolysis 2017; 43(3): 291-301. doi: 10.1007/s11239-016-1461-1.
- Pedersen A B, Sorensen H T, Mehnert F, Johnsen S P, Overgaard S. Effectiveness and safety of different duration of thromboprophylaxis in 16,865 hip replacement patients: a real-word, prospective observational study. Thromb Res 2015; 135(2): 322-8. doi: 10.1016/j.thromres.2014.11.029.
- Petersen P B, Kehlet H, Jorgensen C C, Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group. Safety of in-hospital only thromboprophylaxis after fast-track total hip and knee arthroplasty: a prospective follow-up study in 17,582 procedures. Thromb Haemost 2018; 118(12): 2152-61. doi: 10.1055/s-0038-1675641.
- Pottegård A, Christensen R, Houji A, Christiansen C B, Paulsen M S, Thomsen J L, et al. Primary non-adherence in general practice: a Danish register study. Eur J Clin Pharmacol 2014; 70(6): 757-63. doi: 10.1007/s00228-014-1677-y.
- Edwards N M, Varnum C, Overgaard S, Pedersen A B. Impact of socioeconomic status on the 90- and 365-day rate of revision and mortality after primary total hip arthroplasty: a cohort study based on 103,901 patients with osteoarthritis from national databases in Denmark. Acta Orthop 2021; 92(5): 581-8. doi: 10.1080/17453674.2021.1935487.
- Teng S, Yi C, Krettek C, Jagodzinski M. Smoking and risk of prosthesis-related complications after total hip arthroplasty: a meta-analysis of cohort studies. PLoS One 2015; 10(4): e0125294. doi: 10.1371/journal.pone.0125294.
- Ren X, Ling L, Qi L, Liu Z, Zhang W, Yang Z, et al. Patients’ risk factors for periprosthetic joint infection in primary total hip arthroplasty: a meta-analysis of 40 studies. BMC Musculoskelet Disord 2021; 22(1): 776. doi: 10.1186/s12891-021-04647-1.
Supplementary data
| Outcome | ||||||||||
| Duration of thromboprophylaxis | 0–3 months | 3–6 months | 0.5–1 year | 1–2 years | 2–5 years | |||||
| Events | aHR a (CI) | Events | aHR a (CI) | Events | aHR a (CI) | Events | aHR a (CI) | Events | aHR a (CI) | |
| Revision due to PJI | ||||||||||
| Short | 42 | 1.0 (0.7–1.5) | 5 | 0.6 (0.2–1.7) | 9 | 1.4 (0.6–3.5) | 7 | 0.6 (0.2–1.5) | 5 | 1.1 (0.4–3.1) |
| Medium | 104 | Reference | 18 | Reference | 15 | Reference | 26 | Reference | 17 | Reference |
| Extended | 207 | 1.5 (1.2–2.0) | 9 | 0.3 (0.1–0.7) | 21 | 0.9 (0.5–2.0) | 23 | 0.5 (0.3–0.9) | 21 | 1.0 (0.5–2.0) |
| Revision due to aseptic loosening | ||||||||||
| Short | 8 | 1.0 (0.4–2.8) | 7 | 3.5 (1.0–12.0) | 5 | 0.4 (0.2–1.2) | 17 | 1.3 (0.6–2.5) | 13 | 0.9 (0.5–1.7) |
| Medium | 13 | Reference | 6 | Reference | 24 | Reference | 24 | Reference | 49 | Reference |
| Extended | 31 | 1.4 (0.7–3.0) | 7 | 0.9 (0.3–2.8) | 28 | 0.7 (0.4–1.2) | 66 | 1.5 (0.9–2.5) | 93 | 1.1 (0.8–1.6) |
| All-cause revision | ||||||||||
| Short | 142 | 1.0 (0.8–1.3) | 27 | 0.7 (0.4–1.2) | 37 | 0.9 (0.6–1.4) | 56 | 1.0 (0.7–1.4) | 55 | 0.9 (0.6–1.3) |
| Medium | 270 | Reference | 58 | Reference | 79 | Reference | 112 | Reference | 158 | Reference |
| Extended | 417 | 1.1 (0.9–1.3) | 46 | 0.5 (0.3–0.8) | 100 | 0.8 (0.6–1.1) | 155 | 0.9 (0.7–1.1) | 206 | 0.9 (0.7–1.1) |
| Death | ||||||||||
| Short | 43 | 1.0 (0.6–1.5) | 15 | 0.6 (0.3–1.2) | 41 | 1.0 (0.7–1.5) | 116 | 0.8 (0.6–1.0) | 291 | 0.9 (0.8–1.1) |
| Medium | 68 | Reference | 41 | Reference | 89 | Reference | 250 | Reference | 805 | Reference |
| Extended | 79 | 0.7 (0.5–1.0) | 44 | 0.7 (0.5–1.2) | 117 | 1.0 (0.7–1.3) | 316 | 0.8 (0.7–1.0) | 1,064 | 0.9 (0.8–1.0) |
| a Adjusted HR with 95% confidence intervals (CI), see footnote Table 3. | ||||||||||
| Outcome | Duration of thromboprophylaxis | Adjusted HR a (CI) | |
| Denmark | Norway | ||
| Revision due to PJI | Short | 1.2 (0.8–1.8) | 0.5 (0.1–1.5) |
| Extended | 1.5 (0.9–2.3) | 0.9 (0.7–1.1) | |
| Revision due to aseptic loosening | Short | 1.0 (0.6–1.5) | 1.9 (0.7–5.4) |
| Extended | 1.3 (0.9–2.1) | 1.3 (0.9–1.8) | |
| All-cause revision | Short | 1.0 (0.9–1.2) | 0.7 (0.4–1.4) |
| Extended | 1.1 (0.9–1.4) | 0.8 (0.7–1.0) | |
| Death | Short | 0.9 (0.8–1.0) | 0.7 (0.5–1.1) |
| Extended | 1.0 (0.9–1.2) | 0.9 (0.8–1.0) | |
| a Adjusted HR with 95% confidence intervals (CI), see footnote Table 3. | |||