Early migration in unicompartmental knee arthroplasty: a radiostereometric study of 26 patients with 24 months of follow-up
Jantsje H PASMA 1, Brechtje HESSELING 1,2, Nicole DE ESCH 1,2, Hennie VERBURG 1,2, Dieu D NIESTEN 1,2, and Nina M C MATHIJSSEN 1,2
1 Reinier Haga Orthopedisch Centrum, Zoetermeer, The Netherlands; 2 Department of Orthopedics, Reinier de Graaf Hospital, Delft, The Netherlands
Background and purpose — Aseptic loosening, mainly caused by migration, is one of the most common indications for revisions in unicompartmental knee arthroplasty (UKA). In this study, we investigated the early migration of the Persona Partial Knee (PPK, Zimmer Biomet, Warsaw, IN), a cemented medial fixed-bearing unicompartmental knee prosthesis, and evaluated the clinical results.
Patients and methods — 26 primary PPKs were implanted. Radiographs were obtained direct postoperatively, at 6 weeks, 6, 12 and 24 months postoperatively. Migration of the femoral and tibial component was calculated using model-based radiostereophotogrammetric analysis (mRSA) in terms of translations and rotations. Patient-reported outcome measures (PROMs) were also registered.
Results — At 24 months postoperatively, we found low migration of both the femoral and tibial component in the first 6 months, after which both components stabilized. Only the rotation of the tibial component about the z-axis did not stabilize. All PROMs improved after 24 months compared with preoperative PROMs.
Conclusion — The Persona Partial Knee shows low migration of both the femoral and tibial component and PROMs were improved at 24 months follow-up. Long-term follow-up is needed to investigate the performance of the prosthesis compared with other prostheses.
Citation: Acta Orthopaedica 2022; 93: 914–921. DOI http://dx.doi.org/10.2340/17453674.2022.5672.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-06-03. Accepted: 2022-11-06. Published: 2022-12-19.
Correspondence: j.pasma@rhoc.nl
HV, DDN, NMCM, and BH designed the study. HV, DDN, and NE informed and included patients. HV and DDN performed the surgeries. NE coordinated the follow-up visits. JHP analyzed the data and wrote the manuscript. All authors critically reviewed the manuscript and approved the manuscript for submission.
The authors would like to thank Rianne Oomen and Ian Blom for support in taking and analyzing RSA radiographs.
Handling co-editor: Søren Overgaard
Acta thanks Annette W-Dahl and Svend Erik Østgaard for help with peer review of this study.
Next to osteoarthritis progression, aseptic loosening is one of the most common problems in unicompartmental knee arthroplasty (UKA) and a common indication for revision. This is especially the case in cemented UKA, where aseptic loosening is mainly due to migration (1).
To study the migration and to detect unsatisfactory stability at an early stage, model-based radiostereophotogrammetric analysis (mRSA) is used as a highly accurate and 3-dimensional method of quantifying the motion between an implant and the host bone (2). In total knee arthroplasty (TKA), early and continuous migration, i.e., migration within 2 years, are inversely related with long-term survival and therefore might be used to predict prosthesis survival (3,4). Only one study has investigated the relation between early migration and survival in UKA; results showed a migration of more than 2 mm 12 months after surgery in all revised patients (5).
Previous studies on migration of UKA using mRSA mainly focused on the tibial component (6-11) and showed low migration. However, despite the low migration, 4–30% of the patients had continuous migration of the tibial component, which might result in a higher risk of revision (Table 1) (7-10, 12).
| Reference | Group size | Continuous migration, n (%) |
| Ensini (2013) (15) | 23 | 1 (4) |
| Koppens (2018) (12) | 37 | 11 (30) |
| Kendrick (2015) (10) | 43 | 5 (12) |
| Koppens (2019) (11) | 55 | 3 (6) |
| Linde (2019) (13) | 100 | 19 (19) |
No studies investigated the migration of the Persona Partial Knee (PPK, Zimmer Biomet, Warsaw, IN), a cemented medial fixed-bearing unicompartmental knee replacement system. The shape of this prosthesis is based on morphology, which might result in a better fit. We investigated the migration of both the femoral and tibial component of the PPK during 24 months follow-up and evaluated PROMs results.
Patients and methods
Participants
In this cohort study, 26 patients were included from April 2017 to May 2018 at the Reinier de Graaf Hospital, Delft, the Netherlands. During this period, 45 patients had an indication for primary UKA and were considered for inclusion. Patients who had rheumatoid arthritis or other inflammatory joint disease were excluded. 19 patients were excluded for several reasons (Figure 1).
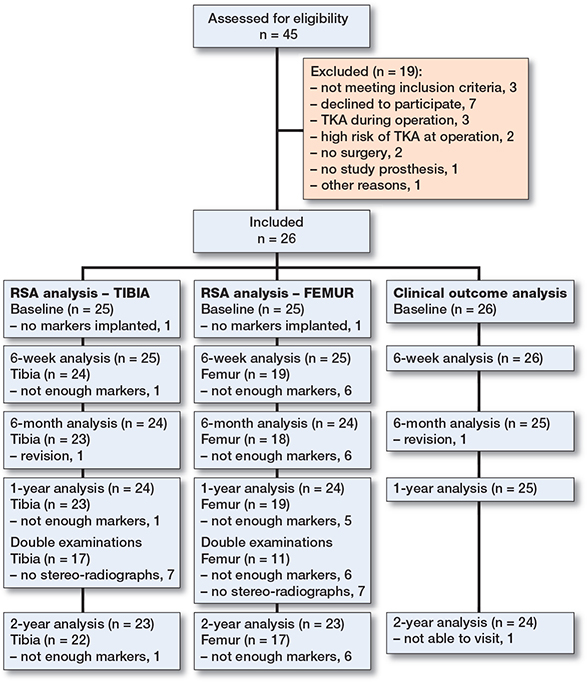
Figure 1. Flow diagram of patient inclusion and follow-up.
Implants and surgery
The PPK is a cemented fixed-bearing UK replacement system limited to the medial tibiofemoral compartment of the knee. It consists of a Co-Cr-Mo alloy femoral component, titanium alloy (Ti-6AI-4V) tibial baseplate and vitamin-E stabilized highly crosslinked polyethylene (VEHXPE) tibial bearing. The design of this prosthesis is based on morphology of the global population, possibly resulting in a more accurate, personalized and anatomical fit.
All patients were admitted on the day of the surgery and received only spinal anesthesia or spinal anesthesia combined with general anesthesia. The surgeries were performed by 2 surgeons (HV and DDN) without navigation or other computer-assisted instruments. Both surgeons are experienced knee surgeons and had previous experience with UKA. The surgeons and surgery personnel were trained by the manufacturer. After bone preparation, 6–9 tantalum marker beads (diameter of 1.0 mm) were inserted in the femur and 6–9 in the tibia. Both components were cemented (Optipac 40 Refobacin Bone Cement R, Zimmer Biomet, Warsaw, IN).
After surgery, a standardized protocol was used for pain medication. At discharge, all patients received a prescription for 1 week of celecoxib, which could be used in addition to paracetamol. If necessary, additional pain medication such as oxycodone was prescribed. Opioid medication was available on request. Patients started with mobilization on the day of surgery. Patients were discharged when they were able to walk 30 m with crutches, to climb stairs, to dress independently, and to go to the toilet independently. In addition, pain relief must have been sufficient before discharge indicated by a Numeric Rating Scale (NRS) for pain below 3 at rest and below 5 during mobilization.
Outcome measures
Clinical examination, standard radiographs, and patient-reported outcome measures (PROMs) were obtained preoperatively, at 6 weeks (±1 week), 6 (±1 week), 12 (±2 weeks), and 24 (±3 weeks) months after surgery. Clinical examination was performed using the clinical part of the Knee Society Score (KSS), consisting of the range of motion, VAS score of pain, and alignment and stability of the medial and lateral collateral ligaments. The KSS is calculated according to the scoring described by Scuderi et al. and ranges from 0 to 100 (13). Patients with a flexion greater than 125° and a stable painless knee can have a score more than 100. Standard radiographs consisted of anteroposterior and lateral view radiographs. Stereo-radiographs to perform mRSA were obtained directly postoperatively after weightbearing and at each follow-up moment.
Patient-reported outcome measures (PROMs)
PROMs were used to investigate the clinical and functional outcomes. The Knee injury and Osteoarthritis Outcome Score (KOOS-PS) is a short measure of physical function for knee osteoarthritis with a minimal score of 0 and a maximal score of 100, in which a higher score indicates more problems (14). The numeric rating scale for pain (NRS pain) is a scale ranging from 0 to 10 on which patients score their pain both at rest and during movement (15). The Oxford Knee Score (OKS) also addresses knee function with a score ranging from 0 to 48, in which a higher score indicates better function (16). EuroQol-5D (EQ-5D-5L) is a general health-related quality of life questionnaire to document the perceived quality of life. The score was calculated using the Dutch tariff and ranges from –0.446 to 1 (17). The EuroQol-5D VAS indicates the overall health on a scale from 0 to 100, in which a higher score indicates a better overall health.
mRSA
Stereo-radiographs were made with a standardized RSA set-up with the patient in supine position with endorotation of at least 20° of the leg. A calibration box (Medis, Leiden, the Netherlands) was used underneath the examination table. The anatomical axis of the leg was parallel to the y-axis of the calibration box. 2 roentgen tubes (1 fixed and 1 portable, i.e., DigitalDiagnost and the MobileDiagnost wDR [Philips, Best, the Netherlands]) were positioned at an angle of 40° to each other and 1.2 meters to the table, resulting in a focus point of 1.5 meters. The images were digitally saved in DICOM format at a resolution of 6.8 pixels/mm and a 16-bit grey-scale resolution.
The analysis was performed with Model-Based RSA software (version 4.2, Medis Specials, LUMC, Leiden, the Netherlands) using computer-aided design (CAD) models of the prosthesis provided by the manufacturer (Zimmer Biomet, Warsaw, IN, USA) and according to the International Organization for Standardization (ISO). For each time point the position of the model in respect of the femur or tibia was calculated. A minimum of 3 bone markers should be visible for analyzing the rotations. Markers were excluded in the case of marker instability (mean error [ME] > 0.35 mm). A mean marker model (MC model) was used to include bone markers in particular stereo-radiographs with < 3 bone markers (18). In the case of < 3 bone markers, a condition number of > 120, and/or a rigid body fitting error of > 0.35 mm, the subject was excluded from analysis.
The precision was calculated using a double examination at 12 months. The precision was defined as the standard deviation (SD) and the bias as the mean of the migration between the 2 examinations with the first examination as a reference. The precision interval (PI) was defined as 1.96 x SD and represents the expected clinical precision; a measured migration within the PI is inconclusive. In case of non-normal distribution only the SD and mean are presented and not the PI (as stated in the ISO).
Implant migration of both the femoral and tibial component were calculated using all 5 stereo-radiographs with the direct postoperative stereo-radiograph as reference. Translations were calculated using the center of gravity of the bone markers as the reference object and the 3-dimensional model of the implant as migrating object. The center of the implant coordinate system lies in the center of the model of the implant. Translations were expressed in mm along the x-, y-, and z-axes. Rotations were expressed in degrees about the x-, y-, and z-axes, as presented in Table 6. The maximum total point motion (MTPM) indicates the length of the translation vector of the point that has the greatest migration.
Statistics
All data was checked against normality. In the case of a normal distribution, data was presented as mean with SD. Otherwise, median with range or number with percentage were presented. Non-normal migration data is presented by mean, median, and range.
Migration results were analyzed using linear mixed models to determine the main effect of time on migration, taking into account the longitudinal nature of the study and missing values. In the case of a significant main effect of time, pairwise comparisons were performed to specify the differences.
Statistical significance was assumed at p < 0.05. All data was analyzed using RStudio (version 1.4.1717) and R (version 4.1.0) (R Foundation for Statistical Computing, Vienna, Austria).
Ethics, registration, funding, data sharing, and disclosures
The study was reviewed and approved by the Ethics Medical Committee (METC Zuidwest Holland, METC-nr 16-031, NL60028.098.16). The study is registered in the Netherlands Trial Register (NL6152). All participants gave written informed consent. This study is funded by Zimmer Biomet. Data cannot be shared. The research department receives grants from Zimmer Biomet and Stryker to perform clinical studies.
Results
Patient characteristics
Table 2 presents the patient characteristics of 26 included patients. The mean (SD) age of the participants was 63 (7.4) years, 15 patients were women, and 11 patients had a left-sided procedure.
No complications occurred during surgery. At 6 weeks, 2 complications were registered; 1 patient had a hematoma and 1 patient had persistent wound drainage, which were not related to the prosthesis. During follow-up, 1 revision occurred after 15 weeks because of a fracture of the tibia following trauma after primary UKA. There were no operative technical issues or signs of infection.
Clinical outcomes
Figure 2 and Table 3 show the results of the clinical examination. Both the KSS and range of motion improved over time (p < 0.001) with stabilization after 6 months. Figure 3 shows the results of the PROMs. All PROMs, except EQ-5D-5L VAS (p = 0.1), improved over time (p < 0.001) and stabilized after 6 months.
| Preoperative | 6 weeks | 6 months | 12 months | 24 months | |
| KSS a | 76 (26–96) | 84 (61–98) | 96 (84–102) | 93 (46–101) | 99 (23–102) |
| Range of motion, ° | 117 (19) | 112 (12) | 126 (6) | 128 (5) | 127 (7) |
| NRS | |||||
| at rest | 4.5 (2.9) | 3.4 (2.9) | 1.5 (1.6) | 1.4 (1.9) | 2.0 (2.4) |
| during movement | 7.2 (1.7) | 4.2 (3.0) | 2.6 (1.9) | 2.4 (2.4) | 2.4 (2.6) |
| OKS | 22 (7.7) | 32 (9.8) | 40 (6.6) | 40 (5.9) | 40 (9.5) |
| KOOS-PS | 53 (19) | 39 (18) | 26 (16) | 27 (12) | 26 (16) |
| EQ-5D-5L a | 0.7 (–0.2 to 0.9) | 0.8 (0.1 to 1.0) | 0.9 (0.2 to 1.0) | 0.9 (0.3 to 1.0) | 0.9 (–0.2 to 1.0) |
| EQ-5D-5L VAS | 67 (22) | 77 (17) | 75 (21) | 79 (15) | 73 (27) |
| a Median (range) due to non-normality | |||||
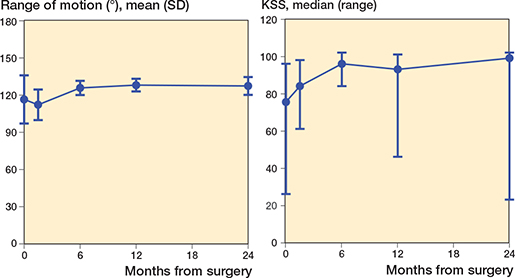
Figure 2. Clinical evaluation presented by the mean (SD) range of motion and median (range) Knee Society Score (KSS), the latter due to non-normality.
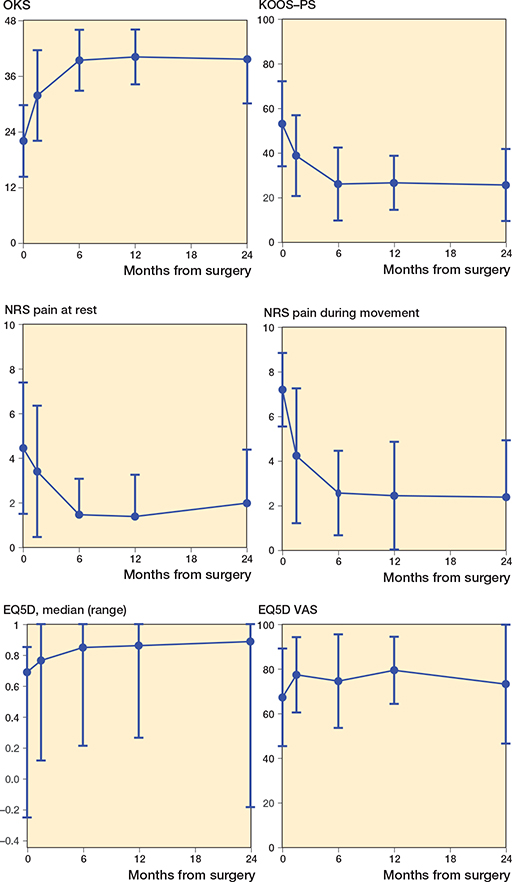
Figure 3. Patient-reported outcome measures presented by the Oxford Knee Score (OKS), Knee injury and Osteoarthritis Outcome Score (KOOS-PS), numeric rating scale (NRS) pain at rest and during movement, the EQ-5D-5L, and the EQ-5D VAS. Data is presented as mean (SD) except for EQ-5D due to non-normality.
mRSA
Precision
Table 4 shows the results of the precision obtained with the double examination performed at 12 months after surgery. Double examinations were obtained from 11 patients for the femoral component and 17 patients for the tibial component.
Migration
Figure 4 and Table 5 present the results of the migration of both the femoral and tibial components. The translation of the femoral component was < 0.17 mm along all 3 axes at 6 weeks after surgery and stabilized at 24 months after surgery (p > 0.2). The rotation of the femoral component was < 0.22° about the 3 axes at 6 weeks after surgery and also stabilized at 24 months after surgery (p > 0.2). All femoral translations and rotations fell within the PIs except for the translation along the y-axis at 24 months postoperatively and the rotation about the x-axis at 6 weeks postoperatively.
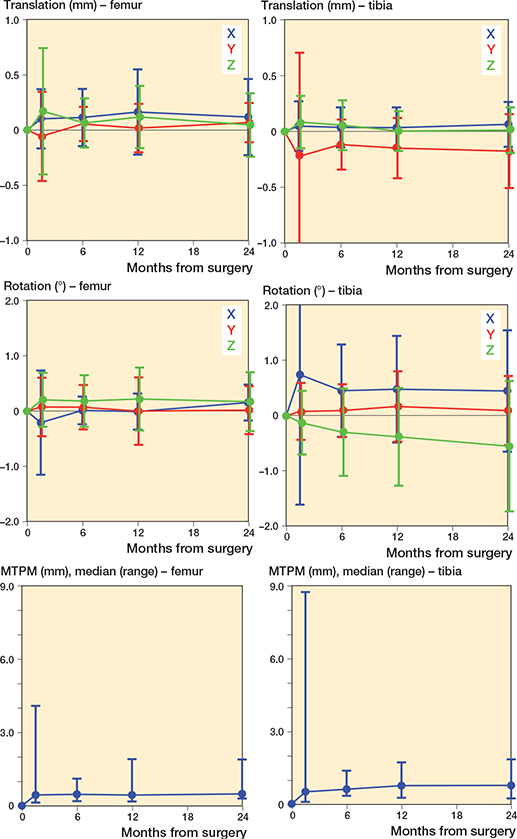
Figure 4. Migration results for both the femoral and tibial component over 2 years presented by the translation along the x-axis (blue), y-axis (red), and z-axis (green), rotations about the x-axis (blue), y-axis (red), and z-axis (green), and the maximum total point motion (MTPM). Data is presented as mean (SD).except for MTPM due to non-normality.
The translation of the tibial component was < 0.22 mm along all 3 axes at 6 weeks after surgery, which stabilized at 24 months after surgery (p > 0.2). The rotation of the tibial component was < 0.75° about the 3 axes at 6 weeks after surgery, which also stabilized at 24 months after surgery, except for the rotation about the z-axis, resulting in a significant effect of time (p = 0.005). At both 12 months and 24 months postoperatively, this rotation was significantly different from 0 (p = 0.02 and p < 0.001, respectively). This rotation also fell outside the PI at 24 months postoperatively. All other tibial translations and rotations fell within the PI, except for the translation along the y-axis at all time points.
The migration results of the femoral component show 1 outlier at 6 weeks after surgery. However, this outlier disappeared on the other time points and is probably due to a measurement error.
Discussion
We investigated the migration of the PPK prosthesis during a follow-up of 24 months and found low migration of both the femoral and the tibial component in the first 6 months, after which both components stabilized. Only the rotation of the tibial component about the z-axis did not stabilize. The precision intervals of our study are comparable with those of other UKA studies (8-10).
Clinical outcomes
PROMs improved within 6 months and stabilized thereafter. All significant changes are also clinically relevant, as they cross the minimal clinically important differences as described in previous studies (19-22). The clinical results are comparable with the results of Parratte et al. and Escudier et al., who also investigated the PPK (i.e., quality of life, function measured by OKS and range of motion) after 12 and 24 months. They found even a better function and quality of life compared with a symmetrical shaped prosthesis (23,24). The results are also comparable with the 2 years results of other studies on UKAs (9,25,26) and the Persona TKA (27).
Migration
The migration of the femoral component is low and mainly falls within the PI. Only the translation along the y-axis at 24 months postoperatively and the rotation about the x-axis at 6 weeks postoperatively are true migrations, as they fell outside the PI. This translation together with the found rotation about the x-axis might be explained by the greater loads during flexion compared with extension.
Only a few studies investigated the migration of the femoral component of UKA (6-9). They also showed a low migration of the femoral component after 24 months. The 5-year migration results were only determined by Campi et al.; no significant migration of the femoral component was found (6). As our study shows comparable results, we expect good results of the femoral component also in the long-term.
The migration of the tibial component mainly consists of translation along the y-axis and rotation about the z-axis. The translation along the y-axis fell outside the PI at all time points. Only at 24 months, the rotation about the z-axis also fell outside the PI. Both indicate true migrations and therefore might be clinically relevant.
The migration is comparable with previous studies in which the migration of the tibial component was investigated (6-10,12). Kendrick et al. showed comparable translation and rotation in all directions, except of the rotation about the x-axis, which was in opposite direction compared to our results, namely in negative direction (7). Ensini et al. also showed low migration of the tibial component. However, it is unclear whether these were in positive or negative directions (12). Koppens et al. showed contradictory results, namely a positive translation along the y-axis but a negative rotation about the x-axis of the tibial component (8).
The negative translation of the tibial component along the y-axis (subsidence) might be explained by the collapse either of the cement structure or of the underlying bone, as the cement already achieves its final shape intra-operatively. Previous studies comparing the migration of cemented and uncemented UKA showed that the tibial component of the uncemented prosthesis subside more compared with the cemented prosthesis in the first year. However, during the second year comparable subsidence was found (6,7).
The negative rotation about the z-axis did not stabilize within 24 months. However, individual data clearly shows a group with continuous migration (7/22) and a group with stabilized migration (15/22), in which continuous migration was defined as a difference of MTPM > 0.2 mm between 12 and 24 months postoperatively (3). This proportion of patients with continuous migration is comparable with other studies (7,9). The group with continuous migration shows, besides a continuous negative rotation about the z-axis, a continuous translation along the y-axis. This indicates that the negative rotation about the z-axis is coincides with the negative translation along the y-axis.
Relation of migration with clinical outcomes
Previous studies on migration in TKA showed a clear relation with clinical outcomes and revision on a later stage and defined thresholds to predict aseptic loosening (3,4). Currently, thresholds are only available for TKA and THA and not (yet) for UKA. We used the thresholds obtained in TKA, as Bruni et al. showed that these thresholds also hold for UKA (5). However, more research is needed to assess whether TKA thresholds are applicable for UKA.
Pijls et al. defined thresholds based on the mean MTPM. Using these thresholds the femoral and tibial component explored in our study are both “at risk” with a MTPM between 0.5 and 1.6 mm, but stable with a MTPM increase of < 0.2 mm between 6 and 12 months and between 12 and 24 months (3). Gudnason et al. showed that in TKA both negative translation along the y-axis (> 0.6 mm) and rotation about the x-axis (> 0.8°) of the tibial component 24 months post-operatively are predictors of subsequent revision due to aseptic loosening (4). In our study, no patients showed a translation along the y-axis above this threshold. However, 5 patients showed a rotation about the x-axis above the threshold of 0.8° at 24 months postoperatively. These patients might be at higher risk for aseptic loosening. They also show a continuous migration of the tibial component. To determine whether the measured migration also leads to revision surgery, we will review all patients 5 years postoperatively.
Limitations
In contrast to previous studies, migration of both the tibial and the femoral component was investigated. However, due to technical errors (i.e., not enough markers visible on the RSA radiographs) not all radiographs could be used to determine the migration of the femoral component (Figure 1). As these technical errors occurred randomly, this did not influence the results.
The number of included patients is relatively low. Previous studies performing mRSA showed that this method is very accurate, which requires a small number of patients to be included to the study (2). However, this number of patients is too small to investigate the relation between migration and clinical outcomes. In addition, long-term follow-up is needed to determine whether the migration found in this study results in revision surgery.
Conclusion
The PPK shows low migration of both the femoral and tibial component and good PROMs at 2 years’ follow-up. Long-term follow-up is needed to gain more insight into the long-term results, to compare those with other prostheses, and to evaluate the risk of continuous migration.
- Tay M L, McGlashan S R, Monk A P, Young S W. Revision indications for medial unicompartmental knee arthroplasty: a systematic review. Arch Orthop Trauma Surg 2022; 142(2): 301-14. doi: 10.1007/s00402-021-03827-x.
- Valstar E R, de Jong F W, Vrooman H A, Rozing P M, Reiber J H. Model-based Roentgen stereophotogrammetry of orthopaedic implants. J Biomech 2001; 34(6): 715-22. doi: 10.1016/s0021-9290(01)00028-8.
- Pijls B G, Plevier J W M, Nelissen R. RSA migration of total knee replacements. Acta Orthop 2018; 89(3):320-8. doi:10.1080/17453674.2018.1443635.
- Gudnason A, Adalberth G, Nilsson K G, Hailer N P. Tibial component rotation around the transverse axis measured by radiostereometry predicts aseptic loosening better than maximal total point motion. Acta Orthop 2017; 88(3): 282-7. doi: 10.1080/17453674.2017.1297001.
- Bruni D, Bragonzoni L, Gagliardi M, Bontempi M, Akkawi I, Raspugli G F, et al. Roentgen stereophotogrammetric analysis: an effective tool to predict implant survival after an all-poly unicompartmental knee arthroplasty: a 10 year follow-up study. Knee Surg Sports Traumatol Arthrosc 2015; 23(11): 3273-80. doi: 10.1007/s00167-014-3106-2.
- Campi S, Kendrick B J L, Kaptein B L, Valstar E R, Jackson W F M, Dodd C A F, et al. Five-year results of a randomised controlled trial comparing cemented and cementless Oxford unicompartmental knee replacement using radiostereometric analysis. Knee 2021; 28: 383-90. doi: 10.1016/j.knee.2020.09.003.
- Kendrick B J, Kaptein B L, Valstar E R, Gill H S, Jackson W F, Dodd C A, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J 2015; 97-B(2): 185-91. doi: 10.1302/0301-620X.97B2.34331.
- Koppens D, Rytter S, Munk S, Dalsgaard J, Sorensen O G, Hansen T B, et al. Equal tibial component fixation of a mobile-bearing and fixed-bearing medial unicompartmental knee arthroplasty: a randomized controlled RSA study with 2-year follow-up. Acta Orthop 2019; 90(6): 575-81. doi: 10.1080/17453674.2019.1639965.
- Koppens D, Stilling M, Munk S, Dalsgaard J, Rytter S, Sorensen O G, et al. Low implant migration of the SIGMA((R)) medial unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2018; 26(6): 1776-85. doi: 10.1007/s00167-017-4782-5.
- Linde K N, Madsen F, Puhakka K B, Langdahl BL, Soballe K, Krog-Mikkelsen I, et al. Preoperative systemic bone quality does not affect tibial component migration in knee arthroplasty: a 2-year radiostereometric analysis of a hundred consecutive patients. J Arthroplasty 2019; 34(10): 2351-9. doi: 10.1016/j.arth.2019.05.019.
- Lindstrand A, Stenström A, Ryd L, Toksvig-Larsen S. The introduction period of unicompartmental knee arthroplasty is critical: a clinical, clinical multicentered, and radiostereometric study of 251 Duracon unicompartmental knee arthroplasties. J Arthroplasty 2000; 15(5): 608-16. doi: 10.1054/arth.2000.6619.
- Ensini A, Barbadoro P, Leardini A, Catani F, Giannini S. Early migration of the cemented tibial component of unicompartmental knee arthroplasty: a radiostereometry study. Knee Surg Sports Traumatol Arthrosc 2013; 21(11): 2474-9. doi: 10.1007/s00167-012-2068-5.
- Scuderi G R, Bourne R B, Noble P C, Benjamin J B, Lonner J H, Scott W N. The new Knee Society Knee Scoring System. Clin Orthop Relat Res 2012; 470(1): 3-19. doi: 10.1007/s11999-011-2135-0.
- Perruccio AV, Stefan Lohmander L, Canizares M, Tennant A, Hawker G A, Conaghan P G, et al. The development of a short measure of physical function for knee OA KOOS-Physical Function Shortform (KOOS-PS): an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008; 16(5): 542-50. doi: 10.1016/j.joca.2007.12.014.
- Hjermstad M J, Fayers P M, Haugen D F, Caraceni A, Hanks G W, Loge J H, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011; 41(6): 1073-93. doi: 10.1016/j.jpainsymman.2010.08.016.
- Haverkamp D, Breugem S J M, Sierevelt I N, Blankevoort L, van Dijk C N. Translation and validation of the Dutch version of the Oxford 12-item knee questionnaire for knee arthroplasty. Acta Orthop 2005; 76(3): 347-52. PMID: 16156462
- Versteegh M M, Vermeulen K M, Evers S M A A, de Wit G A, Prenger R, Stolk E A. Dutch tariff for the five-level version of EQ-5D. Value Health 2016; 19(4): 343-52. doi: 10.1016/j.jval.2016.01.003.
- Kaptein B L, Valstar E R, Stoel B C, Rozing P M, Reiber J H. A new type of model-based Roentgen stereophotogrammetric analysis for solving the occluded marker problem. J Biomech 2005; 38(11): 2330-4. doi: 10.1016/j.jbiomech.2004.09.018.
- Kendrick D B, Strout T D. The minimum clinically significant difference in patient-assigned numeric scores for pain. Am J Emerg Med 2005; 23(7): 828-32. doi: 10.1186/s13018-021-02514-2 10.1016/j.ajem.2005.07.009.
- Singh J A, Luo R, Landon G C, Suarez-Almazor M. Reliability and clinically important improvement thresholds for osteoarthritis pain and function scales: a multicenter study. J Rheumatol 2014; 41(3): 509-15. doi: 10.3899/jrheum.130609.
- Beard D J, Harris K, Dawson J, Doll H, Murray D W, Carr A J, et al. Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. J Clin Epidemiol 2015; 68(1): 73-9.
- Lizaur-Utrilla A, Gonzalez-Parreño S, Martinez-Mendez D, Miralles-Muñoz F A, Lopez-Prats F A, Beard D J, et al. Minimal clinically important differences and substantial clinical benefits for Knee Society Scores: meaningful changes for the Oxford hip and knee scores after joint replacement surgery. Knee Surg Sports Traumatol Arthrosc 2020; 28(5): 1473-8. eng. doi: 10.1007/s00167-019-05543-x.
- Escudier J C, Jacquet C, Flecher X, Parratte S, Ollivier M, Argenson J N. Better implant positioning and clinical outcomes with a morphometric unicompartmental knee arthroplasty: results of a retrospective, matched-controlled study. J Arthroplasty 2019; 34(12): 2903-8. doi: 10.1016/j.arth.2019.07.031
- Parratte S, Sah A, Müller N, Abshagen S. First clinical 1-year outcomes of partial knee arthroplasty with the Persona® Partial Knee. Zimmer Biomet; 2019.28.
- Goh G S, Zeng G J, Khow Y Z, Lo N N, Yeo S J, Liow M H L. No difference in long-term outcomes between men and women undergoing medial fixed-bearing cemented unicompartmental knee arthroplasty: a retrospective cohort study with minimum 10-year follow up. Knee 2021; 30: 26-34. doi: 10.1016/j.knee.2021.03.006.
- Pandit H, Hamilton T W, Jenkins C, Mellon S J, Dodd C A, Murray D W. The clinical outcome of minimally invasive Phase 3 Oxford unicompartmental knee arthroplasty: a 15-year follow-up of 1000 UKAs. Bone Joint J 2015; 97-B(11): 1493-500. doi: 10.1302/0301-620X.97B11.35634.
- Mathijssen N M C, Verburg H, London N J, Landsiedl M, Dominkus M. Patient reported outcomes and implant survivorship after total knee arthroplasty with the Persona knee implant system: two year follow up. BMC Musculoskelet Disord 2019; 20(1): 97. doi: 10.1186/s12891-019-2470-y.