The European Bone and Joint Infection Society definition of periprosthetic joint infection is meaningful in clinical practice: a multicentric validation study with comparison with previous definitions
Ricardo SOUSA 1, Ana RIBAU 2, Pedro ALFARO 3, Marc-Antoine BURCH 4, Joris PLOEGMAKERS 5, Martin MCNALLY 6, Martin CLAUSS 4,7, Marjan WOUTHUYZEN-BAKKER 8, and Alex SORIANO 9
1 Porto Bone and Joint Infection Group (GRIP), Department of Orthopedics, Centro Hospitalar Universitário do Porto and CUF – Hospitais e Clínicas, Portugal; 2 Department of Orthopedics, Centro Hospitalar Universitário do Porto, Porto, Portugal; 3 Visiting fellow at Hospital Clinic, Barcelona, Spain; 4 Orthopedics and Trauma Surgery Department, University Hospital Basel, Switzerland; 5 Department of Orthopedic Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; 6 The Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford University Hospitals, Oxford, United Kingdom; 7 Center for Musculoskeletal Infections and Orthopedics and Trauma Surgery, University Hospital Basel, Switzerland; 8 Department of Medical Microbiology and Infection Prevention, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; 9 Department of Infectious Diseases, University of Barcelona, IDIBAPS, Hospital Clinic of Barcelona, Barcelona, Spain
Background and purpose — A new periprosthetic joint infection (PJI) definition has recently been proposed by the European Bone and Joint Infection Society (EBJIS). The goals of this paper are to evaluate its diagnostic accuracy and compare it with previous definitions and to assess its accuracy in preoperative diagnosis.
Patients and methods — We retrospectively evaluated a multicenter cohort of consecutive revision total hip and knee arthroplasties. Cases with minimum required diagnostic workup were classified according to EBJIS, 2018 International Consensus Meeting (ICM 2018), Infectious Diseases Society of America (IDSA), and modified 2013 Musculoskeletal Infection Society (MSIS) definitions. 2 years’ minimum follow-up was required to assess clinical outcome.
Results — Of the 472 cases included, PJI was diagnosed in 195 (41%) cases using EBJIS; 188 (40%) cases using IDSA; 172 (36%) using ICM 2018; and 145 (31%) cases using MSIS. EBJIS defined fewer cases as intermediate (5% vs. 9%; p = 0.01) compared with ICM 2018. Specificity was determined by comparing risk of subsequent PJI after revision surgery. Infected cases were associated with higher risk of subsequent PJI in every definition. Cases classified as likely/confirmed infections using EBJIS among those classified as not infected in other definitions showed a significantly higher risk of subsequent PJI compared with concordant non-infected cases using MSIS (RR = 3, 95% CI 1–6), but not using ICM 2018 (RR = 2, CI 1–6) or IDSA (RR = 2, CI 1–5). EBJIS showed the highest agreement between pre-operative and definitive classification (k = 0.9, CI 0.8–0.9) and was better at ruling out PJI with an infection unlikely result (sensitivity 89% [84–93], negative predictive value 90% [85–93]).
Conclusion — The newly proposed EBJIS definition emerged as the most sensitive of all major definitions. Cases classified as PJI according to the EBJIS criteria and not by other definitions seem to have increased risk of subsequent PJI compared with concordant non-infected cases. EBJIS classification is accurate in ruling out infection preoperatively.
Citation: Acta Orthopaedica 2023; 94: 8–18. DOI http://dx.doi.org/10.2340/17453674.2023.5670.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-27. Accepted: 2022-11-15. Published: 2023-01-23.
Correspondence: ricardojgsousa@gmail.com
Senior authors (RS, MC, MW, and AS) participated in the design and protocol of the study. AR, PA, MAB, and JP conducted data retrieval in their institutions. RS and AR were responsible for analysis and interpretation of the data. AR performed the statistical analyses. RS drafted the manuscript and all other authors contributed to the interpretation of the data, critically revised, and approved the final manuscript.
Handling co-editors: Eivind Witsø and Robin Christensen
Acta thanks Per Kjærsgaard-Andersen, Inge Skråmm, and Anna Stefánsdóttir for help with peer review of this study.
Periprosthetic joint infection (PJI) is among the most frequent indications for revision of a joint arthroplasty (1).
Accurate diagnosis is the starting point for effective treatment. Nevertheless, diagnosing PJI is a challenging endeavor, and it has been repeatedly shown to be present in a significant proportion of cases thought to be aseptic (2-5). Given that no single test has perfect accuracy, definitive diagnosis must rely on a set of predetermined criteria that constitute any given definition.
In the past decade, there have been many attempts to define criteria by which PJI is diagnosed. Proposed PJI definitions are mostly based on expert opinions or consensus and there are few structured validation studies. The Musculoskeletal Infection Society (MSIS) published a definition in 2011 (6), which was modified and subjected to international review during the first International Consensus Meeting (ICM) on Periprosthetic Joint Infection in 2013 (7). Also in 2013, the Infectious Diseases Society of America (IDSA) published a guideline on diagnosis from an international expert group (8). In 2018 a new definition was presented, with a weighted score and a new category of possibly infected cases (9). This proposal was discussed at the reconvened ICM in 2018 and minor changes were adapted but the final version was supported by only 68% of delegates (10).
Perhaps the major concern with earlier PJI definitions is the potential for lower sensitivity to diagnose the full spectrum of infections. There is now a greater understanding of low-grade infections, which may have been missed in the past (11,12), and how inappropriate treatment may impact outcomes (13-15). With the purpose to overcome this limitation, EBJIS recently proposed a new set of criteria (16). It has recently been shown that it classifies more cases as infected while significantly reducing the number of uncertain diagnoses compared with previously existing definitions (17). However, the question of whether that increased diagnostic sensitivity is exaggerated and offers unacceptable loss of specificity was not addressed.
This study compared the new EBJIS definition with previous major definitions. Diagnostic accuracy was studied using definitive postoperative classification and the rate of recurrent PJI after revision surgery as a proxy to assess correct classification. We also evaluated its usefulness in clinical practice, by comparing classification using preoperatively available information with definitive results.
Patients and methods
This is a multicenter retrospective study of patients who have undergone revision total hip (THA) or knee (TKA) arthroplasty in 4 different European institutions from Barcelona (Spain), Porto (Portugal), Basel (Switzerland), and Groningen (the Netherlands). All consecutive revision arthroplasties performed in a 6-year period between January 1, 2013 and December 31, 2018, regardless of perceived preoperative or definitive infected status, were analyzed. Debridement, antibiotics, and implant retention procedures were not included. The study was approved by the local ethics committee in each center. All centers have dedicated prospective databases in which patients give general consent to being registered.
Data concerning patient demographics and original joint replacement surgery (e.g., indication for surgery, postoperative wound healing problems, etc.) was collected. Detailed clinical information before revision surgery was exhaustively collected from electronic medical records with a special emphasis on variables relevant for the diagnosis of PJI (e.g., presence of sinus tract, history of recent fever or bacteremia, antibiotic therapy at the time of surgery, and blood inflammatory parameters). Preoperative radiographic and nuclear medicine findings as well as synovial fluid investigation results were noted as well as intraoperative findings (e.g., purulence) and definitive microbiologic and histological results. Table 1 (see Appendix) shows a list of all collected variables and diagnostic work-up availability.
Cases without minimum required diagnostics to classify them as septic: < 4 intraoperative microbiology samples (synovial fluid, tissue samples, implant sonication), and no preoperative/intraoperative synovial fluid differential leukocyte count or intraoperative histology were excluded (Figure 1).
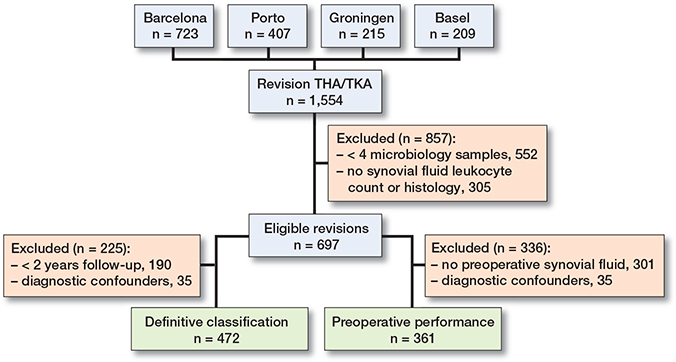
Figure 1. Flowchart of patient selection.
Using available information, all cases were classified using the major PJI definitions: (a) the 2021 European Bone and Joint Infection Society (EBJIS) (16); (b) the 2018 International Consensus Meeting (ICM) (10); (c) the Infectious Diseases Society of America (IDSA) (8) and; (d) the Musculoskeletal Infection Society (MSIS) definition as modified in the 2013 ICM (7). Table 2 (see Appendix) details these definitions and their respective criteria.
All definitions classify the presence of a sinus tract or 2 positive identical cultures as evidence of infection. According to the EBJIS definition, infection was also considered confirmed if total leukocyte count was > 3,000 cells/μL or the proportion of polymorphonuclear neutrophils (PMN) was > 80%, there was growth of > 50 CFU/mL from sonication fluid of any organism, or if histology was positive for infection. Histology was considered positive as defined by the attending pathologist in all definitions. When using the 2013 MSIS criteria and the 2018 ICM criteria, chronic infection thresholds were used. When applying the IDSA definition, infection was also defined by the presence of intraoperative purulence, positive histology, or growth of an uncommon contaminant in a single specimen as explicitly stated in the criteria, but also when a single positive microbiology result of a common contaminant was accompanied by an elevated proportion of PMN or elevated total leukocyte count as suggested in the original paper’s discussion (8). A comprehensive list of considered common and uncommon contaminant microorganisms can be found in Table 4.
To reduce bias, we also excluded cases with potential confounding conditions (i.e., inflammatory arthritis, metalon-metal bearing, periprosthetic fracture, antibiotic within 2 weeks prior to revision surgery, and revision surgery < 6 weeks after index procedure) from further analyses unless there were other confirmatory criteria independent of the underlying condition, such as a sinus tract or positive microbiology (i.e., 2 identical cultures or sonication > 50 CFU/mL) confirming infection.
A minimum follow-up of 2 years after revision surgery was required for assessing outcomes after revision surgery unless the patient developed subsequent PJI or underwent subsequent revision surgery before 2 years. Synovial fluid investigation was required to assess preoperative classification performance. Cases were analyzed whether they were treated for PJI or not. Failure was defined as the following: (i) subsequent PJI as defined by the treating team with or without the need for further surgery or; (ii) the need for subsequent revision surgery considering all causes (infected or aseptic).
Statistics
Descriptive statistics were used to report basic demographics data and the diagnostic workup.
Categorical variables were presented as number of cases and percentages. Contingency tables were performed to illustrate the interrelation between variables. Proportions were compared using a chi-square test and Fisher’s exact test when necessary. Relative risk (RR) with 95% confidence intervals (CI) was calculated when adequate.
Kaplan–Meier survival curves with a Cox regression analysis were used to evaluate events (subsequent PJI and subsequent revision surgery) in time. The log-rank test was used to compare survival between 2 or more independent groups.
Cohen’s weighted kappa coefficient was used to evaluate the concordance between preoperative and final classification. Statistical analyses were performed using IBM SPSS Statistics (version 24.0; IBM Corp, Armonk, NY, USA) with significance defined as a 2-tailed p-value < 0.05.
Contingency tables were performed to assess sensitivity and negative predictive values (NPV) and their 95% confidence intervals (CI).
Ethics, data sharing, funding, and disclosures
A previously defined study protocol was used to obtain local ethics committee approval in every participating institution. Anonymized electronic case report forms were then shared between centers.
This study had no specific funding and none of the authors has any monetary conflicts of interest to disclose. Senior authors of this paper (RS, MMcN, MC, MW, and AS) are or were members of the EBJIS Executive Committee and have previously been involved in the establishment of the EBJIS PJI definition.
Results
During the inclusion period, a total of 1,554 revision hip or knee arthroplasties were performed in the 4 participating centers (723 in Barcelona, 407 in Porto, 209 in Basel, and 215 in Groningen). After applying exclusion criteria, 697 cases (226 THA and 471 TKA) were left for analysis with a mean follow-up of 36 months (range 1–85 months). Figure 1 shows a flowchart of patient selection on 2 different pathways for 2 different analyses: definitive PJI classification comparison and preoperative versus definitive classification results.
Definitive PJI classification comparison
Using all available preoperative and intraoperative test(s) results of the 472 included cases, final PJI classification status according to different definitions was defined (Table 3). There were significant differences among different PJI definitions, with the EBJIS definition classifying the highest number of cases as infected and the MSIS 2013 the least, with a difference of around 10% (p < 0.001). Compared with ICM 2018, the EBJIS definition offered significantly fewer intermediate results: 5% (22/472) likely vs. 9% (42/472) inconclusive, p = 0.01.
Table 4 details definitive microbiology findings according to the different classifications. The proportion of culture-negative infections was significantly different among classifications (p < 0.001). It was higher in EBJIS (28%) than in ICM 2018 (19%) or IDSA (19%), and lowest using the MSIS 2013 (9%). Among patients with single positive cultures, uncommon contaminants were significantly more likely to present with other confirmatory features for infection than common contaminants. There were only 7 cases with isolated cultures growing uncommon contaminants and no confirmatory feature (more detailed information in Table 5, see Appendix).
| Factor | n | No positive cultures | Single positive culture a | 2 positive cultures or sonication (> 50 CFU/mL) | |
| common contaminant b | uncommon contaminant c | ||||
| EBJIS | |||||
| Infection confirmed | 195 | 55 (28) | 24 (12) | 10 (5) | 106 (54) |
| Infection likely | 22 | 3 (14) | 16 (73) | 3 (14) | – |
| Infection unlikely | 255 | 218 (85) | 33 (13) | 4 (2) | – |
| ICM 2018 | |||||
| Infected | 172 | 32 (19) | 23 (13) | 11 (6) | 106 (62) |
| Inconclusive | 42 | 25 (60) | 16 (38) | 1 (2) | – |
| Not infected | 258 | 219 (85) | 34 (13) | 5 (2) | – |
| IDSA | |||||
| Infected | 188 | 36 (19) | 29 (15) | 17 (9) | 106 (56) |
| Not infected | 284 | 240 (85) | 44 (15) | – | – |
| MSIS 2013 | |||||
| Infected | 145 | 13 (9) | 18 (12) | 8 (6) | 106 (73) |
| Not infected | 327 | 263 (80) | 55 (17) | 9 (3) | – |
| a or sonication < 50 CFU/mL. b most coagulase-negative staphylococci spp (S. epidermidis, S. capitis, S. hominis, S. warneri, S. auricularis), viridans group streptococci (S. mitis, S. mutants, S. salivarius), Corynebacterium spp, anaerobic gram-positive bacilli (Cutibacterium acnes), anaerobic gram-positive cocci (Finegoldia magna, Peptococcus spp, Peptostreptococcus spp). c Staphylococcus aureus, Staphylococcus lugdunensis, Enterococcus, Beta-haemolytic Streptococci, Streptococcus anginosus group (S. anginosus, S. constellatus, and S. intermedius), Enterobacteriaceae (Escherichia coli, Proteus spp, Klebsiella spp, Enterobacter spp, Morganella morganii), Pseudomonas aeruginosa, anaerobic gram-negative rods (Bacteroides fragilis, Fusobacterium), Candida spp. |
|||||
Considering the occurrence of subsequent PJI, all classifications demonstrated significantly different outcomes between infected and non-infected cases as expressed in Kaplan–Meier survival curves (Figure 2). Cases classified as infected were more likely to develop subsequent infection over time in all classifications studied. Looking at the need for subsequent revision surgery considering all causes, no significant difference was observed between infected and non-infected cases with any classification.
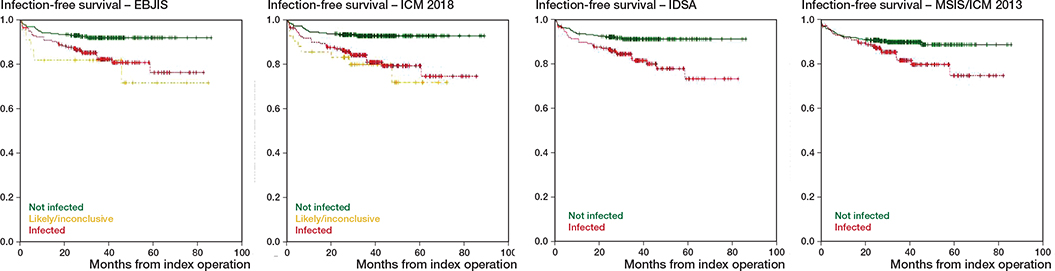
Figure 2. Kaplan–Meier infection-free survival curves for different classifications.
Within EBJIS, the rate of subsequent PJI during followup was significantly lower in the non-infected cohort: 8% (20/255); when compared with the confirmed: 17% (33/195), (RR = 2, CI 1–4); or likely infection: 23% (5/22), (RR = 3, CI 1–10) cohorts. The difference among confirmed and likely infection subgroups was not significant.
Comparing EBJIS subgroups with previous definitions, it is possible to ascertain that cases classified as non-infected within both EBJIS and MSIS have a significantly lower infection rate when compared with those classified as infected in both classifications: 8% (20/255) vs. 17% (25/145), (RR = 2, CI 1–5). The same is true using either the IDSA: 8% (20/250) vs. 18% (31/176), (RR = 2, CI 1–4); or the ICM 2018 definitions: 7% (17/243) vs. 18% (31/170), (RR = 3, CI 2–6) for comparison. More detailed information on outcome of different subgroups of patients as defined by the EBJIS criteria and previous definitions can be found in Table 6 (see Appendix).
Using the EBJIS definition, several patients were classified as likely or confirmed infections among those classified as not infected using other definitions. Compared with MSIS 2013, the rate of subsequent PJI in this subgroup was significantly higher than in the subgroup of concordant non-infected cases: 18% (13/72) vs. 8% (20/255), (RR = 3, CI 1–6). A similar yet not significant trend was also found when confronting it with ICM 2018: 16% (7/45) vs. 7% (17/243), (RR = 2, CI 1–6); but not IDSA: 12% (4/34) vs. 8% (20/250), (RR = 2, CI 1–5).
Interestingly, the rate of subsequent PJI among EBJIS likely or confirmed infections was similar comparing microbiologically confirmed infection with at least 2 identical cultures, single positive cultures (no difference between common or uncommon contaminant), and culture-negative infections: 18% (19/106) vs. 17% (9/53) vs. 17% (10/58) respectively.
Preoperative versus definitive results
361 revisions (316 TKA and 45 THA) with adequate preoperative synovial fluid analysis were included in the study of preoperative versus definitive results (Figure 1). Preoperative and definitive PJI classification status of the included patients according to the different definitions are presented in Table 7.
The EBJIS definition (k = 0.9, CI 0.8–0.9) showed the highest agreement between preoperative and definitive classification, followed by ICM 2018 (k = 0.8, CI 0.8–0.9), IDSA (k = 0.6, CI 0.5–0.7), and MSIS 2013 (k = 0.4, CI 0.3–0.4).
Concerning each classification’s ability to rule out infection prior to revision surgery and using each one’s definitive results for comparison: EBJIS preoperative unlikely result shows 89% (CI 84–93) sensitivity and 90% (CI 85–93) negative predictive value (NPV); ICM 2018 preoperative not infected result shows 85% (CI 79–90) sensitivity and 87% (CI 82–90) NPV; IDSA preoperative not infected result shows 56% (CI 48–65) sensitivity and 77% (CI 74–80) NPV; and MSIS 2013 preoperative not infected result shows 66% (CI 57–74) sensitivity and 85% (CI 81–88) NPV. 8 out of 19 preoperative EBJIS likely and 28 out of 49 ICM 2018 inconclusive preoperative results turned out to be confirmed infections postoperatively.
Using the EBJIS definition, the most common cause for upgrading cases that were misclassified preoperatively as not infected was positive intraoperative cultures, with the majority growing coagulase-negative staphylococci. Table 8 (see Appendix) offers further details concerning these cases.
Discussion
We aimed to study the performance of the newly proposed EBJIS definition and compare it with pre-existing ones.
Our results show that the EBJIS definition classified significantly more cases of revision arthroplasties as infected, compared with all other definitions. It is noteworthy that those cases that are classified as likely or confirmed infection using EBJIS, which would otherwise be called non-infected using previous definitions, do have an unfavorable outcome, suggesting this extra sensitivity is indeed finding genuinely infected cases and does not represent an unacceptable overdiagnosis. The EBJIS definition also performs favorably in the preoperative stage, with a very high agreement between preoperative and definitive classification and the best negative predictive value to rule out infection.
In accordance with previous findings (9,18), the ICM 2018 criteria have also identified more infections than the MSIS 2013. However, our study shows significantly fewer intermediate results using the EBJIS definition compared with the ICM 2018 definition (4.7% vs 8.9%). This is important, as it reduces the number of cases that, after full investigation, still have an undetermined diagnosis but still require a decision on treatment.
Although the IDSA criteria were also more sensitive than MSIS 2013, we found them to be more prone to subjective interpretation and more often to require multidisciplinary discussion, although this is somewhat subjective and cannot be expressed objectively.
In this study, we chose to adopt clinical outcome after a minimum 2 years’ follow-up as a proxy for correct identification of infected cases. Naturally, many scenarios may be responsible for subsequent PJI and previously unrecognized, untreated, persistent infection is only one of them. We chose to use the risk of subsequent PJI in aseptic cases (across definitions) as a reference for comparison. Theoretically, all the alternative potential causes of PJI are present in this cohort of aseptic patients and the increased risk in other subgroups is likely due to unrecognized/untreated PJI. This proxy seems reasonable, as it was shown that patients in the infected cohort were more likely to develop subsequent infection regardless of which classification was used. The outcome of “newly diagnosed” infections was in fact worse than the outcome of the group of patients classified as unlikely to be infected by both EBJIS and previous definitions. One can therefore infer that these are real infections and hypothesize that they would indeed benefit from being treated as such. In fact, their outcome was similar to that of the group of patients in whom both EBJIS and previous definitions classify as infected. This is in line with previous findings, showing that patients with positive findings may be at risk of a poorer outcome, even if they do not qualify as infected in older definitions (13,14).
The EBJIS definition classified more cases as infected when there was no confirmatory microbiological culture (28% culture-negative). This reflects the need for criteria other than bacterial culture to define the presence of infection while newer more sensitive methods of identifying pathogens are developed. This added sensitivity for the EBJIS definition is not at the expense of specificity. Despite the significantly higher rate of culture-negative infections, there is a similar rate of recurrent PJI when comparing microbiologically proven, single positive, or even culturenegative infections. This suggests that these culture-negative cases are in fact infected with organisms that have not been cultured using current methodologies. One can only hypothesize whether new and more sensitive microbiological methods such as next-generation sequencing will make a difference. The ability to identify “difficult to diagnose” infection was one of the main drivers in the development of the new EBJIS definition (16).
Another fundamental aspect of a PJI definition is its ability to serve as a practical guide for clinicians. As such, a key stage is the preoperative period when major decisions regarding the most appropriate course of treatment are made even before relevant diagnostic tests such as intraoperative microbiology or histology are available. Current results show a very favorable performance of the EBJIS definition at this point, with very high agreement between preoperative and definitive postoperative classification. In fact, the EBJIS infection unlikely result was shown to have the highest sensitivity and negative predictive values when compared with previous definitions. This is very helpful in guiding the correct treatment options before revision surgery.
This study is not without limitations. First and foremost, the lack of a true gold standard to clearly differentiate infected from non-infected cases hampers accurate comparison of diagnostic accuracy between different definitions. Previous validation studies have used major criteria (sinus tract and at least 2 positive cultures) as the gold standard but, in our view, this is a perpetuating and self-fulfilling prophecy approach and is not helpful in increasing the sensitivity of the definition. Our approach, to use subsequent infection risk after revision surgery, is novel and perhaps debatable but it allowed for objective comparison between definitions.
Naturally, the study’s retrospective nature may cloak uncertainties concerning the accuracy of clinical information obtained from medical records. It is especially difficult to be certain of the reasoning behind the decision whether to treat each case as infected, especially when diagnosis was not obvious using the older criteria in use during the study period. We hypothesize that there may have been a significant selection bias toward a higher rate of antibiotic treatment after revision surgery in patients with obvious clinical risk factors for infection. The potential effect of such a phenomenon is clearly illustrated in papers that show increased risk of subsequent PJI in cases with positive intraoperative cultures treated with more aggressive antibiotic regimens (5,19). In addition, especially in 1-stage revision, it is not always clear whether the diagnosis of PJI was assumed pre- or postoperatively and whether the surgical procedure would have been different. We therefore declined to draw significant conclusions regarding the effect of “infection treatment” on subsequent clinical outcomes. However important this clearly is, it is unlikely that it would have different impact in the several definitions analyzed.
A minimum diagnostic investigation was established a priori to ensure fair PJI classification status that included intraoperative tests. This automatically excludes from the study the cohort of patients with painful joints not requiring revision surgery. In addition, many revision arthroplasties performed in the participating centers during the study period had to be excluded due to a lack of adequate investigations. This might have affected the estimation of specificity, as probably most of these were judged to be clinically aseptic failures. Although regrettable, the authors believe this reflects actual clinical practice.
A different limitation concerns the diagnostic testing actually performed. Not all cases underwent all diagnostic tests and notably some tests present in the ICM 2018 definition, such as d-dimer, leucocyte esterase, or alpha-defensin, are not part of routine investigation in participating institutions. While this is a possible source of bias, we believe this is overcome by the fact that d-dimer scores in the same category as CRP and both leucocyte esterase and alpha-defensin score in the same category as synovial leukocyte count. Alpha defensin is also part of the EBJIS criteria for confirmed PJI, so would also have been affected in the same way as in ICM. Although most diagnostic tests are objective and can be quantified, some such as histology or even nuclear medicine results were available only as a final interpretation by the attending physician, and adjusting the criterion used to specific interpretation criteria was not possible, thus introducing some classification bias.
We also excluded those cases with conditions that would confound the diagnosis of infection. As such, the findings of this study cannot be extrapolated to this subgroup of patients, who are admittedly difficult to diagnose. It was encouraging that only 35 cases out of 697 had to be excluded for this reason, suggesting that the EBJIS definition is applicable to most patients.
Our study supports that the recently proposed EBJIS PJI definition is meaningful in our clinical practice, with improved detection of low-grade PJI and high specificity. It is easy to apply and is not dependent on adding up several different tests to create a score. It is highly reliable in ruling out infection with minimum clinical and laboratory investigation in the preoperative decision stage. The presence of the middle category of “infection likely” should raise awareness of the high probability of PJI being present and that more comprehensive investigation should be performed.
Conclusion
The EBJIS definition was more sensitive than the other definitions. EBJIS classification is accurate in ruling out infection preoperatively. The EBJIS definition was not designed to dictate any particular treatment for any patient, but our study showed that it was accurate in identifying patients at risk of poor outcomes. Future prospective studies are needed to confirm the findings of our study, and especially to demonstrate the potential advantage of expanding PJI treatment to this larger subgroup of patients that stems from the increased sensitivity. Also, further clarification is needed to better understand whether cases definitively classified as likely infections require the same approach as confirmed infections.
- Patel A, Pavlou G, Mujica-Mota R E, Toms A D. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Joint J 2015; 97-B: 1076-81. doi: 10.1302/0301-620X.97B8.35170.
- Portillo M E, Salvado M, Alier A, Sorli L, Martinez S, Horcajada J P, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res 2013; 471(11): 3672-8. doi: 10.1007/s11999-013-3200-7.
- Ribera A, Morata L, Moranas J, Agullo J L, Martinez J C, Lopez Y, et al. Clinical and microbiological findings in prosthetic joint replacement due to aseptic loosening. J Infect 2014; 69(3): 235-43. doi: 10.1016/j.jinf.2014.05.003.
- Hipfl C, Mooij W, Perka C, Hardt S, Wassilew G I. Unexpected low-grade infections in revision hip arthroplasty for aseptic loosening: a single-institution experience of 274 hips. Bone Joint J 2021; 103-B: 1070-7. doi: 10.1302/0301-620X.103B6.BJJ-2020-2002.R1.
- Jacobs A M E, Benard M, Meis J F, van Hellemondt G, Goosen J H M. The unsuspected prosthetic joint infection: incidence and consequences of positive intra-operative cultures in presumed aseptic knee and hip revisions. Bone Joint J 2017; 99-B: 1482-9. doi: 10.1302/0301-620X.99B11.BJJ-2016-0655.R2.
- Parvizi J, Zmistowski B, Berbari E F, Bauer T W, Springer B D, Della Valle C J, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011; 469(11): 2992-4. doi: 10.1007/s11999-011-2102-9.
- Parvizi J, Gehrke T, International Consensus Group on Periprosthetic Joint Infection: Definition of periprosthetic joint infection. J Arthroplasty 2014; 29: 1331. doi: 10.1016/j.arth.2014.03.009.
- Osmon D R, Berbari E F, Berendt A R, Lew D, Zimmerli W, Steckelberg J M, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56(1): e1-e25. doi: 10.1093/cid/cis803.
- Parvizi J, Tan T L, Goswami K, Higuera C, Della Valle C, Chen A F, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018; 33(5): 1309-14. doi: 10.1016/j.arth.2018.02.078.
- Shohat N, Bauer T, Buttaro M, Budhiparama N, Cashman J, Della Valle C J, et al. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints? Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019; 34: S325-7. doi: 10.1016/j.arth.2018.09.045.
- Li M, Zeng Y, Wu Y, Si H, Bao X, Shen B. Performance of sequencing assays in diagnosis of prosthetic joint infection: a systematic review and meta-analysis. J Arthroplasty 2019; 34: 1514-22. doi: 10.1016/j.arth.2019.02.044.
- Kheir M M, Tan T L, Shohat N, Foltz C, Parvizi J. Routine diagnostic tests for periprosthetic joint infection demonstrate a high false-negative rate and are influenced by the infecting organism. J Bone Joint Surg Am 2018; 100(23): 2057-65. doi: 10.2106/JBJS.17.01429.
- Staats K, Kolbitsch P, Sigmund I K, Hobusch G M, Holinka J, Windhager R. Outcome of total hip and total knee revision arthroplasty with minor infection criteria: a retrospective matched-pair analysis. J Arthroplasty 2017; 32(4): 1266-71. doi: 10.1016/j.arth.2016.11.016.
- Milandt N R, Gundtoft P H, Overgaard S. A single positive tissue culture increases the risk of rerevision of clinically aseptic THA: a national register study. Clin Orthop Relat Res 2019; 477: 1372-81. doi: 10.1097/CORR.0000000000000609.
- Vargas-Reveron C, Soriano A, Fernandez-Valencia J A, Martinez-Pastor J C, Morata L, Munoz-Mahamud E. Prevalence and impact of positive intraoperative cultures in partial hip or knee revision. J Arthroplasty 2020; 35: 1912-16. doi: 10.1016/j.arth.2020.02.025.
- McNally M, Sousa R, Wouthuyzen-Bakker M, Chen A F, Soriano A, Vogely H C, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021; 103-B: 18-25. doi: 10.1302/0301-620X.103B1.BJJ-2020-1381.R1.
- Sigmund I K, Luger M, Windhager R, McNally M A. Diagnosing periprosthetic joint infections: a comparison of infection definitions: EBJIS 2021, ICM 2018, and IDSA 2013. Bone Joint Res 2022; 11: 608-18. doi: 10.1302/2046-3758.119.BJR-2022-0078.R1.
- Guan H, Fu J, Li X, Chai W, Hao L, Li R, et al. The 2018 new definition of periprosthetic joint infection improves the diagnostic efficiency in the Chinese population. J Orthop Surg Res 2019; 14: 151. doi: 10.1186/s13018-019-1185-y.
- Saleh A, Guirguis A, Klika A K, Johnson L, Higuera C A, Barsoum W K. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty 2014; 29: 2181-6. doi: 10.1016/j.arth.2014.07.010.
Appendix
| MSIS 2013 | ||||
| Major criteria | 2 positive periprosthetic cultures with phenotypically identical organisms, OR a sinus tract communicating with the joint | |||
| Minor criteria | (1) Elevated serum C-reactive protein (CRP) AND erythrocyte sedimentation rate (ESR) | |||
| (2) Elevated synovial fluid white blood cell (WBC) count OR ++ change on leukocyte esterase test strip | ||||
| (3) Elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%) | ||||
| (4) Positive histological analysis of periprosthetic tissue | ||||
| (5) A single positive culture | ||||
| PJI is present when 1 of the major criteria exists or 3 out of 5 minor criteria exist. Thresholds for the minor diagnostic criteria for chronic PJI (> 90 days) are: ESR > 30 mm/h; CRP > 10 mg/L; synovial fluid WBC > 3,000 cells/μL; PMN > 80%; histological analysis > 5 neutrophils per high power field in 5 high power fields (×400). | ||||
| IDSA | 1. The presence of a sinus tract that communicates with the prosthesis is definitive evidence of PJI. | |||
| 2. The presence of acute inflammation as seen on histopathologic examination of the periprosthetic tissue at the time of surgical debridement or prosthesis removal as defined by the attending pathologist is highly suggestive evidence of PJI. | ||||
| 3. The presence of purulence without another known etiology surrounding the prosthesis is definitive evidence of PJI. | ||||
| 4. 2 or more intraoperative cultures or combination of preoperative aspiration and intraoperative cultures that yield the same organism (indistinguishable based on common laboratory tests including genus and species identification or common antibiogram) may be considered definitive evidence of PJI. | ||||
| 5. Growth of a virulent microorganism (e.g., S. aureus) in a single specimen of a tissue biopsy or synovial fluid may also represent PJI. One of multiple tissue cultures or a single aspiration culture that yields an organism that is a common contaminant (e.g., coagulase-negative staphylococci, Propionibacterium acnes) should not necessarily be considered evidence of definite PJI and should be evaluated in the context of other available evidence. a | ||||
| a Proportion of PMN > 65% neutrophils or synovial fluid WBC >1,700 cells/μL for total knee arthroplasty or 4,200 cells/μL for total hip arthroplasty as suggested in the original paper were used to define infection when a single positive culture with a common contaminant was present. | ||||
| ICM 2018 | ||||
| Major criteria | 2 positive growths of the same organism using standard culture methods | Infected | ||
| Sinus tract with evidence of communication to the joint or visualization of the prosthesis | ||||
| Minor criteria | Serum CRP > 10 mg/L or d-dimer > 860 μg/L | 2 points | ||
| Elevated serum ESR > 30 mm/h | 1 point | |||
| Elevated synovial WBC > 3,000 cells/μL OR leukocyte esterase ++ OR positive alpha-defensin (signal/cutoff) | 3 points | |||
| Elevated synovial PMN > 70% | 2 points | |||
| Single positive culture | 2 points | |||
| Positive histology | 3 points | |||
| Positive intraoperative purulence (no role in suspected adverse local tissue reaction) | 3 points | |||
| Combined preoperative and postoperative score: ≥ 6 infected, 3-5 inconclusive, and < 3 not infected | ||||
| EBJIS | Infection unlikely ((all findings negative)) | Infection likely (2 positive findings) a | Infection confirmed (any positive finding) | |
| Clinical and blood workup | ||||
| Clinical features | Clear alternative reason for implant dysfunction (e.g., fracture, implant breakage, malposition, tumor) | (1) Radiological signs of loosening within the first 5 years after implantation (2) Previous wound healing problems (3) History of recent fever or bacteremia (4) Purulence around the prosthesis b |
Sinus tract with evidence of communication to the joint or visualization of the prosthesis | |
| C-reactive protein | > 10 mg/L c | |||
| Synovial fluid cytological analysis d | ||||
| Leukocyte count c (cells/μL) | ≤ 1,500 | > 1,500 | > 3,000 | |
| PMN (%) c | ≤ 65% | > 65% | > 80% | |
| Synovial fluid biomarkers | ||||
| Alpha-defensin e | Positive immunoassay or lateral-flow assay e | |||
| Microbiology f | ||||
| Aspiration fluid | Positive culture | |||
| Intraoperative (fluid and tissue) | All cultures negative | Single positive culture g | ≥ 2 positive samples with the same microorganism | |
| Sonication h (CFU/mL) | No growth | > 1 CFU/mL of any organism g | > 50 CFU/mL of any organism | |
| Histology c,i | ||||
| High-power field (400x) | Negative | Presence of ≥ 5 neutrophils in a single HPF | Presence of ≥ 5 neutrophils in ≥ 5 HPF. Presence of visible microorganisms | |
| Other | ||||
| Nuclear imaging | Negative 3-phase isotope bone scan c | Positive WBC scintigraphy j | ||
| a Infection is only likely if there is a positive clinical feature or raised serum C-reactive protein (CRP), together with another positive test (synovial fluid, microbiology, histology, or nuclear imaging). b Except in adverse local tissue reaction (ALTR) and crystal arthropathy cases. c Should be interpreted with caution when other possible causes of inflammation are present: gout or other crystal arthropathy, metallosis, active inflammatory joint disease (e.g., rheumatoid arthritis), periprosthetic fracture, or the early postoperative period. d These values are valid for hips and knee periprosthetic joint infection (PJI). Parameters are only valid when clear fluid is obtained and no lavage has been performed. Volume for the analysis should be > 250 μL, ideally 1 mL, collected in an EDTA containing tube and analyzed in < 1 hour, preferentially using automated techniques. For viscous samples, pre-treatment with hyaluronidase improves the accuracy of optical or automated techniques. In case of bloody samples, the adjusted synovial WBC = synovial WBC observed – [WBC blood / RBC blood x RBC synovial fluid] should be used. e Not valid in cases of ALTR, hematomas, or acute inflammatory arthritis or gout. f If antibiotic treatment has been given (not simple prophylaxis), the results of microbiological analysis may be compromised. In these cases, molecular techniques may have a place. Results of culture may be obtained from preoperative synovial aspiration, preoperative synovial biopsies, or (preferred) from intraoperative tissue samples. g Interpretation of a single positive culture (or < 50 UFC/mL in sonication fluid) must be cautious and taken together with other evidence. If a preoperative aspiration identified the same microorganism, they should be considered as two positive confirmatory samples. Uncommon contaminants or virulent organisms (e.g., Staphylococcus aureus or gram-negative rods) are more likely to represent infection than common contaminants (such as coagulase-negative staphylococci, micrococci, or Cutibacterium acnes). h If centrifugation is applied, then the suggested cut-off is 200 CFU/mL to confirm infection. If other variations to the protocol are used, the published cut-offs for each protocol must be applied. i Histological analysis may be from preoperative biopsy, or intraoperative tissue samples with either paraffin or frozen section preparation. j WBC scintigraphy is regarded as positive if the uptake is increased at the 20-hour scan, compared with the earlier scans (especially when combined with complementary bone marrow scan). |
||||
| EBJIS definitive | EBJIS preoperative | |
| Unlikely | Likely | |
| Likely | 8 Single positive culture (8) AND elevated CRP (3) Early loosening (5) |
– |
| Confirmed | 12 Positive histology (8) ≥ 2 identical cultures (4) |
8 Positive histology (2) ≥ 2 identical cultures (6) |
| Type of microorganisms isolated a | ||
| Coagulase-negative staphylococci | 15 | 4 |
| Streptococcus spp. | 2 | 1 |
| Corynebacterium spp. | 1 | – |
| Anaerobes | – | 2 |
| Candida spp. | – | 1 |
| a Some cases offered > 1 positive sample but grew different species of microorganisms thus not fulfilling criteria to be called confirmed infections and were therefore categorized as single positive cultures. | ||