Performance of thin Vivacit-E hip liners: no relevant wear during ex vivo testing at high acetabular inclination angle
Vesa SAIKKO 1, Perttu NEUVONEN 2, and Aleksi REITO 2
1 Aalto University, Espoo; 2 Coxa Hospital for Joint Replacement, Tampere, Finland
Background and purpose — There is concern among clinicians regarding the performance of thin, highly cross-linked polyethylene acetabular liners at high inclination angles that cause edge contact and high contact stresses. We studied ex vivo wear performance of thin, vitamin-E grafted, highly cross-linked polyethylene (Vivacit-E) liners in relation to high acetabular inclination angle.
Materials and methods — Wear of Vivacit-E acetabular liners (thickness 4.0–5.7 mm) was studied with a validated hip joint simulator at 2 different acetabular inclination angles, 40° (optimal) and 65° (high). The test simulated walking. Wear was evaluated gravimetrically and dimensionally.
Results — At the optimal inclination angle, slight weight gain occurred. At the high angle, the liners lost weight slightly. Due to the minimal weight loss, gravimetric wear rates were difficult to determine. Linear wear was below the detection limit of 0.01 mm.
Conclusion — Even with the high acetabular inclination angle, the prostheses performed well in the present test conditions. The wear rates of the liners were estimated to be clearly below the osteolysis threshold of 0.05 mm/year, below which osteolysis is absent. Since the present hip joint simulator has been shown to produce clinically relevant wear, these ex vivo results are likely to reflect the in vivo behavior of the design.
Citation: Acta Orthopaedica 2022; 93: 901–905. DOI https://doi.org/10.2340/17453674.2022.5359.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-08-22. Accepted: 2022-11-19. Published: 2022-12-02.
Correspondence: vesa.saikko@aalto.fi
VS conducted the study, PN participated in the study design, selected the specimens, and commented on the manuscript, and AR commented on the manuscript.
Handling co-editor: Bart Swierstra
Acta thanks Paul Bills and Dennis Janssen for help with peer review of this study.
In total hip arthroplasty with polyethylene (PE) acetabular liners, a trend in the past 2 decades has been to increase the most common femoral head diameter from 28 mm to 32 or 36 mm to reduce the risk of dislocation (1). Hence, the liner has become thinner, which has been considered acceptable with highly cross-linked polyethylene (XLPE) materials with superior wear resistance (2-4). However, the mechanical properties of XLPE materials are not superior to those of conventional ultra-high molecular weight polyethylene (UHMWPE) (5). Therefore, there is concern among clinicians regarding the performance of thin liners at high acetabular inclination angles with edge contact and high contact stresses. In the past 2 decades, XLPE has replaced UHMWPE as the most used bearing material in the developed countries (according to Orthoplastics, Bacup, UK). Cross-linking is achieved by high doses of gamma or electron beam irradiation, which results in the formation of free radicals. These must be eliminated to avoid oxidation, a reaction that is harmful for mechanical properties. Previously, the elimination was done by various heat treatments, which may also compromise mechanical properties. A trend in the past decade has been to add antioxidant, vitamin E to permanently stabilize XLPE against oxidation (6-9). We studied whether the performance of thin, vitamin-E grafted, highly cross-linked polyethylene (VEXLPE) liners in a modern total hip prosthesis deteriorates significantly when the acetabular inclination angle is increased from optimal to high, and the wear rates with the optimal and high acetabular inclination angles.
Materials and methods
The acetabular components were from the G7 acetabular system and the femoral heads from the VerSys hip system (Zimmer Biomet, Warsaw, IN, USA). According to the manufacturer, the liner was made of vitamin-E blended (grafted), highly cross-linked polyethylene, Vivacit-E, and the cross-linking was achieved by warm electron beam irradiation (100 kGy, with no post-irradiation heat treatment that could compromise mechanical properties). The neutral liner was fully (hemispherically) supported by an acetabular shell made of Ti-6Al-4V (52 mm outer diameter, the most used size in Coxa Hospital for Joint Replacement), i.e., there was no protruding rim (Figure 1). The size E liner was chosen because it enables the use of a 36 mm diameter femoral head, which is clinically the most suitable. The femoral head used was made of CoCr (Zimaloy). Caliper measurements showed that the thickness of the liner at the rim, 65° direction, 40° direction, and apex was 4.0 mm, 5.0 mm, 5.4 mm, and 5.7 mm, respectively. The thickness varies because the inner and outer spherical surfaces of the liner are not concentric. For unobstructed removal of the liner from the shell for periodic, gravimetric wear measurements, the locking lip of the liner was removed. This did not change the position of the liner within the shell, nor did it lead to a possibility of micromotion, due to the dentated shape (Figure 1). The tests included 2 different acetabular inclination (abduction) angles, 40° (optimal) and 65° (high). The high angle value of 65° was chosen to maintain some margin, presumably of a few degrees, until dislocation, which was to be avoided in order to be able to safely perform a 6-week wear test (10,11). The test was intended to be a pure wear test, not a biomechanical stability test. With both angles, 3 bearings (liner + shell + femoral head) were tested simultaneously. In addition, there was 1 loaded, stationary soak control bearing for both angles for the estimation of fluid absorption. 8 similar liners, shells, and femoral heads were tested.
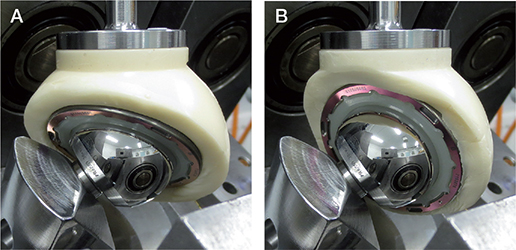
Figure 1. Prostheses installed in HUT-4 hip joint simulator. Acetabular inclination angle, implemented with bone cement, was (A) 40° and (B) 65°. Loading is vertical from above. Femoral head holder had a 12/14 taper similar to that of femoral stem.
The wear test was done with the established 12-station HUT-4 hip joint simulator described elsewhere (10) (Figure 2). The HUT-4 simulator has been shown to produce wear similar to that observed clinically and to that produced under ISO 14242-1 conditions (10-13). The simulator not only reproduced the relevant submicron size distribution of the UHMWPE wear particles (10), but, in former studies, it also correctly reproduced the disastrous wear of large-diameter metal-on-metal prostheses at high acetabular inclination angles (11). Briefly, the motion consisted of sinusoidal flexion–extension (range 46°) and abduction–adduction (range 12°) (Figure 3). There was a phase difference of π/2 to produce the multidirectional relative motion that is essential to the clinically relevant burnishing (14,15). The double-peak load had a maximum of 2.5 kN and a minimum of 0.3 kN. The test frequency was 1.06 Hz. The test was done at a room temperature of 24°C. The lubricant was alpha calf serum (HyClone SH30212, Cytiva, HyClone Laboratories, Logan, UT, USA), diluted 1:1 with ultrapure deionized water. The protein concentration of the lubricant was 20 mg/mL (10). Antibiotic/antimycotic solution (HyClone SV30079) was added to the lubricant (10 ml/L) to suppress microbial growth. The volume of the lubricant in each acrylic lubricant chamber was 450 mL. In the stationary, loaded soak control stations, the lubricant was mixed by a peristaltic pump to prevent sedimentation of proteins.

Figure 2. Test running in HUT-4 hip joint simulator. Note 6 wear test stations and 2 loaded soak control stations, 1 for each acetabular inclination angle.
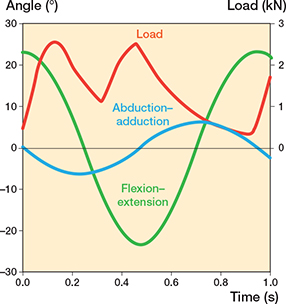
Figure 3. Measured motions and load of HUT-4 hip joint simulator used in present tests.
The test length was 3 million cycles, which may be considered to represent 3 years in vivo. The test was stopped every 500,000 cycles (6 days) for a gravimetric wear measurement of the liners (10). After cleaning, the liners were vacuum desiccated for 30 minutes. The desiccation time of 30 minutes is specified in the ISO 14242-2 standard (16) and has been widely used in orthopedic tribology for decades (17). Before weighing, the weight of the liners was allowed to stabilize overnight in the laboratory atmosphere. The resolution of the balance was 0.01 mg. Fresh serum was used at every restart. For an accurate femoral head alignment, each head was always in the same test station. The liners were circulated among the test stations so that at every restart, the liner was against a different femoral head, i.e., it was against each of the 6 femoral heads for 500,000 cycles. This was done to increase the likelihood that a possible outlier liner truly was an outlier so that the possible deviating behavior was not caused by 1 of the femoral heads or test stations.
An additional, dimensional method of wear measurement was applied as follows. After the test, the thickness change of the liners was measured at the point of theoretical load application in the direction of the load vector using a digital caliper with a resolution of 1 μm. Linear wear was the mean thickness reduction of the wear test liners minus the thickness reduction of the loaded soaked control liner. The detection limit of this dimensional method was estimated to be 0.01 mm, based on numerous repeated measurements, between which the liner was removed from the shell and reinstalled, as in the weighing procedure. The graphic method of Saikko (18) was used to estimate the burnished contact area. The measured mean diameter of the femoral heads was 35.9 mm and the measured mean radius of the bearing surface of the liners was 18.1 mm. Hence, the mean diametral clearance of the joints at the start of the test was 0.3 mm. In the measurement of the liner radius, the method presented in Saikko (19) was used.
Ethics, data sharing, funding, and disclosures
The study did not involve human subjects. Therefore, ethical approval by an ethical review board was not needed. Data produced in the present study is available by request from VS. The study was funded by Aalto University. The authors declare that they have no conflicts of interest related to the present study.
Results
The wear test was uneventful. No liner or femoral head damage occurred. Due to the minimal weight changes, gravimetric wear rates were difficult to determine (Table). The weight changes of the liners were between –2 mg and 2 mg (Figure 4). Nevertheless, a difference in the weight change behavior could be observed between the optimal and high inclination angles. The difference was that at 40° slight weight gain occurred in all liners, whereas at 65° all liners lost weight slightly, including the control liner. The thickness changes caused by wear were below the detection limit of 0.01 mm. The burnished contact area of the 65° liners was on average 35% smaller than that of the 40° liners (Figure 5). The lubricant bulk temperature with the 65° liners was 1.3°C lower on the average than that with the 40° liners, which indicated lower frictional heating at 65°. Microscopy showed mild crisscross scratches on the burnished contact surface and a distinct border between the burnished surface and the original surface with machining marks (Figures 6 and 7). Backside wear of the liners did not occur.
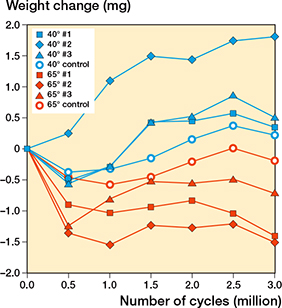
Figure 4. Variation of liner weight with number of cycles. Note that, at the end of the test, weight change was slightly positive in all 40° liners, and slightly negative in all 65° liners, including control the liner.
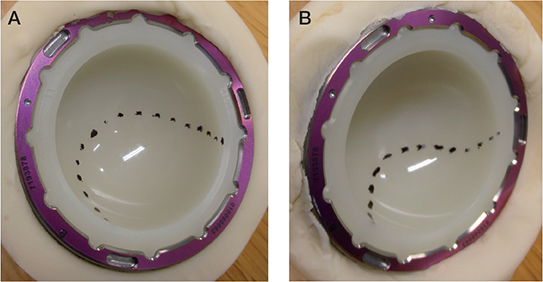
Figure 5. Delineation of liner contact surface. Note burnishing. Acetabular inclination angle was (A) 40° and (B) 65°.

Figure 6. Optical micrograph from liner contact surface. Note mild crisscross scratches caused by multidirectional motion. Acetabular inclination angle was 40°.

Figure 7. Optical micrograph from border of liner contact surface. Note original machining marks in addition to crisscross scratches. Acetabular inclination angle was 40°.
Discussion
We studied wear of thin Vivacit-E liners in G7 total hip prostheses with a validated hip joint simulator at optimal and high inclination angles. There are no prior published, independent wear studies on Vivacit-E. With both angles, the weight changes of the liners were very low, within the range ±2 mg, which indicated not only good wear resistance of Vivacit-E but also good tribological characteristics of the G7 design. For comparison, the average weight of the liners was 12.4 g. Clinically relevant burnishing (14) occurred.
The fluid absorption behavior of the liners explains why the progress of the weight changes was not quite typical (Figure 4). At 40°, the wear test liners gained more weight than the loaded soak control liner. After the test, the 40° wear test liners were heavier than at the start despite periodic vacuum desiccation. In the swing phase with low load, the relative motion may have dragged lubricant into the contact area. After the heel strike, lubricant was mostly squeezed out from the contact area, but a trace amount was squeezed into the liner. Therefore, absorption was facilitated compared with the stationary soak control prostheses. It should be noted that 30 minutes in a vacuum probably dries the liner to a certain depth only, but not completely. Moreover, it is possible that not all absorbed substances, such as lipids, vaporize in a vacuum. At 65°, the weight change behavior was less anomalous. The difference in the weight change behavior between 40° and 65° was likely to be attributable to the 35% difference in the contact area (Table). The contact area was smaller and thus the contact stresses were likely to be higher at 65°. Lubricant absorption may be beneficial for lubrication.
A so-called osteolysis threshold wear rate of 0.05 mm/year has been determined for UHMWPE. Below this limit osteolysis hardly ever occurs (20). As the linear wear of the present liners after 3 million cycles was below 0.01 mm, they appear unlikely to cause any osteolysis. The unproblematic behavior of the Vivacit-E liners even with the high inclination angle was apparently related not only to the good wear resistance but also to the fact that the liners were fully supported by the acetabular shell (Figure 1). The reported cases of thin XLPE liner fractures are for designs that have an unsupported, protruding rim that is ripped off (21). The phenomenon is likely to be exacerbated by a high inclination angle, a shallow acetabular shell, heat treatments, and oxidation.
Usually, extensive cross-linking of orthopedic bearing components is achieved by high doses of gamma irradiation (22). In Vivacit-E, as in its predecessor from which it was developed, Durasul XLPE with post-irradiation heat treatment (Zimmer Biomet, Warsaw, IN, USA), extensive cross-linking is achieved by electron beam irradiation instead. Established facts are that for gamma-irradiated XLPEs and VEXLPEs, measurable wear is always produced against a polished counterface with multidirectional motion and serum lubrication (13,18,23), but not for electron beam irradiated Durasul, which always shows net weight gain (24,25). Nevertheless, the mentioned measurable wear is statistically significantly lower than that of conventional UHMWPE (23,26). For example, the mean wear rate of thin, 54 mm inner diameter, 100 kGy gamma-irradiated VEXLPE liners in test conditions similar to those of the present study was 8.5 mg/1 million cycles at an acetabular inclination angle of 45° and 12.9 mg/1 million cycles at 65°, and the total weight gain of the loaded soak control liner was 2.2 mg (18). In the ISO14242-1 conditions, the mean wear rate of these liners was 7.5 (SD 1.0) mg/1 million cycles at 30°, and the total weight gain of the loaded soak control liner was 5.2 mg (13). Novel insights are that Vivacit-E seems to be similar to Durasul in its wear behavior, which is likely to be due to a superior cross-link density achieved by electron beam irradiation (Dr Werner Schneider, Zimmer Biomet, personal communication). Clinically, Durasul has shown good wear resistance (27,28). The penetration of the femoral head into the Durasul liner was measured to be 0.02 mm/year (27) and 0.03 mm/year (28). Note that penetration is not the same thing as wear because of the unknown proportion of creep. Clinical wear rates for Vivacit-E liners are not yet available.
The test length of 3 million cycles may be considered a limitation but it is sufficient to reliably observe the wear trends, because PEs show very linear wear. Later change in the wear behavior is prevented by the presence of vitamin E, which eliminates oxidation. A hip joint simulator test of 100 million cycles has been done for Vivacit-E liners in the manufacturer’s laboratory, and no change of the very low wear rate, 1.8 (SD 0.2) mg/1 million cycles against 40 mm diameter CoCr heads, occurred during the test (29). Dimensions of the liner and inclination angle were not reported. The tests were done with a hip joint simulator in which the direction of loading was fixed relative to the femoral head (29). In the present simulator, the direction of loading was fixed relative to the liner. The ISO 14242-1 standard covers both methods (12), but they may result in slightly different wear. The principal feature for the reproduction of clinically relevant wear mechanisms is the multidirectional motion, together with protein-based lubrication (10-16). All UHMWPE materials are known for ductility and so their wear is typically highly linear (10,13,18,23-25,29). Very low wear is an exception, because the random variation of the gravimetric method then substantially affects the computed value of the wear rate (23-25). A sample size of 3 is another limitation, which is mainly a cost issue, but the small difference in the weight change behavior between the optimal and high inclination angles could still be shown (Figure 4).
In conclusion, wear tests carried out with a validated hip joint simulator indicated that the thin Vivacit-E liner, extensively cross-linked by warm electron beam irradiation and fully supported by the G7 acetabular shell, had low wear even with a high acetabular inclination angle. The gravimetric wear rate was too low to be reliably determined. The linear wear was clearly below the osteolysis threshold of 0.05 mm/year, below which osteolysis is not observed.
- Goel A, Lau E C, Ong K L, Berry D J, Malkani A L. Dislocation rates following primary total hip arthroplasty have plateaued in the Medicare population. J Arthroplasty 2015; 30(5): 743-6. doi: 10.1016/j.arth.2014.11.012.
- Kuzyk P R T, Saccone M, Sprague S, Simunovic N, Bhandari M, Schemitsch E H. Cross-linked versus conventional polyethylene for total hip replacement: a meta-analysis of randomised controlled trials. J Bone Joint Surg B 2011; 93(5): 593-600. doi: 10.1302/0301-620x.93b5.25908.
- Devane P A, Horne J G, Ashmore A, Mutimer J, Kim W, Stanley J. Highly cross-linked polyethylene reduces wear and revision rates in total hip arthroplasty: a 10-year double-blinded randomized controlled trial. J Bone Joint Surg A 2017; 99(20): 1703-14. doi: 10.2106/JBJS.16.00878.
- de Steiger R, Lorimer M, Graves S E. Cross-linked polyethylene for total hip arthroplasty markedly reduces revision surgery at 16 years. J Bone Joint Surg Am 2018; 100(15): 1281-8. doi: 10.2106/JBJS.17.01221.
- Bracco P, Oral E. Vitamin E-stabilized UHMWPE for total joint implants: a review. Clin Orthop Relat Res 2011; 469(8): 2286-93. doi: 10.1007/s11999-010-1717-6.
- Tsikandylakis G, Mortensen K R, Gromov K, Mohaddes M, Malchau H, Troelsen A. Does the use of the largest possible metal head increase the wear of vitamin E-doped cross-linked polyethylene? Two-year results from a randomized controlled trial. Bone Joint J 2021; 103-B(7): 1206-14. doi: 10.1302/0301-620X.103B7.BJJ-2020-2064.R1.
- Scemama C, Anract P, Dumaine V, Babinet A, Courpied J P, Hamadouche M. Does vitamin E-blended polyethylene reduce wear in primary total hip arthroplasty: a blinded randomised clinical trial. Int Orthop 2017; 41(6): 1113-18. doi: 10.1007/s00264-016-3320-2.
- Hemmilä M, Laaksonen I, Matilainen M, Eskelinen A, Haapakoski J, Puhto A-P, et al. Implant survival of 2,723 vitamin E-infused highly crosslinked polyethylene liners in total hip arthroplasty: data from the Finnish Arthroplasty Register. Acta Orthop 2021; 92(3): 316-22. doi: 10.1080/17453674.2021.1879513.
- Kjærgaard K, Varnum C, Ding M, Overgaard S. Revision risk of total hip arthroplasty with vitamin E doped liners: results from the Danish Hip Arthroplasty Register. J Arthroplasty 2022; 37(6): 1136-42. doi: 10.1016/j.arth.2022.02.007.
- Saikko V. A 12-station anatomic hip joint simulator. Proc Inst Mech Eng H 2005; 219(6): 437-48. doi: 10.1243/095441105x34419.
- Saikko V. Effect of wear, acetabular cup inclination angle, load and serum degradation on the friction of a large diameter metal-on-metal hip prosthesis. Clin Biomech 2019; 63: 1-9. doi: 10.1016/j.clinbiomech.2019.02.005.
- ISO 14242-1:2014/Amd 1:2018 Implants for surgery — wear of total hip-joint prostheses — Part 1: Loading and displacement parameters for wear-testing machines and corresponding environmental conditions for test. Geneva: International Organization for Standardization.
- Saikko V, Morad O, Viitala R. Modification of a simplified hip joint simulator into an ISO 14242-1 compliant design and a comparison of wear test results. J Tribol 2022; 144(5): 051703. doi: 10.1115/1.4052942.
- McKellop H A, Campbell P, Park S-H, Schmalzried T P, Grigoris P, Amstutz H C, et al. The origin of submicron polyethylene wear debris in total hip arthroplasty. Clin Orthop Relat Res 1995; 311: 3-20.
- Calonius O, Saikko V. Force track analysis of contemporary hip simulators. J Biomech 2003; 36(11): 1719-26. doi: 10.1016/S0021-9290(03)00166-0.
- ISO 14242-2:2016 Implants for surgery — wear of total hip-joint prostheses — Part 2: Methods of measurement. Geneva: International Organization for Standardization.
- Clarke I C, Starkebaum W, Hosseinian A, McGuire P, Okuda R, Salovey R, et al. Fluid-sorption phenomena in sterilized polyethylene acetabular prostheses. Biomaterials 1985; 6(3): 184-8. doi: 10.1016/0142-9612(85)90007-9.
- Saikko V. Wear and friction of thin, large-diameter acetabular liners made from highly cross-linked, vitamin-E-stabilized UHMWPE against CoCr femoral heads. Wear 2019; 432-3: 202948. doi: 10.1016/j.wear.2019.202948.
- Saikko V. A simulator study of friction in total replacement hip joints. Proc Inst Mech Eng H 1992; 206(4): 201-11. doi: 10.1243/PIME_PROC_1992_206_292_02.
- Dumbleton J H, Manley M T, Edidin A A. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 2002; 17(5): 649-61. doi: 10.1054/arth.2002.33664.
- Ast M P, John T K, Labbisiere A, Robador N, Gonzales Della Valle A. Fractures of a single design of highly cross-linked polyethylene acetabular liners: an analysis of voluntary reports to the United States Food and Drug Administration. J Arthroplasty 2014; 29(6): 1231-5. doi: 10.1016/j.arth.2013.12.022
- Kurtz S M, editor. UHMWPE biomaterials handbook. 3rd ed. Amsterdam: Elsevier; 2015.
- Saikko V. Performance analysis of an orthopaedic biomaterial 100-station wear test system. Proc Inst Mech Eng C 2010; 224(3): 697-701. doi: 10.1243/09544062JMES1626.
- Saikko V, Calonius O, Keränen J. Effect of counterface roughness on the wear of conventional and crosslinked ultrahigh molecular weight polyethylene studied with a multidirectional motion pin-on-disk device. J Biomed Mater Res 2001; 57(4): 506-12. doi: 10.1002/1097-4636(20011215)57:4<506::aid-jbm1196>3.0.co;2-h.
- Saikko V, Calonius O, Keränen J. Wear of conventional and cross-linked ultra-high-molecular-weight polyethylene acetabular cups against polished and roughened CoCr femoral heads in a biaxial hip simulator. J Biomed Mater Res 2002; 63(6): 848-53. doi: 10.1002/jbm.10471.
- Saikko V, Morad O, Viitala R. Effect of temperature on UHMWPE and VEXLPE friction and wear against CoCr in noncyclic tests. Wear 2022; 490-1: 204190. doi: 10.1016/j.wear.2021.204190.
- Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A. New polyethylenes in total hip replacement: a ten- to 12-year follow-up study. Bone Joint J 2013; 95-B(3): 326-32. doi: 10.1302/0301-620x.95b3.29456.
- Georis P, Thirion T, Gillet P. Clinical and radiological results with a 36-mm cobalt-chrome prosthetic head, cross-linked Durasul liners associated with Allofit cups: a more than 10-year follow-up period. Hip Int 2020; 30(4): 446-51. doi: 10.1177/1120700019869829.
- Popoola O O, Orozco Villasenor D A, Fryman J C, Mimnaugh K, Rufner A. High cycle in vitro hip wear of and in vivo biological response to vitamin E blended highly crosslinked polyethylene. Biotribology 2018; 16: 10-16. doi: 10.1016/j.biotri.2018.09.001.