Subtherapeutic levels of cefuroxime inside a cannulated pedicle screw used in spine surgery: results from a porcine microdialysis study
Magnus A HVISTENDAHL 1,2, Pelle HANBERG 1,2, Maiken STILLING 1–3, Alexander Emil KASPERSEN 1, Kristian HØY 1,3, and Mats BUE 1–3
1 Department of Clinical Medicine, Aarhus University, Aarhus N; 2 Aarhus Denmark Microdialysis Research (ADMIRE), Orthopedic Research Laboratory, Aarhus University Hospital, Aarhus N; 3 Department of Orthopedic Surgery, Aarhus University Hospital, Aarhus N, Denmark
Background and purpose — Minimally invasive spine surgery has continuously evolved for specific surgical procedures and patient populations to lower morbidity and the risk of postoperative bacterial infection. Perioperative antibiotic prophylaxis is an important preventive measure and local tissue concentrations can be quantified with microdialysis. Insertion of spinal implants induces tissue trauma and inflammation, which may affect antibiotic proximate implant concentrations. We compared perioperative cefuroxime concentrations inside a cannulated pedicle screw used in minimally invasive spine surgery with the opposite non-instrumented vertebral pedicle.
Materials and methods — Microdialysis catheters were placed inside a cannulated pedicle screw and in the opposite non-instrumented vertebral pedicle of the same vertebra (L1) in 8 female pigs through a posterior lumbar surgical approach. Following a single-dose intravenous cefuroxime administration (1.5 g), dialysates and plasma were dynamically sampled over 8 hours. The primary endpoint was time above the cefuroxime clinical breakpoint minimal inhibitory concentration for Staphylococcus aureus of 4 µg/mL (T>MIC4).
Results — Median T>MIC4 was 0 h (range 0–0) inside the cannulated pedicle screw, 1.6 h (range 1.1–2.4) in non-instrumented vertebral pedicle, and 1.9 h (range 1.9–2.9) in plasma.
Conclusion — A single-dose intravenous cefuroxime administration provided low and subtherapeutic concentrations for prevention of infection inside a cannulated pedicle screw in the lumbar spine. Therapeutic concentrations were achieved in the opposite non-instrumented vertebral pedicle up to 1.5–2 h. Therefore, additional prophylactic strategies may be considered in cannulated instrumented spine surgery, especially in high-risk patients. Alternative dosing regimens seem relevant in lumbar spine surgery lasting longer than 1.5 h.
Citation: Acta Orthopaedica 2022; 93: 874–879. DOI http://dx.doi.org/10.2340/17453674.2022.5276.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-06-20. Accepted: 2022-11-02. Published: 2022-11-28.
Correspondence: maghvi@clin.au.dk
MAH, PH, MS, AEK, KH, and MB designed the study. All authors planned the study. KH performed all surgical procedures. MAH and PH placed all microdialysis catheters. MAH, PH, AEK, and MB contributed to data collection. MAH, PH, MS, KH, and MB performed the data analyses. MAH drafted the manuscript. All authors interpreted the data and results and contributed to the completion of the final manuscript.
The authors would like to thank Department of Clinical Biochemistry and Immunology, Hospital Lillebaelt, Vejle, Denmark for helping with the chemical analyses.
Handling co-editors: Eivind Witsø and Philippe Wagner
Acta thanks Thomas Andersen for help with peer review of this study.
Minimally invasive spine surgery has continuously evolved for specific surgical procedures and patient populations to lower morbidity and postoperative infection rates (1,2). Infection rates following minimally invasive spine surgery are reported to be between 0.1% and 6% (1,2), with a tendency towards higher rates in instrumented surgery (2,3), compared with 0.2–16% in traditional open spine surgery (4). In minimally invasive spine surgery, application of cannulated pedicle screws is essential to ensure correct percutaneous placement. However, the cannulation constitutes a functional internal dead space and introduction of implants enhances the bacterial virulence (5). This increases the risk of acquiring postoperative bacterial bone infection related to spinal implants (implant-associated vertebral osteomyelitis) (6) and therapeutic perioperative antibiotic prophylaxis is therefore central in lowering the risk. Cefuroxime, a second-generation cephalosporin with few side effects, is widely used for perioperative antibiotic prophylaxis in spine surgery, as its antimicrobial spectrum covers the most frequent bacteria causing infection after spine surgery (7,8). The pharmacokinetic tool, microdialysis, allows for dynamic real-time perioperative sampling of the unbound fraction of cefuroxime and has the potential to estimate perioperative proximate implant concentrations (9,10). We compared the perioperative concentrations of cefuroxime inside a cannulated pedicle screw with the opposite non-instrumented vertebral pedicle using microdialysis, following a single-dose intravenous cefuroxime administration of 1.5 g. We hypothesized that introduction of a commonly applied cannulated pedicle screw induces subtherapeutic perioperative proximate internal implant concentrations.
Materials and methods
The study was carried out at the Department of Clinical Medicine, Aarhus University, Aarhus, Denmark in corporation with the Department of Orthopedic Surgery, Aarhus University Hospital, Aarhus, Denmark. The Department of Clinical Biochemistry and Immunology, Hospital Lillebaelt, Vejle, Denmark performed the chemical analyses.
Overview
8 female pigs (mixed Duroc and Danish Landrace-Yorkshire, weight 74–77 kg, age 5 months) were included in an experimental microdialysis study. The pigs were anesthetized (propofol 30–50 mL/h, fentanyl 15–25 mL/h) and kept physiologically stable, normothermic, and normotensive throughout the study. Following the study period (8 h), all pigs were killed by an overdose of pentobarbital. With reference to reduction, refinement, and replacement (the 3Rs), different data attained from the same pigs were published recently (11).
Study procedures
Surgical procedure and placements of microdialysis catheters
By a posterior approach, with the pigs in prone position, the lumbar vertebral arch, facet joints, spinous and transverse processes were surgically exposed (L1–L5). With fluoroscopic guidance, a sharp pedicle awl, followed by a lumbar ball handle probe, was used to make a hole in the right vertebral pedicle of vertebra L1 (depth 30 mm, diameter 2 mm) in a transpedicular direction penetrating into the vertebral corpus. Next, a thread was made by hand with a selfdrilling tap prior to placement of a cannulated pedicle screw (Medtronic CD Horizon Longitude Multi Axial Screw, length 30 mm, outer diameter 5.5 mm, internal diameter 1.6 mm; Medtronic plc, Minneapolis, MN, USA). A drill hole (diameter 2 mm, depth 30 mm) was made in the opposite vertebral pedicle with a transpedicular approach penetrating into the vertebral corpus of the left vertebral pedicle of vertebra L1. Two microdialysis catheters (membrane length 10 mm) were placed inside the cannulated pedicle screw and the opposite non-instrumented drill hole (vertebral pedicle), respectively (Figure 1). The catheters were fixed to adjacent soft tissue with sutures to prevent dislocation. The skin incision was closed and microdialysis precision pumps were connected to all catheters. A 30 min equilibration period was allowed and individual catheter calibration with an internal standard method was performed (12).
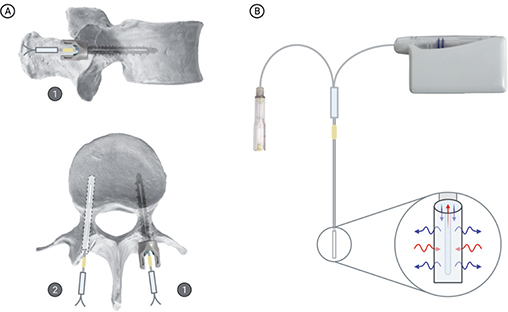
Figure 1. (A) Schematic illustration of the placement of microdialysis catheters (1) inside the cannulation of the cannulated pedicle screw of the right vertebral pedicle and (2) inside a drill hole in the left vertebral pedicle. (B) Overview of the microdialysis method with a precision pump, vial for collection of dialysates, and a catheter with illustration of diffusion across the semipermeable membrane. Created with BioRender.com.
Study medicine and administration
After completed microdialysis equilibration, a single dose of 1.5 g cefuroxime (Fresenius Kabi) was administrated intravenously over 10 min, marking time 0 of the following sampling period.
Sampling of microdialysates and plasma
Dialysates were collected every 30 min in the first 4 hours (0–4 h) and every 60 min in the last 4 hours (4–8 h). Venous blood samples were collected from a central venous catheter placed in the jugular vein at the midpoint of every sampling interval (0–8 h). Blood samples were refrigerated for a maximum of 8 h and thereafter centrifuged (4,000 rpm for 10 min at 5°C). Plasma was subsequently separated with pipettes and stored in Eppendorf tubes in a freezer (–80°C) for later analysis. Dialysates from the microdialysis catheters were collected in microvials and immediately stored in a freezer (–80°C) until analysis. A total sampling period of 8 hours resulted in 12 dialysates from each microdialysis catheter and 12 blood samples from each pig.
Microdialysis
Microdialysis is a pharmacokinetic tool that allows for dynamic real-time sampling of unbound cefuroxime concentrations in the extracellular space (13). Only a fraction of the absolute cefuroxime tissue concentration is reflected in the dialysates upon sampling. This fraction is called relative recovery and calibration methods are needed to estimate absolute cefuroxime tissue concentrations (14). In this study, retrodialysis by drug with the validated internal standard of meropenem (0.9% NaCl holding 5 µg/mL meropenem) was used to determine individual catheter relative recovery based on the meropenem dialysate concentrations (12). Thus, quantification of both meropenem and cefuroxime was performed within the same sample. A more thorough description of the microdialysis technique and calibration procedure can be found elsewhere (13).
Microdialysis equipment from M Dialysis AB (Stockholm, Sweden) was used: 63 Microdialysis Catheters (membrane length 10 mm, molecular weight cutoff 20 kDa) and 107 Microdialysis Precision Pumps (flow rate 1.0 µL/min).
Cefuroxime and meropenem quantification
Cefuroxime and meropenem concentrations in dialysates and free concentrations of cefuroxime in plasma were quantified by ultra-high performance liquid chromatography tandem mass spectrometry (11). For cefuroxime, the intermediate precision for the internal controls were 14.2%, 9.6%, 2.6%, and 3.9% for targets 0.01, 0.05, 5.0, and 10.0 µg/mL, respectively. For meropenem, the intermediate precision of the internal controls was 16.6%, 3.9%, and 5.6% for targets 0.05, 5.0, and 10.0 µg/mL, respectively. The lower limit of quantification for cefuroxime and meropenem were 0.01 µg/mL and 0.05 µg/mL, respectively.
Endpoints
For time-dependent antibiotics like cefuroxime, it is recommended that target-site concentrations as a minimum exceed the minimal inhibitory concentration (MIC) for relevant bacteria (15) throughout the surgical procedure (7,16). Staphylococccus aureus remains the most common etiology in post-operative bacterial infection, and for cefuroxime the clinical breakpoint MIC value for S. aureus is 4 µg/mL (15). The primary endpoint of the study was time above the cefuroxime clinical breakpoint MIC (T>MIC) of 4 µg/mL (T>MIC4), and secondary endpoints were T>MIC for S. aureus with higher susceptibility (1 and 2 µg/mL).
Statistics
All cefuroxime dialysate concentrations were set to the midpoint of their respective sampling interval prior to statistical analysis. Values below the lower limit of quantification were set to zero. Relative recovery and absolute concentrations of cefuroxime were calculated individually for each microdialysis catheter. T>MIC was evaluated by linear interpolation (Microsoft Excel; Microsoft Corp, Redmond, WA, USA) in relation to relevant cefuroxime MIC values (1, 2, and 4 µg/mL) for Staphylococcus aureus (15). The data was subsequently transferred to Stata 17 (StataCorp, College Station, TX, USA), which was used for all subsequent analyses. AUC0-last was estimated by the trapezoid rule. The penetration was defined as the tissue AUC0-last to plasma AUC0-last ratio. Values and corresponding 95% confidence interval (CI) below 100% were defined as incomplete. Cmax was calculated as the maximum concentration of all measured tissue concentrations. Within each compartment, Quantile–Quantile (QQ) plot of the residuals were used to check for normality. Since not all data followed a normal distribution, T>MIC is presented as median with corresponding range for all compartments as well as mean with 95% confidence interval (CI) where appropriate. The differences in T>MIC and the pharmacokinetic parameters between the investigated compartments were clearly demonstrated by median with range and mean with 95% CI, respectively, and thus no further comparative statistics were applied. Thus, T>MIC are presented as median with range and mean with 95% CI in Table 1 while the pharmacokinetic parameters (AUC0-last, Cmax and penetration (AUCtissue/AUCplasma) are presented as mean with 95% CI in Table 2.
| Cannulated pedicle screw (n = 8) | Non-instrumented vertebral pedicle (n = 8) | Plasma (n = 8) | |
| Median T>MIC (range) | |||
| 1 μg/mL | 0 (0–7.4) a | 3.2 (2.7–4.8) | 3.4 (3.3–4.8) |
| 2 μg/mL | 0 (0–4.0) b | 2.4 (1.9–3.4) | 2.7 (2.6–3-9) |
| 4 μg/mL | 0 (0–0) c | 1.6 (1.1–2.4) | 1.9 (1.9–2-9) |
| Mean T>MIC (CI) | |||
| 1 μg/mL | – | 3.5 (2.9–4.2) | 3.7 (3.3–4.1) |
| 2 μg/mL | – | 2.5 (2.1–2.9) | 2.8 (2.5–3.2) |
| 4 μg/mL | – | 1.7 (1.4–2.0) | 2.1 (1.8–2.4) |
| a 2 pigs reached a concentration > 1 μg/mL. | |||
| b 1 pigs reached a concentration > 2 μg/mL. | |||
| c No pig reached a concentration > 4 μg/mL. | |||
Ethics, funding, and disclosures
The study was approved by the Danish Animal Experiments Inspectorate (License no. 2017/15-0201-01184) and conducted in compliance with existing regulations by the European Union Directive for Protection of Animals used for Scientific Purposes (2010/63/EU) as well as the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. This work was supported by internal funding and unrestricted project grants from the Carl and Ellen Hertz’ Grant and the A.P. Møller Foundation. There are no conflicts of interest to disclose.
Results
All pigs completed the study, and all plasma samples and dialysates were successfully collected. Mean relative recovery was 49% (SD 12) and 57% (SD 6) for the cannulated pedicle screw and the opposite non-instrumented vertebral pedicle, respectively. Concentration-time profiles of mean cefuroxime concentrations for all investigated compartments are illustrated in Figure 2.
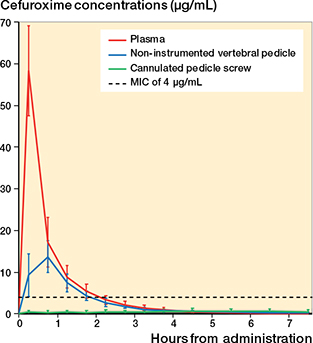
Figure 2. Concentration-time profiles of mean cefuroxime concentrations in plasma, the non-instrumented vertebral pedicle, and the cannulated pedicle screw. Vertical error bars represent 95% confidence intervals. MIC: minimal inhibitory concentration.
T>MIC
T>MIC in hours is presented in Table 1. Median T>MIC4 were 0 h (range 0–0) in the cannulated pedicle screw, 1.6 h (range 1.1–2.4) in the opposite non-instrumented vertebral pedicle, and 1.9 h (range 1.9–2.9) in plasma. For lower MIC values (1–2 µg/mL), no differences were found in T>MIC between plasma and the non-instrumented vertebral pedicle as illustrated by the 95% CI. Inside the cannulated pedicle screw, only 2 and 1 out of 8 pigs presented concentrations above 1 and 2 µg/mL, respectively, resulting in median T>MIC of 0 h (range 0–7.4) for MIC of 1 µg/mL and 0 h (range 0–4.0) for MIC of 2 µg/mL. This was significantly lower compared with both the non-instrumented vertebral pedicle and plasma. In addition to median values, mean estimates of T>MIC are presented where appropriate in Table 1.
Pharmacokinetic parameters
Results for key pharmacokinetic parameters are given in Table 2. The penetration of cefuroxime was incomplete to both the cannulated pedicle screw 8% (CI 0–19) and the non-instrumented vertebral pedicle 48% (CI 39–58).
Discussion
We evaluated perioperative cefuroxime concentrations inside a cannulated pedicle screw used in minimally invasive spine surgery and in the opposite non-instrumented vertebral pedicle of the same lumbar vertebra after a single-dose intravenous administration (1.5 g). The main finding was low and subtherapeutic cefuroxime concentrations within the cannulated pedicle screw, where no pigs reached concentrations above the cefuroxime clinical breakpoint MIC for Staphylococcus aureus of 4 µg/mL, and only 2 out of 8 pigs presented concentrations above 1 µg/mL inside the screw. All pigs reached concentrations above 4 µg/mL in the opposite non-instrumented vertebral pedicle.
When implants are used for bone stabilization, it is important to prevent colonization of infection-inducing bacteria on the implant surface (biofilm formation) to ensure integration and avoid loosening. Integration is a complex process and requires among other things a sufficient immune response by the host and apparent infection-free conditions around the implant. The internal dead space of the cannulated pedicle screw may induce an environment with different physiological diffusion characteristics in comparison with vital tissue, presumably with a low oxygen tension and pH. Such conditions are suitable for bacterial proliferation and biofilm formation (17) and a stagnant internal dead space may not be affected by systemic cefuroxime dosing regimens. Thus, to provide a more therapeutic protection of the internal surface of the screw, application of active and/or passive surface modifications of the screw, local antibiotic delivery systems, or intrawound application of antibiotic powder may be considered to reach high local concentrations (18), especially in high-risk patients. Another theoretical optimization could rely on the choice of pedicle screw. Whereas the commonly employed cannulated pedicle screws in minimally invasive spine surgery may be limited by their access points for cefuroxime entry (screw tip and top), fenestrated pedicle screws hold several additional access points. Accordingly, comparable studies assessing the effect of screw fenestration and the impact of in-screw cement application on the antibiotic proximate implant concentrations are warranted.
Although we used tissue-sparing techniques when placing the pedicle screws, obligate local inflammation and microstructural bone damage will follow screw insertion (19) and may affect the local distribution of cefuroxime to the proximity of the outer surface of the screw. In this context, cefuroxime penetration has previously been found to be negatively affected by the presence of infection and inflammation (10,20). Due to anatomical limitations of the pedicle, we were unable to quantify cefuroxime concentrations in the same vertebral pedicle as the screw. Whether the cefuroxime concentrations measured in the opposite non-instrumented vertebral pedicle of the same lumbar vertebra could serve as a proxy for the outer surface protection of the cannulated pedicle screw needs further investigation. A recent porcine study has demonstrated that the presence of a cannulated screw impaired the penetration of meropenem and vancomycin into cancellous bone (tibial metaphysis) adjacent to the screw (average distance from screw 3 mm) (21). This supports further analytical discussion as regards the extent to which the mechanical stress (following screw insertion) affects the local environment and thus antibiotic protection of the screw. As this subject is of significant interest for several orthopedic subspecialities, future studies are needed to further clarify proximate implant outer surface antibiotic concentrations.
The limited access points for entry may be the primary explanation for the low cefuroxime concentrations inside the screw. While these concentrations were low, therapeutic concentrations (> 4 µg/mL) were reached for 1.5–2 hours in the opposite non-instrumented vertebral pedicle. However, this time span is shorter than the duration of many instrumented minimally invasive spine procedures. Therefore, alternative dosing regimens should be considered in order to comply with the recommended guidelines for perioperative antibiotic prophylaxis. Based on the results of this study and existing literature, an additional cefuroxime bolus injection after 1.5 hours of surgery or continuous infusion seem like suitable alternatives to improve T>MIC (22).
Previous porcine studies have investigated cefuroxime concentrations in non-instrumented cervical and lumbar vertebral cancellous bone (11,22,23). Cefuroxime penetrations were described to be incomplete into both cervical (anterior surgical approach) and lumbar corpus vertebral cancellous bone (posterior surgical approach) with lowest concentrations and shortest T>MIC4 in lumbar vertebral cancellous bone. Specifically, the cefuroxime T>MIC4 was reported to be in the range of 1.3–2.0 h for lumbar vertebral cancellous bone and 2.0–3.0 h for cervical vertebral cancellous bone, which is comparable to 1.2–2.4 h for the non-instrumented vertebral pedicle in the present study. These differences throughout the vertebral column may be reasoned by a heterogenous and anatomicdependent blood supply and effect of the surgical exposure, or a combination of both. These variations should be kept in mind by the operating surgeon.
Although pigs are generally viewed as good experimental study animals due to their human resemblance in terms of anatomy and physiology, interspecies and age-related differences do exist (24). Previous studies on cefuroxime concentrations in bone tissue have demonstrated lower concentrations in pigs compared with humans, possibly due to differences in volume of distribution and turnover of cefuroxime such as faster elimination, which seems to result in longer T>MIC in humans (25). Therefore, the presented experimental results and their translational potential must be interpreted with this in mind. As a practical but important limitation to the study, the surgical exposure was an open posterior lumbar approach, in contrast to a minimally invasive surgical exposure. To ensure accurate and correct placement of the microdialysis catheters preventing structural damage of the catheters, an open posterior lumbar spine exposure was performed. This may affect the overlying soft tissue, potentially reducing blood supply to the lumbar vertebral column (26). However, reduced blood supply to the vertebral pedicle and corpus (anterior column) may be unlikely during posterior surgical exposure or at least the effect of this on proximate antibiotic implant concentrations would be equally detectable in the opposite pedicle. Moreover, the inside of the cannulated pedicle screw is a non-biological dead space and may not be affected by this.
Conclusion
A single-dose intravenous administration of 1.5 g cefuroxime provided subtherapeutic concentrations (< 4 µg/mL) for prevention of colonization inside a cannulated pedicle screw commonly used in minimally invasive spine surgery. In the opposite non-instrumented vertebral pedicle, cefuroxime concentrations were therapeutic for up to 1.5–2 h. Therefore, additional prophylactic strategies in minimally invasive instrumented spine surgery of the lumbar spine may be considered, especially in high-risk patients. Alternative cefuroxime dosing regimens seems relevant in lumbar spine surgery lasting more than 1.5 h.
- O’Toole J E, Eichholz K M, Fessler R G. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 2009; 11: 471-6. doi: 10.3171/2009.5.Spine08633.
- de Bodman C, Miyanji F, Borner B, Zambelli P Y, Racloz G, Dayer R. Minimally invasive surgery for adolescent idiopathic scoliosis: correction of deformity and peri-operative morbidity in 70 consecutive patients. Bone Joint J 2017; 99-b: 1651-7. doi: 10.1302/0301-620x.99b12.Bjj-2017-0022.R2.
- McGirt M J, Parker S L, Lerner J, Engelhart L, Knight T, Wang M Y. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine 2011; 14: 771-8. doi: 10.3171/2011.1.Spine10571.
- Zhou J, Wang R, Huo X, Xiong W, Kang L, Xue Y. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2020; 45: 208-16. doi: 10.1097/brs.0000000000003218.
- Arciola C R, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 2018; 16: 397-409. doi: 10.1038/s41579-018-0019-y.
- Abdul-Jabbar A, Berven S H, Hu S S, Chou D, Mummaneni P V, Takemoto S, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976) 2013; 38: E1425-31. doi: 10.1097/BRS.0b013e3182a42a68.
- Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg 2008; 16: 283-93. doi: 10.5435/00124635-200805000-00007.
- Smith B R, LeFrock J L. Cefuroxime: antimicrobial activity, pharmacology, and clinical efficacy. Ther Drug Monit 1983; 5: 149-60.
- Tøstesen S K, Hanberg P, Bue M, Thillemann T M, Falstie-Jensen T, Tøttrup M, et al. Weight-based cefuroxime dosing provides comparable orthopedic target tissue concentrations between weight groups: a microdialysis porcine study. APMIS 2022; 130(2): 111-8. doi: 10.1111/apm.13198.
- Tøttrup M, Bue M, Koch J, Jensen L K, Hanberg P, Aalbæk B, et al. Effects of implant-associated osteomyelitis on cefuroxime bone pharmacokinetics: assessment in a porcine model. J Bone Joint Surg Am 2016; 98: 363-9. doi: 10.2106/jbjs.O.00550.
- Hvistendahl M A, Bue M, Hanberg P, Kaspersen A E, Schmedes A V, Stilling M, et al. Cefuroxime concentrations in the anterior and posterior column of the lumbar spine: an experimental porcine study. Spine J 2022; 22(9): 1434-41. doi: 10.1016/j.spinee.2022.05.010.
- Hanberg P, Bue M, Öbrink-Hansen K, Kabel J, Thomassen M, Tøttrup M, et al. Simultaneous retrodialysis by drug for cefuroxime using meropenem as an internal standard: a microdialysis validation study. J Pharm Sci 2020; 109: 1373-9. doi: 10.1016/j.xphs.2019.11.014.
- Joukhadar C, Müller M. Microdialysis. Clin Pharmacokinet 2005; 44: 895-913. doi: 10.2165/00003088-200544090-00002.
- Kho C M, Enche Ab Rahim S K, Ahmad Z A, Abdullah N S. A review on microdialysis calibration methods: the theory and current related efforts. Mol Neurobiol 2017; 54: 3506-27. doi: 10.1007/s12035-016-9929-8.
- European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC distribution website (cefuroxime). [cited 24 May 2022; Available from: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=58&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50.
- Bratzler D W, Houck P M. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004; 38: 1706-15. doi: 10.1086/421095.
- Metsemakers W J, Fragomen A T, Moriarty T F, Morgenstern M, Egol K A, Zalavras C, et al. Evidence-based recommendations for local antimicrobial strategies and dead space management in fracture-related infection. J Orthop Trauma 2020; 34: 18-29. doi: 10.1097/bot.0000000000001615.
- Rodríguez-Merchán E C, Davidson D J, Liddle A D. Recent strategies to combat infections from biofilm-forming bacteria on orthopaedic implants. Int J Mol Sci 2021; 22: 10243. doi: 10.3390/ijms221910243
- Steiner J A, Ferguson S J, van Lenthe G H. Screw insertion in trabecular bone causes peri-implant bone damage. Med Eng Phys 2016; 38: 417-22. doi: 10.1016/j.medengphy.2016.01.006.
- Jensen L K, Koch J, Henriksen N L, Bue M, Tøttrup M, Hanberg P, et al. Suppurative inflammation and local tissue destruction reduce the penetration of cefuroxime to infected bone implant cavities. J Comp Pathol 2017; 157: 308-16. doi: 10.1016/j.jcpa.2017.10.001.
- Vittrup S, Stilling M, Hanberg P, Tøstesen S K, Knudsen M B, Kipp J O, et al. Concentrations of co-administered vancomycin and meropenem in the internal dead space of a cannulated screw and in cancellous bone adjacent to the screw: evaluated by microdialysis in a porcine model. Injury 2022; 53: 2734-40. doi: 10.1016/j.injury.2022.06.008.
- Hanberg P, Bue M, Jørgensen A R, Thomassen M, Öbrink-Hansen K, Søballe K, et al. Pharmacokinetics of double-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone: a randomized microdialysis study. Spine J 2020; 20: 1327-32. doi: 10.1016/j.spinee.2020.03.006.
- Hanberg P, Bue M, Birke Sørensen H, Søballe K, Tøttrup M. Pharmacokinetics of single-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone determined by microdialysis. Spine J 2016; 16: 432-8. doi: 10.1016/j.spinee.2015.11.031.
- Swindle M M, Makin A, Herron A J, Clubb F J, Jr., Frazier K S. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012; 49: 344-56. doi: 10.1177/0300985811402846.
- Hanberg P, Bue M, Kabel J, Jørgensen A R, Jessen C, Søballe K, et al. Effects of tourniquet inflation on peri- and postoperative cefuroxime concentrations in bone and tissue. Acta Orthop 2021; 92(6): 746-52. doi: 10.1080/17453674.2021.1942620.
- Crock H V, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop Relat Res 1976; (115): 6-21.