No difference in implant survival between 28-mm M2a RingLoc metal-on-metal and metal-on-polyethylene total hip arthroplasty: results from the Finnish Arthroplasty Register
Antton PALOMÄKI 1, Matias HEMMILÄ 1, Markus MATILAINEN 2, Antti ESKELINEN 3, Jaason HAAPAKOSKI 4, Ari-Pekka PUHTO 5, Jukka KETTUNEN 6, Konsta PAMILO 3, Mikko MANNINEN 7, Anna VASARA 8, and Keijo T MÄKELÄ 1
1 Department of Orthopaedics and Traumatology, Turku University Hospital, Turku, and University of Turku; 2 Turku PET Centre, Turku and Faculty of Science and Engineering, Åbo Akademi University, Turku; 3 Coxa Hospital for Joint Replacement, Tampere; 4 National Institute for Health and Welfare, Helsinki; 5 OYS Centre for Musculoskeletal Surgery, Oulu University Hospital, Oulu; 6 Department of Orthopaedics and Traumatology, Kuopio University Hospital, Kuopio; 7 Orton Hospital, Helsinki; 8 Department of Orthopaedics and Traumatology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
Background and purpose — Long-term outcome of small head (28 mm) metal-on-metal (MoM) total hip arthroplasty (THA) is available mainly for Metasul devices (Sulzer Medica, Winterthur, Switzerland). Biomet MoM THA was frequently used in Finland. Therefore, we assessed long-term survivorship of the M2a 28-mm RingLoc MoM THA (Biomet, Warsaw, IN, USA) and compared it with the metal-on-polyethylene (MoP) RingLoc THA from the same manufacturer.
Patients and methods — We conducted a register study based on THAs from the Finnish Arthroplasty Register performed between January 1, 2000 and December 31, 2007. 290 28-mm head M2a MoM THAs and 1,647 28-mm head MoP THAs (reference group) were included. The endpoint was revision for any reason, or revision for aseptic loosening, osteolysis, liner wear, or metallosis as one group. Kaplan–Meier survival estimates were calculated, and revision risks were assessed using a Cox multiple regression model, both with 95% confidence intervals (CI).
Results — No difference was found in the 15-year Kaplan–Meier survivorship between the 28-mm head M2a RingLoc MoM THA and the reference group for any reason for revision (87.7% [82.9–92.1] and 83.3% [81.0–85.3], respectively). The adjusted hazard ratio (HR) for any reason for revision for the MoM THA group compared with the reference group was at least equal or better (0.70 [0.48–1.02]). Both groups presented similar survival for revision for aseptic loosening of the cup, osteolysis, liner wear, or metallosis, at 96.2% (92.7–98.0) and 95.4% (93.9–96.5), respectively.
Interpretation — In the long-term survival there was no difference between the M2a 28-mm RingLoc MoM THA and 28-mm MoP THA. Further follow-up regimens for M2a 28-mm RingLoc THA patients may be unnecessary, but long-term metal ion and radiological data is needed before any formal suggestions.
Citation: Acta Orthopaedica 2022; 93: 854–858. DOI http://dx.doi.org/10.2340/17453674.2022.5252.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-02-18. Accepted: 2022-10-11. Published: 2022-11-24.
Correspondence: antton.palomaki@tyks.fi
AP, MH, JH, KM: planning the study, analysis of the data, and writing the manuscript; MM calculating the statistics and revision of the manuscript; AE, A-PP, JK, MM, AV: analysis of the data and revision of the manuscript.
Handling co-editors: Søren Overgaard and Philippe Wagner
Acta thanks Alister Hart and Claus Varnum for help with peer review of this study.
Polyethylene wear and osteolysis of metal-on-polyethylene (MoP) total hip arthroplasty (THA) generated the rise of second-generation metal-on-metal (MoM) bearing surfaces in the 1990s (1,2). The absence of polyethylene particles and the reported low wear rate were the drivers for the increased usage of MoM THA (3). After promising short-term results, alarming implant failures and adverse reactions to metal debris (ARMD) were notoriously reported for large diameter head (LH) MoM THA (4-6). Unlike LH MoM, small-head (SH) MoM THA with 28mm head diameter has been associated with satisfactory survivorship and fewer development of ARMD (7-11). However, there is only very limited data concerning survivorship of the second-generation small head MoM THAs other than the Metasul bearing THA (Sulzer Medica, now Zimmer Biomet, Warsaw, IN, USA, and Winterthur, Switzerland), which was introduced in 1988.
Biomet (Biomet, Warsaw, IN, USA) introduced a 28-mm head M2a RingLoc THA in the late 1990s. M2a bearing surfaces were made of wrought high carbon Co-Cr-Mo alloy in accordance with ASTMF-1537/ISO 5832-12. The M2a RingLoc liner was manufactured by molding a block of conventional polyethylene (ArCom, Biomet) around a highly polished metal alloy bearing insert (12). The M2a RingLoc THA was the most common SH MoM device in Finland. Long-term survivorship data of this device is scarce. Therefore, we assessed the long-term survival of the uncemented SH M2a RingLoc THA, and compared it with that of metal-on-polyethylene (MoP) RingLoc THA from the same manufacturer.
Patients and methods
Our study is based on data from the Finnish Arthroplasty Register (FAR). Management and activity of FAR has been presented previously (13). All arthroplasty units including private hospitals deliver THA data, thus coverage of hospitals is 100%. Completeness of primary THA exceeds 95%, and completeness of revision THA is 81% (14). The data content of the register was scrutinized and revised in May 2014. The updated data includes information on ARMD as reason for revision.
Study population
Between January 2000 and December 2007, 290 M2a 28-mm RingLoc MoM THAs (study group) and 1,647 MoP 28-mm RingLoc THAs (reference group) were recorded, with follow-up until December 31, 2017. THAs with the head size other than 28 mm and head size material other than metal were excluded. Revision was identified as change or removal of at least one component in the hip.
Surgery
In both groups the cup component was always Vision RingLoc with a conventional ArCom polyethylene liner. Only uncemented stems were included. A Bi-Metric stem was used in 92% of cases in the study group and 99% in the control group.
Statistics
The endpoint was revision for any reason (primary), or revision for aseptic loosening, osteolysis, liner wear, or ARMD (secondary, composite of all these mentioned revision causes). Prior register revision in May 2014, revisions performed for osteolysis and wear were coded as performed for “other reason.” Therefore, revisions performed for other reason prior May 2014 are included in the analyses of secondary outcome. Patients were censored for any other event than the outcome, or at the end of the follow up. The Kaplan–Meier (K–M) survival estimates with the 95% confidence interval (CI) were calculated for both groups at 1, 5, 10, and 15 years for any reason for revision and for loosening of the cup, osteolysis, liner wear, or ARMD. The survival curves were compared using a log-rank test.
In theory, inclusion of bilateral cases in a survival analysis violates the basic assumption that all cases are independent. However, several reports have shown that the effect of including bilateral cases in studies of implant survival is negligible (15,16). Therefore, after careful consideration we decided to include bilateral cases (128) in the analyses.
We adjusted the estimated revision risks in the Cox multiple regression model by sex and diagnosis (primary osteoarthritis, rheumatoid arthritis, other). Age group (18–54, 55–65, > 65) was stratified. The second analysis was performed for loosening of the cup, osteolysis, liner wear, or ARMD as endpoint. Sex and diagnosis were also adjusted for in the Cox model, and age group was stratified.
Proportional hazards (PH) assumption was checked by visual inspection of the K–M curves, and using a Kolmogorov-type supremum test and graphically using Schoenfeld residuals. If the PH assumption for a variable was not fulfilled in the Cox model, the model was stratified by that instead. Stratification in Cox models means that all level combinations of the stratified variables are allowed to have a different baseline hazard, and the hazard ratios for the other variables (those that meet the PH assumption) are then optimized for these. Without stratification we would assume that the baseline hazards were the same for all levels of such variables. Results of the Cox regression analysis are presented with the hazard ratio (HR) and 95% CI.
All analyses were performed using SAS software, Version 9.4 (SAS Institute, Cary, NC, USA).
Ethics, funding, data sharing, and disclosures
Ethical approval was granted on June 13, 2017 by National Institute for Health and Wellfare (Dnro THL/926/5.05.00/2017). The authors declare no conflicts of interest. The study was funded by the Finnish Arthroplasty Association and the Finnish Government Research Grant. Data sharing is not possible.
Results
Demographics
Table 1 presents the demographic data of the 2 groups. Mean follow-up time of the study group was 13.3 years (range 0.8–18.4) and that of the reference group 12.1 years (range 0–18.5). Reasons for revision are presented in Tables 2 (prior the data contents revision May 15, 2014) and 3 (May 15, 2014 onwards). Mortality during the study period of the M2A group was 17% and that of the reference group 31%. The number of patients with bilateral THA was 128, and in 19 patients THA was performed simultaneously.
| Factor | M2a group (290 hips) | Reference group (1,647 hips) |
| Male sex | 83 (29) | 827(50) |
| Diagnosis | ||
| Primary osteoarthritis | 185 (64) | 1,399 (85) |
| Rheumatoid arthritis | 30 (10) | 56 (3) |
| Other | 75 (26) | 192 (12) |
| Age group | ||
| 18–54 | 124 (43) | 233 (14) |
| 55–65 | 106 (37) | 614 (37) |
| > 65 | 60 (20) | 800 (49) |
| Status at end of follow-up (including dead) | ||
| Not revised | 256 (88) | 1,413 (86) |
| Revised | 34 (12) | 234 (14) |
| Stem model | ||
| Bi-Metric | 267 (92) | 1,629 (99) |
| Other a | 23 (8) | 18 (1) |
| Operation year | ||
| 2000–2003 | 187 (65) | 1,102 (67) |
| 2004–2007 | 103 (35) | 545 (33) |
| a ABG II, Accolade I, BFX, BIHAPRO, CDH, CLS, Lubinus SP II, Mallory-Head, Taperlock. | ||
Revision for any reason
There was no difference in the 15-year survivorship with revision for any reason as endpoint between the groups, at 87.7% (82.9–91.2) for the study group and 83.3% (81.0–85.3) for the reference group (Table 4 and Figure 1). In the Cox regression analysis, there was no difference in the risk of revision of the study group compared with the reference group (HR 0.70 [0.48–1.02]) (Table 5), i.e., results were inconclusive in terms of this endpoint.
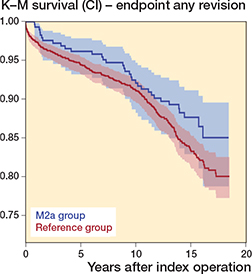
Figure 1. Kaplan–Meier survival for the M2a group (blue) and the reference group (red) with 95% CI levels with revision for any reason as the endpoint. Logrank p = 0.1.
Revision for aseptic loosening of the cup, osteolysis, liner wear, or ARMD
There was no difference in the 15-year survivorship with revision for aseptic loosening of the cup, osteolysis, liner wear, or ARMD as endpoint between the groups: study group 96.2% (92.7–98.0) and reference group 95.4% (93.9–96.5) (Table 4 and Figure 2). In the Cox regression analysis, there was no difference in the risk of revision of the study group compared with the reference group (HR 0.72 [0.36–1.45]) (Table 5), i.e., results were inconclusive in terms of this endpoint.
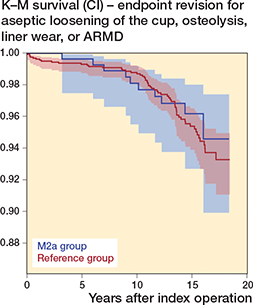
Figure 2. Kaplan–Meier survival for the M2a group (blue) and the reference group (red) with 95% CI levels with revision for aseptic loosening of the cup, osteolysis, liner wear, or ARMD as the endpoint. Logrank p = 0.6.
Discussion
We found that, in the long term, there was no difference between the M2a 28-mm head MoM THA and 28-mm head MoP THA.
Previous reports focus mainly on devices under the same product umbrella with notable differences. Van der Veen et al. (12) reported similar 96% implant survival rates at 13 years when comparing 23 cemented M2a THAs and 33 cemented MoP THAs. A Stanmore cemented stem was used in both groups. In the current study, all acetabular and femoral components were uncemented, although the bearing couple was the same as in the report of van der Veen. However, when using uncemented Biomet cups (PE+M2a or PE) the liner was attached to the acetabular component by RingLoc mechanism, producing intraoperative modularity comparable to the monoblock Stanmore cup. Implant survival in our study for both M2a and MoP THA groups was slightly inferior compared with those of van der Veen et al. (12) but their study patients initially participated in a single-center trial whereas our data represents nationwide results. Difference in fixation method may also have some minor bias on the results.
Lombardi et al. (17) reported on 300 hips with M2a Taper bearing THA with a wrought cobalt-chromium tapered insert that was impacted into a titanium porous plasma spray-coated outer shell and articulated with a wrought cobalt-chromium modular femoral head (28 or 32 mm). Also, this M2a device differs notably from our study device. The M2a insert used in the study of Lombardi et al. adds an extra metal-on-metal surface between the cup (titanium alloy) and the insert (cobaltchromium). Further, in our study the head size was always 28 mm. Their 10-year survival rate was 96%, 15-year rate 92%, and 19-year rate 73%. ARMD was found in 5% and this was the main reason for revision in 70% of re-operations. Again, our population-based implant survival overall was slightly inferior in both the MoM and MoP groups. However, ARMD revisions in our data were scarce. There were 2 revisions performed for ARMD during the new register era, and 3 revisions performed for “other reason” before the FAR register reformation. If the latter were true ARMD cases (not for example leg-length discrepancy etc.), the worst-case scenario for ARMD revision is 1.7% (5 of 290), and the best-case scenario 0.7% (2 of 290). At most 19% of all revisions in our study were performed for ARMD. Avoiding the extra metalon-metal surface in the RingLoc MoM device may reduce metallosis compared with the tapered system from the same manufacturer.
De Steiger et al. (18) assessed 15-year survivorship of MoM THA using small heads (≤ 32 mm) compared with large heads based on the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). The cumulative percentage revision for SH MoM THA was 8.5%, whereas that for LH MoM THA was 27%. The authors concluded that although implant survival of the SH MoM THA was substantially greater than that of the LH MoM THA, over time there was also a gradual increase in the diagnosis of ARMD for the SH MoM THA. However, 78% of the study devices had a Metasul bearing couple. There were only 90 Biomet RingLoc devices and 54 Biomet Taperloc devices among an overall 4,838 SH MoM THAs, so the conclusions are not directly adaptable for the M2a SH devices. Contrary to their results, no increase ARMD revisions was detected in the current study including RingLoc SH MoM THA only. We are not aware of any larger series of 28-mm RingLoc MoM THA than the current one.
We acknowledge that our study has several limitations. First, the study setting was retrospective, although the data was prospectively collected. Unfortunately, we do not have any radiographic or MRI data to detect osteolysis or metallosis, or data on the whole-blood cobalt or chromium levels of the SH MoM THA patients. However, it has been stated previously that the prevalence of pseudotumors may be similar in patients with a well-functioning hip prosthesis and patients with a painful hip. Although MRI is useful for surgical planning, the presence of a cystic pseudotumor may not necessarily indicate the need for revision arthroplasty (19). In LH ReCap-M2a MoM THA, patients’ blood metal ion level decreased in the long-term follow-up. Also patient satisfaction was excellent or good in 76% of patients in spite of marginally elevated blood-metal ions (20).
Data on patient comorbidities was not included either. There was a difference in mortality between the 2 groups (17% for M2a and 31% for the reference group), suggesting that there may be some confounding by indication. Further, we could only assess revision operation as the outcome, because we do not have data on patient-reported outcome measures. Some patients may have experienced pain or other implant-related problems without undergoing a revision. Moreover, the completeness of data of the revision operations in FAR has varied between 80% and 90%, so some revisions are missing. However, we do not think that these issues notably influenced our results and the message. Our findings are implant specific, and not generalizable to other SH MoM THA brands.
In conclusion, there was no difference in the long-term implant survival between the SH M2a RingLoc THA and MoP RingLoc THA from the same manufacturer. This is the largest series of this device we are aware of. Revisions for ARMD were infrequent. Further follow-up regimens for M2a 28-mm RingLoc THA patients may be unnecessary, but long-term metal ion and radiological data are needed before any formal suggestions.
- Carlsson A S, Gentz C F. Mechanical loosening of the femoral head prosthesis in the Charnley total hip arthroplasty. Clin Orthop Relat Res 1980; 147: 262-70.
- Jasty M J, Floyd W E, Schiller A L, Goldring S R, Harris W H. Localized osteolysis in stable, non-septic total hip replacement. J Bone Joint Surg (Am) 1986; 68: 912-19. doi: 10.2106/00004623-198668060-00014.
- Lombardi A V, Mallory T H, Alexiades M M, Cuckler J M, Faris P M, Jaffe K A, et al. Short-term results of the M2a-Taper metalon-metal articulation. J Arthroplasty 2001; 16: 122.8. doi: 10.1054/arth.2001.29307.
- AOANJRR Hip, Knee & Shoulder Arthroplasty; Annual Report 2007. Available at https://aoanjr.sahmri.com
- NJR Annual Report; 2009. Available at https://www.njrcentre.org.uk
- Ollivere B, Darrah C, Barker T, Nolan J, Porteous M J. Early clinical failure of the Birmingham metal-on-metal hip resurfacing is associated with metallosis and soft-tissue necrosis. J Bone Joint Surg (Br) 2009; 91: 1025-30. doi: 10.1302/0301-620X.91B8.21701.
- Delaunay C P, Bonnomet F, Clavert P, Laffargue P, Migaud H. THA Using metal-on-metal articulation in active patients younger than 50 years. Clin Orthop Relat Res 2008; 466: 340-6. doi: 10.1007/s11999-007-0045-y.
- Saito S, Ishii T, Mori S, Hosaka K, Ootaki M, Tokuhashi Y. Longterm results of Metasul metal-on-metal total hip arthroplasty. Orthopedics 2010; 33(8). doi: 10.3928/01477447-20100625-11.
- Randelli F, Banci L, D’Anna A, Visentin O, Randelli G. Cementless Metasul metal-on-metal total hip arthroplasties at 13 years. J Arthroplasty 2012; 27: 186-92. doi: 10.1016/j.arth.2011.04.015.
- Delaunay C P, Putman S, Puliéro B, Bégin M, Migaud H, Bonnomet F. Cementless total hip arthroplasty with Metasul bearings provides good results in active young patients: a concise followup. Clin Orthop Relat Res 2016; 474: 2126.33. doi: 10.1007/s11999-016-4920-2.
- Jin S-Y, Jin J-Y, Kang J-K, Yoon T-R, Park K-S. Minimum 15-year results of Metasul 28-mm metal-on-metal total hip arthroplasty in patients younger than 50 years of age. J Orthop Surg Res 2021; 16(1): 218. doi:10.1186/s13018-021-02352-2.
- van der Veen H C, Reininga I H, Zijlstra W P, Boomsma M F, Bulstra S K, van Raay J J. Pseudotumours, cobalt and clinical outcome in small head metal-on-metal versus conventional metal-on-polyethylene total hip arthroplasty. HIP Int 2020; 30: 56-63. doi: 10.1177/1120700019832877.
- Palomäki A, Hemmilä M, Laaksonen I, Matilainen M, Eskelinen A, Haapakoski J. Implant survival of 6,080 Tritanium cups in primary total hip arthroplasty. J Bone Joint Surg 2020; 102: 1177-85. doi: 10.2106/JBJS.19.00874.
- FAR. Finnish Arthroplasty Register. Available online from: https://www.thl.fi/far.
- Lie S A, Engesæter L B, Havelin L I, Gjessing H K, Vollset S E. Dependency issues in survival analyses of 55 782 primary hip replacements from 47 355 patients. Stat Med 2004; 23: 3227-40. doi: 10.1002/sim.1905.
- Robertsson O, Ranstam J. No bias of ignored bilaterality when analysing the revision risk of knee prostheses: analysis of a population based sample of 44,590 patients with 55,298 knee prostheses from the National Swedish Knee Arthroplasty Register. BMC Musculoskelet Disord 2003; 4: 1. doi: 10.1186/1471-2474-4-1.
- Lombardi A V, Berend K R, Adams J B, Satterwhite K L. Adverse reactions to metal on metal are not exclusive to large heads in total hip arthroplasty. Clin Orthop Relat Res 2016; 474: 432-40. doi: 10.1007/s11999-015-4539-8.
- de Steiger R, Peng A, Lewis P, Graves S. What is the long-term survival for primary THA with small-head metal-on-metal bearings? Clin Orthop Relat Res 2018; 476: 1231-7. doi: 10.1007/s11999.0000000000000209.
- Hart A J, Satchithananda K, Liddle A D, Sabah S A, McRobbie D, Henckel J, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses. J Bone Joint Surg 2012; 94: 317-25. doi: 10.2106/JBJS.J.01508.
- Pietiläinen S, Linnovaara A, Venäläinen M S, Mäntymäki H, Laaksonen I, Lankinen P, et al. Median 10-year whole blood metal ion levels and clinical outcome of ReCap-M2a-Magnum metalon-metal total hip arthroplasty. Acta Orthop 2022; 93: 444-50. doi: 10.2340/17453674.2022.2510.