Study protocol: The DAICY trial—dual versus single-antibiotic impregnated cement in primary hemiarthroplasty for femoral neck fracture—a register-based cluster-randomized crossover-controlled trial
Sebastian MUKKA 1, Nils P HAILER 2, Michael MÖLLER 3,4, Max GORDON 5, Stergios LAZARINIS 2, Cecilia ROGMARK 6,7, Ollie ÖSTLUND 8, Olof SKÖLDENBERG 5, Olof WOLF 2,4, and The DAICY study group a
1 Department of Surgical and Perioperative Science (Orthopaedics), Umeå University, Umeå; 2 Department of Surgical Sciences, Orthopaedics, Uppsala University, Uppsala; 3 Institute of Clinical Science, Sahlgrenska Academy, University of Gothenburg, Gothenburg; 4 Swedish Fracture Register, Registercentrum Västra Götaland, Gothenburg; 5 Department of Clinical Sciences at Danderyd Hospital, Division of Orthopaedics, Karolinska Institutet, Stockholm; 6 Department of Orthopedics, Lund University, Skåne University Hospital, Malmö; 7 Swedish Arthroplasty Register, Registercentrum Västra Götaland, Gothenburg; 8 Uppsala Clinical Research Center, Uppsala, Sweden
a The DAICY study group: Region Norrbotten, Sunderby Hospital: Jonas Åhlander, Danyar Barneh; Region Västerbotten, Skellefteå Hospital: Mark Kruse; Region Västerbotten, Lycksele Hospital: Lycksele: Maria Thorén Örnberg, Algirdas Petrauskas; Region Västerbotten, Umeå University Hospital: Jonas Sundkvist; Region Västernorrland, Örnsköldsvik Hospital: Thomas Stålarm; Region Jämtland-Härjedalen, Östersund Hospital: Lars Holgén; Region Dalarna, Falu Hospital: Anna Berggren; Region Halland, Halmstad Hospital: Daniel Stam, Henrik Bjerome Ahlin; Region Blekinge, Blekinge Hospital Karlskrona: Olof Leonardsson; Region Stockholm, Södersjukhuset: Leif Mattisson; Region Västra Götaland, Södra Älvsborg Hospital: Per-Erik Johansson; Region Västra Götaland, Sahlgrenska University Hospital, Mölndal: Per Hulenvik; Region Värmland, Karlstad Hospital: Per Fischer, Fredrik Sundström
Background and purpose — Older patients with a displaced femoral neck fracture (FNF) are often treated with a cemented primary hemiarthroplasty (HA). The DAICY trial investigates whether high-dose dual-impregnated antibiotic-loaded cement (DIAC) including gentamicin and clindamycin can reduce the risk of periprosthetic joint infection (PJI) in comparison with low-dose single-impregnated gentamicin antibiotic-loaded cement (SIAC), in patients ≥ 60 years treated with a cemented HA for a displaced FNF.
Study design — The trial is a national, multicenter, register-based, cluster-randomized, crossover trial. Patients ≥ 60 years with a non-pathological, displaced FNF (Type Garden 3–4/AO 31-B2 or B3) suitable for HA according to local guidelines are eligible for inclusion. Participating orthopedic departments will be randomized to start with either SIAC (control group) or DIAC treatment (intervention group) for 2 years. After 2 years, the study departments will then change to the other treatment arm for the remaining 2 years of the study. Approximately 7,000 patients will be included. The study is pragmatic in that the choice of implant brands, surgical approach and peri- and postoperative protocols follow the local routines of each participating department. All outcome variables will be retrieved after linkage of the study cohort to the following Swedish registers: the Fracture Register, the Arthroplasty Register, the National Patient Register and the Prescribed Drug Registry
Outcome — The primary outcome will be periprosthetic joint infection of the index joint within 1 year after surgery. Secondary outcomes will be any reoperation on the index joint, mortality within 90 days and 1 year, resistance patterns of causative bacteria in cases of PJI, and health economics.
Potential added value — This trial is designed to support or refute the efficacy of DIAC used in patients with a displaced FNF, potentially reducing PJI and resource allocation.
Start of the trial and estimated duration — The DAICY trial started recruiting patients in January 2022 and will continue recruiting for approximately 4 years. Complete follow-up expected in 5 years.
Citation: Acta Orthopaedica 2022; 93: 794–800. DOI http://dx.doi.org/10.2340/17453674.2022.4819.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-05-17. Accepted: 2022-09-14. Published: 2022-10-05.
Correspondence: sebastian.mukka@umu.se
All authors contributed to the design of the trial and shared in reviewing the manuscript. OÖ performed the sample size and power calculations analysis and statistical analysis plan. SM managed the ethical applications and drafted the manuscript. All authors have given their final approval of the version to be published and agree to be accountable for all aspects of the work.
Acta thanks Jan-Erik Gjertsen for help with peer review of this study protocol.
Most patients with a displaced femoral neck fracture (FNF) are treated with arthroplasty, with hemiarthroplasty (HA) as the most commonly used procedure (1,2). With a growing elderly population, hip fractures are expected to increase steadily. Approximately 4,000 patients in Sweden are treated annually with HA for an FNF (3). In Sweden, cemented arthroplasty in FNF is used almost exclusively because of improved functional outcomes and lower risk for reoperations when compared with uncemented HA (4-6).
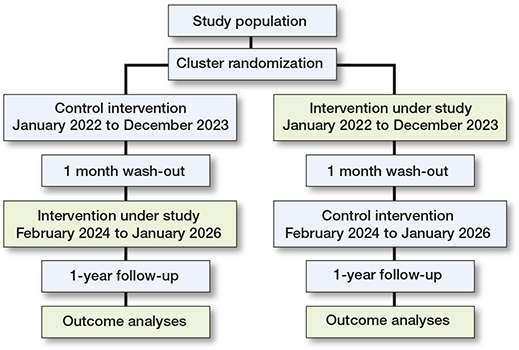
Illustration of the study design
Individuals with FNF treated with HA are particularly susceptible to periprosthetic joint infection (PJI); the incidence reported in the literature is as high as 7% worldwide, with a 1-year mortality rate of up to 50% in those suffering infection (7-9).
Perioperative antibiotic prophylaxis administered parenterally and through the bone cement used for implant fixation has been proven to reduce the incidence of PJI (10,11). However, there is no consensus on the antibiotic content or type and dose of the bone cement. Antibiotic-loaded bone cement with lowdose gentamicin is standard practice in Sweden (12). There is some evidence that using high-dose dual antibiotic-impregnated bone cement including high-dose gentamycin (DIAC) in combination with clindamycin reduces the incidence of PJI compared with single-impregnated antibiotic-loaded cement (SIAC) (13). In a well-conducted, manufacturer-subsidized, quasi-randomized study of 848 individuals treated with HA, the use of DIAC reduced the rate of infection compared with standard low-dose single antibiotic-loaded bone cement (13). The reduction of PJI is by an extended inhibition of bacterial growth and biofilm formation (14). The main concern of the routine use of DIAC is the development of antimicrobial resistance patterns, higher cost, and potential toxicity. However, there has been no compelling evidence to support these claims (15,16).
This register-based, cluster-randomized, crossover-controlled trial aims to investigate whether DIAC reduces the incidence of PJI after HA due to a displaced FNF in patients ≥ 60 years compared with single-impregnated antibiotic bone cement (SIAC) at 1 year postoperatively. As secondary endpoints, we will analyze the risk of any reoperation, antibiotic suppression, resistance pattern of PJI, mortality, and whether the use of DIAC is cost-efficient.
Patients and methods
Study design
The DAICY trial is a multicenter, register-based, cluster-randomized, crossover-controlled trial. The participating orthopedic departments are randomized 1:1 to start with either the intervention (DIAC) or control (SIAC) arm (Figure). After the first period of 2 years (January 1, 2022–December 31, 2023) is completed and a wash-out period of 1 month, the study departments will change such that the patients included in the remaining 2 years (February 1, 2024–January 31, 2026) will be assigned the opposite treatment arm. All patients with a displaced FNF who are eligible for HA treatment according to local guidelines will be included.
Study patients and eligibility criteria
All patients based on age (≥ 60 years), type of fracture (type 3 or 4 according to the Garden classification of hip fractures, AO types 31-B2 or B3), and eligible for HA will be included by the participating departments. Exclusion criteria will be previous contralateral hip HA in this study, pathological or stress fracture, not available to participate in the intervention (e.g., sensitivity to any of the components in the bone cement), and patients who have actively marked their hospital charts with an added privacy notice. The participating departments were recruited by the first author (SM).
Randomization and blinding
Before the start of the study, the participating departments were randomized by code to treatment sequence (AB; DIAC–SIAC), BA; SIAC–DIAC). Participants allocated to the AB study arm receive treatment DIAC first, followed by treatment SIAC, and vice versa in the BA arm. The code and the sequence of intervention was performed by an independent statistician (OÖ) at Uppsala Clinical Research Center. The study is open label; patients and physicians will be aware of treatment allocation.
Surgical intervention
The trial design is pragmatic, implying that the choice of implant brands, surgical approach, and pre-, peri-, and postoperative routines will be based on the participating hospitals’ preferences. The study protocol requires all participating departments to maintain their chosen regimen across intervention and control groups. Routine clinical care, including regional or general anesthesia, will be used. All participants will receive perioperative prophylactic intravenous antibiotics in accordance with current protocols agreed at each department.
The Lubinus SP2 (Waldemar Link), Exeter (Stryker), and MS-30 (Zimmer Biomet) stems are the main components used in the Swedish departments in the treatment of FNF patients (12). The local standard stem type for HA due to FNF will be used in each participating department.
Trial intervention
Group 1 (control): Cemented HA with low-dose SIAC. The cement used will be Heraeus Palacos R+G (Hanau, Germany) or Zimmer/Biomet Optipac cement. Both types of cement contain gentamicin 0.5 g per 40 g of cement mix.
Group 2 (intervention): Cemented HA with high-dose DIAC. The cement used will be Heraeus Copal G+C (Hanau, Germany), containing 1 g gentamicin and 1 g clindamycin per 40 g of cement mix.
Patient withdrawal from the trial
Participants are free to withdraw from the Swedish Fracture Register (SFR) and thereby from the trial with no adverse consequences of any kind.
Primary endpoint–periprosthetic joint infection
The primary endpoint is the occurrence of any PJI of the index joint within 1 year of surgery. The definition of PJI will be that the treating physicians had determined the presence of a PJI and started treatment accordingly (reoperation, suppressive antibiotics, or combinations thereof). The occurrence of PJI is assessed by linking study participants with Swedish national health data registers by using the unique Swedish personal identification number (PIN).
We will use the SFR, National Patient Register (NPR), Swedish Arthroplasty Register (SAR), and the Swedish Prescribed Drug Register (SPDR) to identify potential PJIs by the registration of any of the ICD codes (version 10) or NOMESCO codes indicative of this complication (Table 1, see Supplementary data). In cases when these codes are present, a review of the medical records will be made to identify patients and confirm the presence of PJI. This review would capture any reoperation records for surgery related to the index hip fracture, details of antibiotics prescribed, and microbiology reports if samples of the suspected infected tissues around the hip were sent for analysis.
Secondary outcome variables
Any reoperation
Reoperation is defined as the occurrence of any surgical procedure performed on the previously treated hip within 1 year after surgery. The occurrence of reoperation is assessed by linking study participants with the SAR and NPR as described above and will be defined by registration of at least 1 of the specified ICD or NOMESCO codes (Table 1, see Supplementary data).
Antibiotic suppression
Antibiotic prescription information will be obtained from the SPDR at 120 days and 1-year post-surgery by linking the study cohort to the SPDR (Table 2, see Supplementary data).
Mortality
Dates of death will be identified through the PIN and retrieved from the Swedish Tax Agency, the governmental body responsible for the population register in Sweden. This is embedded in the SFR, and a script is run every night, making it possible to estimate real-time mortality rates. 90-day and 1-year mortality rates will be presented.
Resistance patterns of infections
All patients with infections identified at the primary endpoint will be assessed for antibiotic resistance profiles. We will use the SFR, NPR, SAR, and SPDR to identify potential PJIs, verified by accessing patient medical records. A review of medical records would capture details of antibiotics prescribed and microbiology reports if samples of the suspected infected tissues around the hip were sent for analysis. This outcome parameter is planned for separate analysis and publication.
Cost-effectiveness
Procedural admissions costs for reoperations will also be collected from all participating departments to allow for basic health economic calculations. This outcome parameter is planned for separate analysis and publication.
Data collection
The SFR is a national quality register for managing fractures and treatment. Data on patient and fracture characteristics, injury mechanism, and fracture treatment are recorded in each affiliated department via a pre-specified digital form by the treating physician. Femoral fractures are classified according to the 2007 AO/OTA classification system (17). The number of departments affiliated with the SFR has increased gradually, and since January 1, 2021 all orthopedic departments in Sweden are engaged in the SFR, with a coverage of 100%. The completeness for hip and femur fractures was 80.9% in comparison with the NPR during 2020.
The SAR is a national quality register for surgery with hip arthroplasty in Sweden. SAR covers 100% of all hospitals performing arthroplasty in Sweden, with a completeness of approximately 96% for hemiarthroplasties. The completeness of revision procedures for HA and total hip arthroplasty is about 93% (18).
The NPR collects data on diseases, surgical treatments, and medical care measures using coding according to the Swedish ICD system. It is mandatory by law for privately and publicly funded hospitals to deliver data to the NPR. All inpatient hospitals and outpatient visits are included from private and public caregivers. The NPR has high validity, especially for surgical procedures (19).
The SPDR is a national register to which all pharmacies in Sweden are obliged to report and include all dispensed outpatient prescribed drugs. Data on the prescribed drug, the amount, the amount dispensed, the date of both prescription and dispensing, the specialty of the prescribing doctor, the level of care, and the instruction from the prescribing physician are included in the register.
Baseline data on age, sex, injury mechanism, fracture classification, time of diagnosis (defined as the time for obtaining radiographs of the fracture), and the start of surgical treatment (date and time) are collected in the SFR. Data on surgical approach, bone cement and brand, stem and head components, cognitive status, and ASA class are recorded in the SAR.
After study completion, the study cohort obtained from the SFR will be linked to the SAR, the NPR, and SPDR to form the research database and ensure high completeness for PJI (20). Patients with a potentially identified PJI through the linkage process will be confirmed by reviewing the medical records.
Data quality assurance
A study monitor will have regular contact with all participating departments to confirm that the team at each participating unit adheres to the study protocol and assists locally responsible investigators. A locally responsible study coordinator will ensure that all personnel involved in the treatment of trial subjects at each participating unit are adequately informed and trained regarding protocol requirements and that the standardization of surgery is maintained. The steering committee will not have access to outcomes until the database is locked.
Estimated sample size and power
Power was calculated for the proposed trial design and primary analysis using simulation. In addition to the hypothesized intervention effect, power depends on the number of clusters, the number of yearly operations at each cluster, PJI frequency in the control group, and intra-cluster and intracluster period correlation.
10,000 data sets were randomly generated from a model with individual outcome probabilities
logit(Pr{Yijk = 1|ci, pij}) = µ + βττij + βππj + ci + pij,
where Yijk is the indicator for PJI in patient k in period j in cluster i. τij indicates DIAC treatment in cluster i in period j, and πj is the period indicator. For each simulated data set, the cluster and cluster-period effects ci and pij were sampled from normal distributions with variance selected to provide the assumed intra-cluster and intra-cluster-period correlations. The overall period effect βπ is unimportant for the simulations and was set to 0. The intercept µ and treatment effect βτ were set to provide the assumed control and intervention arm outcome probabilities.
The updated simulation model includes the 15 verified participating hospitals/departments, using their recorded annual number of operations for 2019 (64, 132, 209, 294, 224, 97, 67, 144, 71, 45, 32, 94, 97, 112, and 65). Hence, the 4-year trial is assumed to include approximately 7,000 patients.
The 1-year risk of PJI in the study population is presumed to be 3% in the control group. This estimate, considered conservative, is based on the observed PJI incidence rate in Sweden (21,22). Power was calculated under the hypothesis that a 1.5% 1-year risk of PJI in the intervention group would be considered the minimal clinically significant difference to motivate a general change in routines and recommendations from SIAC to DIAC.
Thus, the trial is designed with the ability to detect a statistically significant difference of 1.5% (from assumed 3%) in the incidence of PJI between the groups.
There is no available data to estimate the correlations for intra-cluster and intra-cluster periods in PJI. For this reason, we simulated power under 3 scenarios: small correlations (0.01 within cluster and 0.008 within cluster period), medium correlations (0.05 within cluster and 0.04 within cluster-period), and large correlations (0.1 within cluster and 0.08 within cluster-period) (23). For the small, medium ,and large scenarios, the power to obtain a 2-sided p < 0.05 for the hypothesis of no difference was 90%, 86%, and 80%, respectively.
Statistics
Generally, all statistical analyses will account for the cluster-randomized crossover design to ensure correct type I error rates and 95% confidence intervals (CIs). Analyses will be conducted using the intention-to-treat principle, including all eligible patients with available follow-up data within a study department according to allocated treatment. The threshold of statistical significance will be set at a 2-sided p-value of 0.05 and CIs.
As primary analysis, the difference in risk of PJI after the intervention treatment compared with the control treatment will be estimated using linear regression for aggregated cluster-period data, with the proportion of events within a cluster-period as the dependent variable and treatment, cluster, period, and proportion of females within the cluster-period as independent variables. An estimated difference in risk with CIs and a 2-sided p-value will be presented.
With the register-based follow-up, we assume that follow-up will be nearly complete and the quality of data sufficient with confirmation of data in an individual medical chart review. If a patient has incomplete follow-up and a value for the primary outcome is missing, the patient will be excluded from further analysis.
Since the need for a cemented HA is not affected by the current trial arm at the site, we assume that inclusion in the analysis population is not affected by the randomized treatment. We expect minimal missing data for identified patients, and the primary analysis using observed cases assumes missingness at random. In addition, sensitivity analyses will be performed using imputation of missing data, also exploring informative missingness and death before PJI as a competing risk.
Secondary event outcomes will be analyzed and described in the same way as the primary outcome. The anticipated model for these secondary analyses will be logistic regression for individual data adjusted for cluster, period, and sex as fixed factors and cluster-period as a random factor. Choice of distribution(s) for the cluster-period remains to be decided, as well as computational details to obtain confidence intervals. Numerical randomization tests for a range of fixed odds ratios may be used to obtain CIs, because the number of cluster-periods could be too small for asymptotic methods.
Supplementary and sensitivity analyses will be performed for all event endpoints. An analysis of the primary outcome, including all intention-to-treat patients, will be performed using multiple imputations for each outcome based on individual patient characteristics (details described in the statistical analysis plan). Sensitivity analyses to investigate the impact of handling death as a non-event and analyzing death as a secondary outcome will include analyses of the composite of PJI and death. As a sensitivity analysis for the asymptotic approximation in the linear model, a 2-sided p-value for no difference in proportion will be obtained using Monte Carlo randomization inference based on the likelihood ratio.
For the supplementary analysis, treatment contrasts as odds ratios will be presented for all event outcomes with CIs. An appropriate method, accounting for the cluster-crossover design, will be pre-defined in the statistical analysis plan. While the linear model for aggregated data used for the primary analysis is often robust, asymptotic tests based on other models’ risk-inflated Type I error rates when the number of clusters is small will be undertaken (24). An additional supplementary analysis of time-to-event will be performed for mortality, using a method accounting for the cluster-allocated crossover design.
Allocated and actually performed treatments will be described in a CONSORT diagram, and additional per-protocol analyses will be undertaken as sensitivity analyses. Secondary outcomes will be presented without formal multiplicity adjustment.
For all event outcome variables, pre-defined subgroup/interaction analyses to assess the homogeneity of the treatment contrast will be performed for age, sex, ASA class (I–II or III–IV) and procedural characteristics (type of stem, head, surgical approach). For categorical subgroup indicators, events will be described in each subgroup in the same way as for the entire population. Treatment contrast in each subgroup will be estimated using a linear model at the cluster level, with proportions summarized by cluster-period and subgroup indicator as the dependent variable. The independent variables will be treatment, cluster, period, and subgroup, as well as the interaction between treatment and subgroup, and presented with nominal 95% CI for each subgroup and the interaction p-value.
For health economic studies, analyses of cost per quality-adjusted life-year will be described in a separate analysis plan.
Ethics, data sharing plan, funding, potential conflicts of interests, and dissemination
The study was approved by the Swedish Ethical Review Authority (Approval No. 2020-04815, date of issue October 22, 2020, amendments approved November 1, 2021 and January 12, 2022). The results from the study will be distributed to the medical community through presentations and publications in a scientific, peer-reviewed medical journal.
Individual informed consent was waived by the Swedish Ethical Review Authority because of the nature of the study, which posed no additional risks to patients. Study information is available on each participating department’s web page and posted in the ward.
The dataset analyzed in this study is not publicly available as the study was approved to ensure the confidentiality of the patients’ data included in the study. We are amenable to sharing data but are legally restricted from sharing the data publicly according to the law on Public Access and Secrecy, chapter 21, paragraph 7 and chapter 25, paragraph 1 (https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/offentlighets%2D%2Doch-sekretess-lag-2009400_sfs-2009-400). Any person interested in the data set may contact Umeå University and the corresponding author to find ways to share data according to Swedish law and regulations. It is also possible for individuals interested in this data to apply directly to the Center of Registers, Västra Götaland (URL: http://registercentrum.se/), a process that involves approval by the Swedish Ethical Review Authority.
The trial is supported by a grant from the Swedish Research Council (VR 2021-00349), the Swedish Medical Association (SLS-943141, SLS-943154), Uppsala-Örebro Regional Research Council (RFR 968753), and grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (RV-939240, RV-939383). The funding body has no authority over study design, data collection management, interpretation of data, analysis, or writing of manuscripts. The formal sponsor is Region Västerbotten, Sweden.
SM reports lecturer fees from Johnson & Johnson. NPH reports institutional support and lecturer fees from Waldemar Link GmbH and Zimmer Biomet. OW reports lecturer fees from Link Sweden and Smith & Nephew and consulting fees from Anatomica. MM reports lecturer fees from DePuy Synthes. None of the other authors declare any conflict of interest. MG declares no conflicts of interest. OS is principal investigator for clinical trials founded by institutional support but receives no personal support from Zimmer Biomet, Link Sweden, and Swemac. CR reports lecturer fees from Waldemar Link GmbH Sweden. SL reports lecturer fees from Waldemar Link GmbH and Heraeus Medical GmbH. OÖ declares no conflicts of interest.
The results from the study will be distributed through presentations and publication in a scientific peer-review medical journal after completion, preliminarily during 2028.
Study start and duration
The DAICY trial started recruiting patients in January 2022 and will continue recruiting for approximately 4 years and follow-up will be completed in 5 years.
Discussion
The DAICY trial aims to provide evidence to support or refute the use of DIAC in patients with a displaced FNF treated with HA. There is limited high-level evidence regarding the potentially reduced risk of PJI using DIAC (13), but there could be an increased risk of developing antimicrobial resistance patterns (15,16). Thus, before routine use of DIAC, a large, national, independent study of its efficacy is warranted. The present study is an independent academic study that, together with the meticulously designed and ongoing WhiTE 8 trial in the UK, will provide evidence on the use of DIAC (25). The potential strength of the present trial is the independent academic study setting, a longer follow-up (1 year versus 90 days), and the collecting of outcome data through a national quality register to ensure high completeness.
Strengths and limitations
This is a large-scale cluster randomized trial with a sample size large enough to gain sufficient power to detect a potential effect of DIAC. The study set up with pragmatic, broad inclusion criteria and a register-based study design also addresses the issue regarding the lack of external validity inherent in conventional randomized controlled study designs (26). External validity guarantees that the findings are generalizable and can be applied to other contexts and groups outside the study design. The SFR, supplying the main register platform used for the inclusion of patients, is a population-based register (27,28), and the linkage of the study cohort with the SAR, NPR, and SPDR is being performed to access additional procedural details and baseline data outcomes. The high coverage and completeness of the SAR and NPR provide valid and reliable outcomes, and specificity is secured with the confirmation of PJI in the medical charts (12,19). The independent academic setting and the 1-year follow-up further strengthen the study’s results and generalizability.
The study has limitations that need to be addressed. First, the cluster-randomized study design might introduce bias (e.g., selection bias, baseline imbalance, loss of clusters). However, the design improves the feasibility of including approximately 7,000 patients, who are generally recommended to undergo surgery within 24 hours of admission, and where a large proportion of these patients suffer from cognitive impairment. The cluster-randomized design also makes the study robust to unforeseen events (e.g., the Covid-19 pandemic).
The participating departments are recommended not to change routines (i.e., prophylactic antibiotic regimes, waiting time for surgery, experience of the treating surgeon); however, such changes could be detected in the SFR and SAR. During the crossover between the intervention and control there could be patients receiving the “wrong” type of cement at the start of each cluster period. However, the large sample size should counteract unintended crossover effects. The linkage to SAR will help ensure that the type of cement used is correct.
In summary, this cluster-randomized study with its register-based nationwide sample and pragmatic design investigates the use of DIAC in patients undergoing primary HA for a displaced FNF.
Reference
- Bhandari M, Swiontkowski M. Management of acute hip fracture. N Engl J Med 2017; 377(21): 2053-62. doi: 10.1056/NEJMcp1611090.
- Sundkvist J, Bruggeman A, Sayed-Noor A, Moller M, Wolf O, Mukka S. Epidemiology, classification, treatment, and mortality of adult femoral neck and basicervical fractures: an observational study of 40,049 fractures from the Swedish Fracture Register. J Orthop Surg Res 2021; 16(1): 561. doi: 10.1186/s13018-021-02701-1.
- Swedish Hip Arthroplasty Register. Annual Report 2021; 2022 [cited 2022]. Available from: https://registercentrum.blob.core.windows.net/slr/r/Svenska-Ledprotesregistret-Arsrapport-2021-HJljVnlWvF.pdf.
- Parker M J, Cawley S. Cemented or uncemented hemiarthroplasty for displaced intracapsular fractures of the hip: a randomized trial of 400 patients. Bone Joint J 2020; 102-B(1): 11-16. doi: 10.1302/0301-620X.102B1.BJJ-2019-1041.R1.
- Viberg B, Pedersen A B, Kjaersgaard A, Lauritsen J, Overgaard S. Risk of mortality and re-operation in hip fracture patients undergoing cemented versus uncemented hemiarthroplasty: a population-based study from Danish National Registries. Bone Joint J 2022; 104-B(1): 127-33. doi: 10.1302/0301-620X.104B1.BJJ-2021-0523.R1.
- Fernandez M A, Achten J, Parsons N, Griffin X L, Png M E, Gould J, et al. Cemented or Uncemented hemiarthroplasty for intracapsular hip fracture. N Engl J Med 2022; 386(6): 521-30. doi: 10.1056/NEJMoa2108337.
- Edwards C, Counsell A, Boulton C, Moran C G. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br 2008; 90(6): 770-7. doi: 10.1302/0301-620X.90B6.20194.
- Guren E, Figved W, Frihagen F, Watne L O, Westberg M. Prosthetic joint infection: a devastating complication of hemiarthroplasty for hip fracture. Acta Orthop 2017; 88(4): 383-9. doi: 10.1080/17453674.2017.1301009.
- Noailles T, Brulefert K, Chalopin A, Longis P M, Gouin F. What are the risk factors for post-operative infection after hip hemiarthroplasty? Systematic review of literature. Int Orthop 2016; 40(9): 1843-8. doi: 10.1007/s00264-015-3033-y.
- Voigt J, Mosier M, Darouiche R. Systematic review and meta-analysis of randomized controlled trials of antibiotics and antiseptics for preventing infection in people receiving primary total hip and knee prostheses. Antimicrob Agents Chemother 2015; 59(11): 6696-707. doi: 10.1128/AAC.01331-15.
- Zhang J, Zhang X Y, Jiang F L, Wu Y P, Yang B B, Liu Z Y, et al. Antibiotic-impregnated bone cement for preventing infection in patients receiving primary total hip and knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2019; 98(49): e18068. doi: 10.1097/MD.0000000000018068.
- Swedish Hip Arthroplasty Register. Annual Report 2019; 2020 [cited 2022 Mar 06]. Available from: https://registercentrum.blob.core.windows.net/shpr/r/-rsrapport_H-ftprotes_2019_pdf_FINAL-B1lgibPvrv.pdf.
- Sprowson A P, Jensen C, Chambers S, Parsons N R, Aradhyula N M, Carluke I, et al. The use of high-dose dual-impregnated antibiotic-laden cement with hemiarthroplasty for the treatment of a fracture of the hip: the Fractured Hip Infection trial. Bone Joint J 2016; 98-B(11): 1534-41. doi: 10.1302/0301-620X.98B11.34693.
- Ensing G T, van Horn J R, van der Mei H C, Busscher H J, Neut D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin Orthop Relat Res 2008; 466(6): 1492-8. doi: 10.1007/s11999-008-0203-x.
- Thornes B, Murray P, Bouchier-Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg Br 2002; 84(5): 758-60. doi: 10.1302/0301-620x.84b5.11907.
- Tyas B, Marsh M, Oswald T, Refaie R, Molyneux C, Reed M. Antibiotic resistance profiles of deep surgical site infections in hip hemiarthroplasty; comparing low dose single antibiotic versus high dose dual antibiotic impregnated cement. J Bone Jt Infect 2018; 3(3): 123-9. doi: 10.7150/jbji.22192.
- Knutsson S B, Wennergren D, Bojan A, Ekelund J, Moller M. Femoral fracture classification in the Swedish Fracture Register: a validity study. BMC Musculoskelet Disord 2019; 20(1): 197. doi: 10.1186/s12891-019-2579-z.
- Swedish Hip Arthroplasty Register. Annual Report 2018; 2019.
- Ludvigsson J F, Andersson E, Ekbom A, Feychting M, Kim J L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. doi: 10.1186/1471-2458-11-450.
- Lindgren J V, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of re-operations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord 2014; 15: 384. doi: 10.1186/1471-2474-15-384.
- Mellner C, Eisler T, Knutsson B, Mukka S. Early periprosthetic joint infection and debridement, antibiotics and implant retention in arthroplasty for femoral neck fracture. Hip Int 2017; 27(4): 349-53. doi: 10.5301/hipint.5000467.
- Mukka S, Mellner C, Knutsson B, Sayed-Noor A, Skoldenberg O. Substantially higher prevalence of postoperative peri-prosthetic fractures in octogenarians with hip fractures operated with a cemented, polished tapered stem rather than an anatomic stem. Acta Orthop 2016; 87(3): 257-61. doi: 10.3109/17453674.2016.1162898.
- Forbes A B, Akram M, Pilcher D, Cooper J, Bellomo R. Cluster randomised crossover trials with binary data and unbalanced cluster sizes: application to studies of near-universal interventions in intensive care. Clin Trials 2015; 12(1): 34-44. doi: 10.1177/1740774514559610.
- Morgan K E, Forbes A B, Keogh R H, Jairath V, Kahan B C. Choosing appropriate analysis methods for cluster randomised crossover trials with a binary outcome. Stat Med 2017; 36(2): 318-33. doi: 10.1002/sim.7137.
- Agni N R, Costa M L, Achten J, O’Connor H, Png M E, Peckham N, et al. A randomized clinical trial of low dose single antibiotic-loaded cement versus high dose dual antibiotic-loaded cement in patients receiving a hip hemiarthroplasty after fracture: a protocol for the WHiTE 8 COPAL study. Bone Jt Open 2021; 2(2): 72-8. doi: 10.1302/2633-1462.22.BJO-2020-0174.
- Mukka S, Sjoholm P, Chammout G, Kelly-Pettersson P, Sayed-Noor A S, Skoldenberg O. External validity of the HOPE-Trial: hemiarthroplasty compared with total hip arthroplasty for displaced femoral neck fractures in octogenarians. JB JS Open Access 2019; 4(2): e0061. doi: 10.2106/JBJS.OA.18.00061.
- Moller M, Wolf O, Bergdahl C, Mukka S, Rydberg E M, Hailer N P, et al. The Swedish Fracture Registe: ten years of experience and 600,000 fractures collected in a National Quality Register. BMC Musculoskelet Disord 2022; 23(1): 141. doi: 10.1186/s12891-022-05062-w.
- Wennergren D, Ekholm C, Sandelin A, Moller M. The Swedish fracture register: 103,000 fractures registered. BMC Musculoskelet Disord 2015; 16: 338. doi: 10.1186/s12891-015-0795-8.
Supplementary data