The learning curve of the direct anterior approach is 100 cases: an analysis based on 15,875 total hip arthroplasties in the Dutch Arthroplasty Register
Rinne M PETERS 1,2, Bas L E F TEN HAVE 3, Kyrill RYKOV 3, Liza VAN STEENBERGEN 4, Hein PUTTER 5, Marijn RUTGERS 6, Stan VOS 7, Bob VAN STEIJNEN 8, Rudolf W POOLMAN 9,10, Stephan B W VEHMEIJER 11, and Wierd P ZIJLSTRA 1
1 Department of Orthopedic Surgery, Medical Center Leeuwarden; 2 Department of Orthopedic Surgery, University Medical Center Groningen; 3 Department of Orthopedic Surgery, Martini Hospital, Groningen; 4 Dutch Arthroplasty Register, ‘s Hertogenbosch; 5 Department of Medical Statistics and Bioinformatics Statistics, Leiden University Medical Center; 6 Department of Orthopedic Surgery, HAGA Hospital, The Hague; 7 Department of Orthopedic Surgery, Noordwest Ziekenhuisgroep, Alkmaar; 8 Department of Orthopedic Surgery, Antonius ziekenhuis, Sneek; 9 Department of Orthopedic Surgery, OLVG, Amsterdam; 10 Department of Orthopedic Surgery, Leiden University Medical Center; 11 Department of Orthopedic Surgery, Reinier de Graaf, Delft, the Netherlands
Background and purpose — In the last decade, the direct anterior approach (DAA) for total hip arthroplasty (THA) has become more popular in the Netherlands. Therefore, we investigated the learning curve and survival rate of the DAA in primary THA, using data from the Dutch Arthroplasty Register (LROI).
Patients and methods — We identified all patients who received a primary THA using the DAA in several high-volume centers in the Netherlands between 2007 and 2019 (n = 15,903). Procedures were ordered per surgeon, using date of operation. Using the procedure number, operations were divided into 6 groups based on the number of previous procedures per surgeon (first 25, 26–50, 51–100, 101–150, 151–200, > 200). Data from different surgeons in different hospitals was pooled together. Revision rates were calculated using a multilevel time-to-event analysis.
Results — Patients operated on in group 1–25 (hazard ratio [HR] 1.6; 95% CI 1.1–2.4) and 26–50 (HR 1.6; CI 1.1–2.5) had a higher risk for revision compared with patients operated on in group > 200 THAs. Between 50 and 100 procedures the revision risk was increased (HR 1.3; CI 0.9–1.9), albeit not statistically significant. From 100 procedures onwards the HR for revision was respectively 1.0 (CI 0.6–1.6) and 0.8 (CI 0.5–1.4) for patients in operation groups 101–150 and 151–200. Main reasons for revision were loosening of the stem (29%), periprosthetic infection (19%), and dislocation (16%).
Interpretation — We found a 64% increased risk of revision for patients undergoing THA using the DAA for the first 50 cases per surgeon. Between 50 and 100 cases, this risk was 30% increased, but not statistically significant. From 100 cases onwards, a steady state had been reached in revision rate. The learning curve for DAA therefore is around 100 cases.
Citation: Acta Orthopaedica 2022; 93: 775–782. DOI http://dx.doi.org/10.2340/17453674.2022.4802.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-25. Accepted: 2022-08-25. Published: 2022-09-27.
Correspondence: rinnepeters@gmail.com
The authors thank M. Gademan, PhD, statistician of the Leiden University Medical Center (LUMC). for her assistance in performing the statistical analysis.
Authors contributed to (1) study design and study protocol, (2) gathered data, (3) analyzed data, (4) initial draft, and (5) final draft. RMP and WPZ contributed to 1–5; LvS contributed to 2, 3, and 5; HP contributed to 3, 4, and 5 KR; MR, CJV, BvS, RWP, SBWV, and BtH contributed to 1, 4, and 5.
Acta thanks Harald Brismar and Kirill Gromov for help with peer review of this study.
The decision on surgical approach to perform a THA is predominantly determined by the surgeon’s preference and local hospital standards, given the lack of compelling evidence in favor of one approach (1-4).
In the Netherlands, the posterolateral (PL) approach is the most frequently used approach (50% in 2020) for primary THA. However, in the last decade the direct anterior approach (DAA) has become more popular, while the use of direct lateral (DL) and anterolateral approaches diminished. In 2010, the DAA was used in 5% of cases compared with 41% in 2020 (5). A similar trend was seen in Norway, where the PL approach is most frequently used (72%), with a gradual increase in the DAA over the last 5 years (9.3% in 2021). In contrast, in Sweden the PL (59%) and DL in lateral (32%) or supine (8.4%) position have been dominant since 2005 (6-7).
Potential benefits of the DAA include early mobilization, reduced postoperative length of stay, and low dislocation rates (1-2,8). Conflicting data exists as to whether this technique is associated with a lower risk of revision due to dislocation and whether it is muscle-sparing (3,9-11). However, the approach is technically demanding with a significant learning curve even for experienced surgeons (12-15). Furthermore, DAA seems to be associated with increased risk of femoral stem revision (e.g., loosening and periprosthetic femoral fracture [PFF]) in the medium term (9,16-18). Lastly, femoral exposure might be difficult, and damage to the lateral femoral cutaneous nerve (LFCN) has been described (19-20).
For surgeons considering a switch to DAA, it is important to know how long their expected learning curve will be before reaching a steady state. There is no consensus on the length of this learning curve for experienced surgeons. Therefore, we investigated the learning curve and survival rate of the DAA using LROI data.
Patients and methods
Data collection
The LROI collects data on all patients undergoing THA in the Netherlands since 2007. The LROI covers all Dutch hospitals, which has resulted in a completeness of > 98% for primary THAs since 2010, with high validity of the data (5,21). As surgeons are blinded in the LROI and the number of surgeons per hospital cannot be assessed, we were unable to assess the learning curve for the DAA on a nationwide scale. Therefore, we started a collaboration with 6 high-volume DAA arthroplasty hospitals in the Netherlands, so individual surgeon data could be unblinded. All primary THAs implanted via DAA in these 6 centers between 2007 and 2019 were included (n = 15,903). Other approaches (posterolateral, anterolateral, DL), hip hemi-arthroplasties, hip resurfacing arthroplasties, metal-on-metal THA, and revision procedures were excluded.
Patient characteristics, procedure information, and implant details were retrieved from the register. Data from the LROI are matched with the national insurance database on healthcare (22), to obtain information on the vital status.
Learning curve
To assess the learning curve for the DAA, revision rates were calculated for the different phases of the learning curve. Within the 6 hospitals all procedures were ordered per surgeon, using the date of operation. Procedures were divided into 6 groups based on the number of previous procedures per surgeon (1–25, 26–50, 51–100, 101–150, 151–200, and > 200). Thereafter, data from different surgeons and hospitals was pooled; all THAs performed within the first 25 procedures per surgeon were pooled together. The other groups (26–50, 51–100, 101–150, 151–200, and > 200) were created similarly. Revision rates for the different groups were compared in order to assess the learning curve and survival rate of the DAA.
During the data exploration we noticed that in all centers there were surgeons with only a few procedures, which could possibly be the result of registration errors. Therefore, THAs performed by surgeons with < 10 procedures were excluded to increase the reliability of the data. Subsequently, the total number of procedures was 15,875.
Statistics
Survival time was calculated as the time from primary THA to first revision arthroplasty for any reason, death of the patient, or January 1, 2020 (end of follow-up). A revision arthroplasty is defined as any change (insertion, replacement, or removal) of 1 or more components. Standard survival analysis treats death simply as censored information but this approach over-estimates revision rates (23). Therefore, the crude cumulative incidence of revision was calculated using competing risk analyses where death was considered to be a competing risk. Crude revision percentages within 5 and 9 years were estimated according to the different operation groups. Differences in revision rates were compared.
Furthermore, we had to take into account the nested structure of the dataset. Each patient belongs to 1 of the 6 hospitals. In theory, differences between patient outcomes could in fact be attributable to differences between the various hospitals. To correct for this random center effect and account for correlation among patients within hospitals, a 2-level frailty model was used. According to the hierarchical structure of the data, patients were defined as level 1, and hospitals as level 2, and a multilevel Cox regression analysis (time-to-event analysis) was performed (24,25). Specifically, a gamma frailty model with frailty effects for hospitals was fit (26). The model was adjusted for age, sex, ASA score, BMI, fixation type, and femoral head size. P-values below 0.05 were considered statistically significant. For the 95% confidence intervals (CI), we assumed that the number of observed cases followed a Poisson distribution. Data analysis was performed in an unblinded manner. Analyses were performed using SPSS 22.0 (IBM Corp, Armonk, NY, USA) and R statistics (R Foundation for Statistical Computing, Vienna, Austria.
Ethics, funding, and potential conflicts of interests
The study was approved by the board and scientific advisory committee of the LROI and the Medical Ethics Committee of the University Medical Center Groningen (no. METc 2021/280). The dataset was processed in compliance with the regulations of the LROI governing research on registry data. Data from the LROI cannot be shared due to data privacy policy regulations. No funding was received. No conflicting interests were declared.
Results
15,875 THAs were included in the analysis (Table 1, see Supplementary data). The mean follow up was 4.0 years (maximum 11.5 years). The THAs were performed by 43 different orthopedic surgeons across the 6 hospitals (respectively 14, 15, 4, 4, 4, and 2 per hospital). The number of procedures per hospital varied from 881 to 6,128 (Table 2). 43 different surgeons were included in the 1–25 procedure group, while 19 surgeons were present in the > 200 subgroup (Table 3, see Supplementary data).
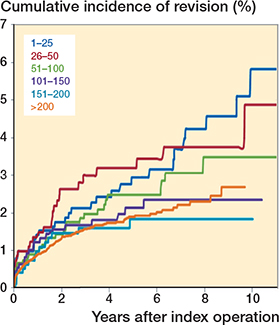
Cumulative incidence of revision according to operation group after primary THA using the direct anterior approach (n = 15,875).
Overall crude cumulative incidence of revision
297 THAs were revised (1.9%). The overall 5-year revision rate for all causes for the first 25 procedures per surgeon was 2.9% (CI 2.0–4.4) (if surgeons with < 10 cases are included as well, the 5-year revision rate rises to 3.4%). At 5 years, a lower cumulative incidence of revision was found when the surgeon had performed 151–200 (1.8%; CI 1.1–3.1) or more than 200 (1.9%; CI 1.6–2.2) previous DAA THAs. At 9 years, the crude revision rate was lower when the surgeon had performed 101–150 (2.3%; 1.5–3.6), 151–200 (1.8%; CI 1.1–3.1) or > 200 (2.7%; CI 2.1–3.5) THAs compared with first (1–25) and second 25 (26–50) cases (respectively 5.6%; CI 3.1–6.7 and 3.7%; CI 2.5–5.6) (Table 4, Figure). However, none of these differences were statistically significant.
Reason for revision
The most common reason for revision was loosening of the femoral component (29%), followed by periprosthetic infection (19%), dislocation (16%), and periprosthetic fracture (14%). In the early (< 25) phase of the learning curve, femoral loosening (33%) and periprosthetic fractures (15%) were the 2 most common revision causes; in the 100–200 phase, these were periprosthetic fracture (29%) and infection (29%), and in the final phase (> 200) femoral loosening (28%) and infection (22%). However, there were no statistically significant differences in reasons for revision between the groups (Table 5)
| Operation groups | |||||||
| 1–25 | 26–50 | 51–100 | 101–150 | 151–200 | >200 | Total | |
| Revised | 33 (3.2) | 26 (3.1) | 33 (2.4) | 21 (1.9) | 15 (1.5) | 169 (1.6) | 297 (1.9) |
| Indication for revision a | |||||||
| Loosening of stem | 11 | 11 | 7 | 4 | 6 | 48 (28) | 87 (29) |
| Infection | 3 | 4 | 3 | 6 | 3 | 37 (22) | 56 (19) |
| Dislocation | 4 | 4 | 4 | 4 | 0 | 30 (18) | 46 (16) |
| Periprosthetic fracture | 5 | 4 | 4 | 6 | 2 | 20 (12) | 41 (14) |
| Loosening of cup | 3 | 4 | 4 | 1 | 3 | 15 (8.9) | 30 (10) |
| Girdlestone | 1 | 1 | 0 | 1 | 1 | 13 (7.7) | 17 (5.7) |
| Cup/liner wear | 0 | 0 | 1 | 0 | 0 | 5 (3.0) | 6 (2.0) |
| Peri-articular ossification | 0 | 0 | 2 | 0 | 0 | 2 (1.2) | 4 (1.3) |
| Other | 7 | 5 | 9 | 4 | 2 | 26 (15) | 53(18) |
| a A patient may have more than 1 reason for revision or reoperation. | |||||||
Overall adjusted multilevel analysis
Multivariable survival analysis demonstrated that patients in groups 1–25 and 26–50 had a higher risk of revision compared with patients in group > 200 (respectively HR 1.6; CI 1.1–2.4 and 1.6; CI 1.1–2.5). When orthopedic surgeons had performed more than 50 DAA THAs, the risk of revision was 26% higher (compared with > 200 group; HR 1.3; CI 0.9–1.9), albeit not statistically different. Above 100 procedures the hazard risk for revision further dropped to respectively 0.98 (CI 0.6–1.6) and 0.8 (CI 0.5–1.4) for patients in groups 101–150 and 151–200 (Table 6).
| Operation groups (procedures) | Hazard ratio (95% CI) a |
| 1–25 | 1.6 (1.1–2.4) b |
| 26–50 | 1.6 (1.1–2.5) b |
| 51–100 | 1.3 (0.9–1.9) |
| 101–150 | 1.0 (0.6–1.6) |
| 151–200 | 0.8 (0. –1.4) |
| > 200 | 1.0 (reference) |
| a Adjusted for age, sex, ASA score, BMI, head size, fixation method, and hospital in which the operation was performed. | |
| b p < 0.05. | |
Furthermore, a statistically lower risk of revision was found for patients with a normal BMI (18.5–25) compared with patients with mild obesity (HR 0.7; CI 0.5–1.0), while severe obesity resulted in a 19% increased risk of revision (HR 1.2; CI 0.3–4.8). However, the latter was not statistically significant, perhaps due to low numbers (n = 94) (data not shown).
Lastly, small femoral head components (22–28 mm) resulted in an increased risk of revision compared with 32 mm heads (HR 1.6; CI 1.0–2.4). Reversed hybrid fixation technique (cemented cup with uncemented stem) also increased the risk of revision (HR 1.9; 1.1–3.1) compared with cementless fixation (data not shown).
Discussion
We found a 64% increased risk of revision for patients undergoing THA using the DAA for the first 50 cases per surgeon. Between 50 and 100 cases, this risk was around 30% increased, but not statistically different. From 100 cases onwards, a steady state had been reached in the revision rate. The learning curve for the DAA THA therefore is around 100 cases.
Previous literature
In general, the learning curve for surgical procedures can be defined as the number of times a task must be repeated before a steady state of outcome is reached (27).
During this learning curve, extra time, attention, and the support of an experienced surgeon may be needed to prevent early complications during this transition. After gaining experience, a reduction in operation time, perioperative blood loss, and a lower risk of complications were described (4). Our findings are in accordance with the literature. De Steiger et al. (14) found that 50 or more procedures should be performed by a surgeon before the rate of revision is not different from a surgeon performing 100 or more anterior THAs. That was based on results from 5,499 THAs registered in the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). A systematic review assessing the learning curve, safety, and accuracy of the DAA demonstrated a reduction in operation time and fluoroscopy time after the first 100 THAs (28). This was in accordance with findings of Nairn et al. (4). Their systematic literature review revealed a substantial learning curve for the DAA and a relative plateau in mean operation time after 100 cases.
Adequate positioning of THA components is important to reduce the risk of instability, component wear and impingement, resulting in dislocation or revision surgery (4). For surgeons switching from PL to DAA, changing from lateral decubitus into supine position could result in difficulties in regard to implant positioning during the early phases of the learning curve of the DAA (e.g., excessive acetabular anteversion or malpositioning of the stem). Kobayashi et al. (29) investigated implant positioning in the first 80 consecutive THA cases performed using the DAA with fluoroscopic assistance in comparison with their previous 80 THAs placed via the posterolateral approach. In the DAA group, the femoral component was more frequently positioned in flexion (than neutral) compared with the PL approach, although using fluoroscopy seemed to decrease the complications such as PFF during these first cases. Other studies described an increased risk of stem revision (for PFF or loosening) in patients operated on through the DAA compared with the posterolateral approach (16,30). A possible explanation is that surgical exposure of the femur is more complex with the DAA than with the PL approach. However, our data did not demonstrate an increased risk for femoral-sided complications during the first stages of the learning curve, possibly due to small numbers.
Case-mix and surgically modifiable factors
A high percentage of our patients were aged < 75 years (72%), female (68%), and had a low ASA score (I–II in 87%) and BMI (< 30 in 79%) compared with the total population of THA patients in the Netherlands (5). The adjusted analysis demonstrated a trend towards a lower risk of revision in patients with a normal BMI. This was consistent with previous findings in the general population (all approaches) where severe obesity and a high ASA score were identified as the strongest predictors for short-term revision after primary THA (31).
Therefore, it might be wise to take this into account during the first stages of the learning curve (e.g., perform the operation on patients with an acceptably low ASA without [severe] obesity).
As described, we included ASA score, BMI, fixation technique, age, and sex in our analysis as confounding variables since they might result in an increased risk for revision due to specific reasons. For example, there may be more revisions due to a PFF for uncemented THAs or more revisions due to a periprosthetic infection in patients with a high ASA score or severe obesity (31).
For the surgically modifiable factors, we found a higher risk of revision in patients with a small femoral head. This was in accordance with previous studies. Over the years (and thus learning curve), trends towards the use of larger femoral heads have resulted in improved survival rates. For example, a 60% increased risk of revision due to dislocation for small femoral heads (22–28 mm) compared to 32 mm femoral heads was demonstrated previously (9).
Overall, the most frequently used bearing type was metal-on-polyethylene (39%) with some variation across the groups. In the > 200 procedures subgroup, ceramic-on-polyethylene and ceramic-on-ceramic were relatively more frequently registered compared with the 0–25 subgroup (31–35% and 16–28% respectively) (Table 1, see Supplementary data). These findings are in line with the trend toward increased usage of larger femoral heads, cementless fixation, and advanced bearing surfaces over the past decade (9,32).
Limitations
There are some notable limitations to this study. Our data does not contain information on early postoperative complications that did not result in revision surgery such as dislocation and closed reduction, PFF in need of open reduction and internal fixation, or DAIR procedures without a component exchange. In addition, other perioperative parameters could not be monitored, e.g., duration of procedure, surgical instruments, component position, length of stay, opioid use or perioperative pain, occurrence of LFCN palsy, and intraoperative blood loss. Furthermore, the known limitations and risks of bias for observational studies are present for this study. Moreover, this data reflects the experience of 6 (high volume) hospitals, limiting the overall generalizability of the results. Nonetheless, the number of cases in these 6 ‘early adaptor’ centers make up 32% of the nationwide number of DAA THAs in the LROI. Furthermore, the surgical experience of the surgeon at the start of the learning curve is unknown. In our experience, there might be a difference between ‘first- and second-generation’ surgeons. Second-generation surgeons might have a shorter learning curve due to the fact that they have been taught about pearls and pitfalls by first-generation surgeons. In addition, it is not known if the operation was performed by an orthopedic surgeon or a resident. However, recent literature suggests that complication rates, revision percentages, and mortality rates are similar in THAs performed by a resident as primary surgeon compared with surgeries performed by an orthopedic surgeon (33).
Implications for further research
Future studies should focus on process assessment of learning curves when introducing new surgical approaches. Process assessment is supplementary to outcome assessment learning curve studies and has the benefit of needing a smaller sample size. This reduces the potential hazard to patients when introducing a new surgical technique. Given the low quality of previous studies, future studies should focus on learning curves and outcome relevant for patients, comparing new surgical techniques such as direct superior approach (34). In addition, future studies could focus on the learning curve of second-generation surgeons, who might have a shorter learning curve because they are supported by more experienced DAA surgeons (35). In our data, 43 different surgeons were encountered in the 1–25 group, while for the > 200 subgroup 19 surgeons were present. We could only speculate on the reason why surgeons may have “given up.” This could be for a multitude of reasons, e.g., simply because they have not reached the next phase of the learning curve at the time of data collection, or departure to another hospital (which might not be included in our data selection, e.g., private practice), or end of residency.
Implications for clinical practice
Patients and surgeons should discuss the need for shifting toward a new surgical approach given the risk a learning curve yields to patients. Given the lack of clear superior results of one surgical approach over the other for THA, and the increased risk (HR 1.6) of revision surgery (and hence complications) during the first 50 (to 100) patients when mastering the DAA, one should question the true need to switch surgical approach. Based on the latest LROI data, the all-cause survival rates of the DAA, posterolateral, direct lateral, and anterolateral approaches are all within 96–97% at 9-year follow-up, and the absolute differences in PROM improvement between approaches are only small (36). These data should be balanced against the increased risks to patients during the DAA learning curve. If a switch to DAA is chosen, some authors suggest this should be reserved for well-trained, high-volume surgeons and centers, perhaps avoiding very muscular and morbidly obese patients (37). Furthermore, when a switch in surgical approach is deliberated, one could consider training the DAA when in residency (35), following a dissection course, performing a number of procedures together with an experienced surgeon or the use of intraoperative fluoroscopy assistance. An appropriate amount of time should be planned, and the changes should be limited to the approach (in other words, do not simultaneously switch to a different prosthesis design as well). Lastly, the rest of the OR team (nurses, anesthesia staff) should be involved in the decision to change the approach.
In conclusion, using Dutch arthroplasty register data we found a clear learning curve for the DAA in THA. There was a significantly increased risk of revision for the first 50 cases per surgeon. Between 50 and 100 cases, the risk was still slightly elevated. From 100 cases onwards, a steady state had been reached. We therefore estimate the learning curve for direct anterior approach THA to be around 100 cases. Orthopedic surgeons can use these results in addition to the existing literature, when making decisions whether to use or to make the transition to the DAA.
Reference
- Amlie E, Havelin L I, Furnes O, Baste V, Nordsletten L, Hovik O, et al. Worse patient-reported outcome after lateral approach than after anterior and posterolateral approach in primary hip arthroplasty: a cross-sectional questionnaire study of 1,476 patients 1–3 years after surgery. Acta Orthop 2014; 85(5): 463-9.
- Higgins B T, Barlow D R, Heagerty N E, Lin T J. Anterior vs. posterior approach for total hip arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015; 30(3): 419-34.
- Huerfano E, Bautista, M, Huerfano M, Nossa J M. Use of surgical approach is not associated with instability after primary total hip arthroplasty: a meta-analysis comparing direct anterior and posterolateral approaches. J Am Acad Orthop Surg 2021; 29(22): e1126-e1140.
- Nairn L, Gyemi L, Gouveia K, Ekhtiari S, Khanna V. The learning curve for the direct anterior total hip arthroplasty: a systematic review. Int Orthop 2021; 45: 1971–82.
- LROI report. Annual report Dutch Arthroplasty Register; 2021.
- Norwegian Arthroplasty Register. Report; 2021.
- Swedish Arthroplasty Register. Annual report; 2021.
- Graves S C, Dropkin B M, Keeney B J, Lurie J D, Tomek I M. Does surgical approach affect patient-reported function after primary THA? Clin Orthop Relat Res 2016; 474(4): 971-81.
- Zijlstra W P, De Hartog B, Van Steenbergen L N, Scheurs B W, Nelissen R G H H. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty, Acta Orthop 2017; 88 (4): 392-401.
- Aggarwal V K, Elbuluk A, Dundon J, Herrero C, Hernandez C, Vigdorchik J M, et al. Surgical approach significantly affects the complication rates associated with total hip arthroplasty. Bone Joint J. 2019; 101-B(6): 646-51.
- Rykov K, Meys T W G M, Knobben B A S, Sietsma M S, Reininga I H F, Ten Have B L E F. MRI assessment of muscle damage after the posterolateral versus direct anterior approach for THA (Polada Trial): a randomized controlled trial. J Arthroplasty 2021; 36(9): 3248-58.e1.
- Goytia R N, Jones L C, Hungerford M W. Learning curve for the anterior approach total hip arthroplasty. J Surg Orthop Adv 2012; 21: 78–83.
- Muller D A, Zingg P O, Dora C. Anterior minimally invasive approach for total hip replacement: five-year survivorship and learning curve. Hip Int 2014; 24: 277-83.
- De Steiger R N, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 2015; 473: 3860-6.
- Den Hartog Y M, Mathijssen N M, Vehmeijer S B. The less invasive anterior approach for total hip arthroplasty: a comparison to other approaches and an evaluation of the learning curve—a systematic review. Hip Int 2016; 26(2): 105-20.
- Hoskins W, Bingham R, Lorimer M, Hatton A, de Steiger R N. Early rate of revision of total hip arthroplasty related to surgical approach: an analysis of 122,345 primary total hip arthroplasties. J Bone Joint Surg Am 2020; 102(21): 1874-82.
- Australian Orthopaedic Association National Joint Replacement Registry Annual report. Adelaide: AOA; 2021.
- Janssen L, Wijnands K A P, Janssen D, Janssen M W H E, Morrenhof J W. Do stem design and surgical approach influence early aseptic loosening in cementless THA? Clin Orthop Relat Res 2018; 476(6): 1212-20.
- Goulding K, Beaule P E, Kim P R, Fazekas A. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res 2010; 468(9): 2397-404.
- Dahm F, Aichmair A, Dominkus M, Hofstaetter J G. Incidence of lateral femoral cutaneous nerve lesions after direct anterior approach primary total hip arthroplasty: a literature review. Orthop Traumatol Surg Res 2021; 107(8): 102956.
- van Steenbergen L N, Denissen G A, Spooren A, van Rooden S M, van Oosterhout F J, Morrenhof J W, et al. More than 95% completeness of reported procedures in the population-based Dutch Arthroplasty Register. Acta Orthop 2015; 86(4): 498-505.
- Vektis. Available from: http://www.vektis.nl; 2018.
- Lacny S, Wilson T, Clement F, Roberts D J, Faris P D, Ghali W A, Marshall D A. Kaplan–Meier survival analysis overestimates the risk of revision arthroplasty: a meta-analysis. Clin Orthop Relat Res 2015; 473 (11): 3431-42.
- Yau K. Multilevel models for survival analysis with random effects. Biometrics 2001; 57(1): 96-102.
- Singer J D, Willet J B. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press; 2003. Chapter 15.
- Balan T A, Putter H. A tutorial on frailty models. Stat Methods Med Res 2020; 29(11): 3424-54.
- van Oldenrijk J, Schafroth M U, Bhandari M, Runne W C, Poolman R W. Time-action analysis (TAA) of the surgical technique implanting the Collum Femoris Preserving (CFP) hip arthroplasty. TAASTIC trial identifying pitfalls during the learning curve of surgeons participating in a subsequent randomized controlled trial (an observational study). BMC Musculoskelet Disord 2008; 9: 93.
- Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics 2008; 31(12 Suppl. 2): orthosupersite.com/view.asp?rID=37187.
- Kobayashi H, Homma Y, Baba T, Ochi H, Matsumoto M, Yuasa T, et al. Surgeons changing the approach for total hip arthroplasty from posterior to direct anterior with fluoroscopy should consider potential excessive cup anteversion and flexion implantation of the stem in their early experience. Int Orthop 2016; 40(9): 1813-19.
- Angerame M R, Fehring T K, Masonis J L, Mason J B, Odum S M, Springer B D. Early failure of primary total hip arthroplasty: is surgical approach a risk factor? J Arthroplasty 2018; 33(6): 1780-5.
- Peters R M, van Steenbergen L N, Stewart R E, Stevens M, Rijk P C, Bulstra S K, Zijlstra W P. Patient characteristics influence revision rate of total hip arthroplasty: American Society of Anesthesiologists score and body mass index were the strongest predictors for short-term revision after primary total hip arthroplasty. J Arthroplasty 2020; 35(1): 188-92.
- Peters R M, van Steenbergen L N, Stevens M, Rijk P C, Bulstra S K, Zijlstra W P. The effect of bearing type on the outcome of total hip arthroplasty. Acta Orthop 2018; 89(2): 163-9.
- Bron D M, Wolterbeek N, Poolman R W, Kempen D H R, Delawi D. Resident training does not influence the complication risk in total knee and hip arthroplasty Acta Orthop 2021; 92(6): 689-94.
- Duijnisveld B J, van den Hout J A A M, Wagenmakers R, Koenraadt K L M, Bolder S B T. No learning curve of the direct superior approach in total hip arthroplasty. Orthop Surg 2020; 12(3): 852-60.
- Müller D A, Zingg P O, Dora C. Anterior minimally invasive approach for total hip replacement: five-year survivorship and learning curve. Hip Int 2014; 24(3): 277-83.
- Peters R M, van Beers L W A H, van Steenbergen L N, Wolkenfelt J, Ettema H B, Ten Have B L E F, et al. Similar superior patient-reported outcome measures for anterior and posterolateral approaches after total hip arthroplasty: postoperative patient-reported outcome measure improvement after 3 months in 12,774 primary total hip arthroplasties using the anterior, anterolateral, straight lateral, or posterolateral approach. J Arthroplasty 2018; 33(6): 1786-93.
- Hallert O, Li Y, Brismar H, Lindgren U. The direct anterior approach: initial experience of a minimally invasive technique for total hip arthroplasty. J Orthop Surg Res 2012; 7: 17.
Supplementary data
| n (%) | Operation groups | ||||||
| 1–25 | 26–50 | 51–100 | 101–150 | 151–200 | >200 | Total | |
| 1,024 (6.5) | 827 (5.2) | 1,390 (8.8) | 1,111 (7.0) | 1,005 (6.3) | 10,518 (66.3) | 15,875 | |
| Age b | |||||||
| < 60 | 164 (16) | 134 (16) | 232 (17) | 189 (17) | 161 (16) | 1,984 (19) | 2,864 (18) |
| 60–74 | 554 (54) | 463 (56) | 744 (54) | 608 (55) | 520 (52) | 5,627 (54) | 8,516 (54) |
| ≥ 75 | 304 (30) | 230 (28) | 414 (30) | 313 (28) | 323 (32) | 2,907 (28) | 4,491 (28) |
| Sex | |||||||
| Male | 344 (34) | 260 (31) | 460 (33) | 373 (34) | 311 (31) | 3,569 (34) | 5,317 (34) |
| Female | 674 (66) | 566 (69) | 930 (67) | 736 (66) | 694 (69) | 6,947 (66) | 10,547 (66) |
| ASA score b | |||||||
| I | 248 (25) | 192 (24) | 313 (23) | 283 (26) | 257 (26) | 2,636 (25) | 3,929 (25) |
| II | 591 (60) | 519 (65) | 847 (62) | 656 (60) | 571 (58) | 6,418 (62) | 9,602 (62) |
| III–IV | 144 (15) | 83(11) | 197 (15) | 153 (14) | 155 (16) | 1,356 (13) | 2,088 (13) |
| Diagnosis | |||||||
| OA | 947 (94) | 762 (93) | 1,287 (93) | 1,034 (94) | 921 (92) | 9,740 (93) | 14,691 (93) |
| Non-OA | 60 (6.0) | 58 (7.1) | 98 (7.1) | 67 (6.1) | 76 (7.6) | 744 (7.1) | 1,103 (7.0) |
| Previous operation a | |||||||
| Yes | 12 (1.2) | 12 (1.5) | 28 (2.0) | 27 (2.5) | 24 (2.5) | 241 (2.3) | 344 (2.2) |
| No | 1,002 (98) | 807 (99) | 1,351 (98) | 1,072 (98) | 935 (98) | 10,142 (98) | 15,309 (98) |
| Smoking status | |||||||
| Yes | 67 (10) | 54 (11) | 125 (14) | 83 (12) | 92 (12) | 961 (11) | 1,382 (11) |
| No | 584 (90) | 441 (89) | 741 (86) | 635 (88) | 666 (88) | 7,689 (89) | 10,756 (89) |
| BMI | |||||||
| ≤ 18.5 | 5 (0.8) | 2 (0.4) | 6 (0.7) | 1 (0.1) | 9 (1.2) | 72 (0.8) | 95 (0.8) |
| > 18.5–25 | 211 (33) | 189 (38) | 312 (36) | 244 (34) | 262 (34) | 3,127 (36) | 4,345 (36) |
| > 25–30 | 287 (45) | 225 (45) | 373 (44) | 317 (44) | 337 (44) | 3,713 (42) | 5,252 (43) |
| > 30–40 | 130 (20) | 81 (16) | 160 (19) | 146 (20) | 162 (21) | 1,769 (20) | 2,448 (20) |
| > 40 | 3 (0.5) | 1 (0.2) | 6 (0.7) | 9 (1.3) | 3 (0.4) | 72 (0.8) | 94 (0.8) |
| Charnley score a | |||||||
| A | 286 (47) | 224 (47) | 375 (47) | 301 (44) | 276 (41) | 3,370 (41) | 4,832 (42) |
| B1 | 211 (34) | 170 (36) | 267 (33) | 256 (38) | 239 (36) | 2,680 (33) | 3,823 (34) |
| B2 | 104 (17) | 79 (17) | 147 (18) | 113 (17) | 148 (22) | 1,941 (24) | 2,532 (22) |
| C | 13 (2.1) | 6 (1.3) | 11 (1.4) | 10 (1.5) | 7 (1.0) | 187 (2.3) | 234 (2.0) |
| ODEP rating stem | |||||||
| < 5A | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (0.1) | 6 (0.0) |
| ≥ 5A | 1,012 (99) | 819 (99) | 1,380 (99) | 1,100 (99) | 996 (99) | 10,459 (99) | 15,766 (99) |
| Unknown | 12 (1.2) | 8 (1.0) | 10 (0.7) | 11 (1.0) | 9 (0.9) | 53 (0.5) | 103 (0.6) |
| ODEP rating cup a | |||||||
| < 5A | 2 (0.2) | 1 (0.1) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 663 (6.3) | 668 (4.2) |
| ≥ 5A | 1,018 (99) | 825 (100) | 1,389 (100) | 1,110 (100) | 1,001 (100) | 9,820 (93) | 15,163 (96) |
| Unknown | 4 (0.4) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 4 (0.4) | 35 (0.3) | 44 (0.3) |
| Configuration stem a | |||||||
| Anatomic | 683 (67) | 558 (68) | 954 (69) | 823 (74) | 774 (77) | 8,212 (78) | 12,004 (76) |
| Shoulder | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) |
| Other c | 341 (33) | 268 (32) | 436 (31) | 288 (26) | 231 (23) | 2,306 (22) | 3,870 (24) |
| Head size, mm a | |||||||
| 22–28 | 422 (41) | 320 (39) | 458 (33) | 349 (31) | 267 (27) | 2,276 (22) | 4,092 (26) |
| 32 | 366 (36) | 297 (36) | 595 (43) | 462 (42) | 456 (45) | 4,789 (46) | 6,965 (44) |
| 36 | 236 (23) | 210 (25) | 334 (24) | 300 (27) | 282 (28) | 3,452 (33) | 4,814 (30) |
| ≥ 38 | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.0) |
| Fixation a | |||||||
| Cemented | 305 (30) | 242 (29) | 397 (29) | 263 (24) | 210 (21) | 1,959 (19) | 3,376 (21) |
| Cementless | 531 (52) | 470 (57) | 788 (57) | 700 (63) | 663 (66) | 7,390 (70) | 10,542 (67) |
| Reversed hybrid | 167 (16) | 107 (13) | 180 (13) | 138 (12) | 121 (12) | 985 (9.4) | 1,698 (11) |
| Hybrid | 11 (1.1) | 3 (0.4) | 16 (1.2) | 6 (0.5) | 9 (0.9) | 119 (1.1) | 164 (1.0) |
| Unknown | 3 (0.3) | 3 (0.4) | 4 (0.3) | 3 (0.3) | 2 (0.2) | 43 (0.4) | 58 (0.4) |
| Bearing type a | |||||||
| Metal-on-PE | 541 (53) | 388 (47) | 623 (45) | 467 (42) | 411 (41) | 3,786 (36) | 6,216 (39) |
| Cer-on-PE | 314 (31) | 290 (35) | 518 (37) | 437 (39) | 389 (39) | 3,636 (35) | 5,584 (35) |
| Cer-on-cer | 168 (16) | 137 (17) | 241 (17) | 203 (18) | 204 (20) | 2,961 (28) | 3,914 (25) |
| OxZ-on-PE | 0 (0.0) | 12 (1.5) | 7 (0.5) | 2 (0.2) | 1 (0.1) | 135 (1.3) | 157 (1.0) |
| Cer-on-metal | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.0) |
| Period | |||||||
| 2007–2011 | 310 (30) | 228 (28) | 231 (17) | 145 (13) | 144 (14) | 798 (7.6) | 1,856 (12) |
| 2012–2015 | 306 (30) | 249 (30) | 514 (37) | 489 (44) | 454 (45) | 3,870 (37) | 5,882 (37) |
| 2016–2019 | 408 (40) | 350 (42) | 645 (46) | 477 (43) | 407 (41) | 5,850 (56) | 8,137 (51) |
| a p < 0.0001, b p < 0.05. c Missing or cemented. | |||||||
| OA: osteoarthritis, ODEP: Orthopaedic Data Evaluation Panel, PE: polyethylene. Cer: ceramic. | |||||||
| OxZ: oxidized zirconium/oxinium. | |||||||