Calcium phosphate bone cement and metaphyseal corrective osteotomies in the upper extremity: long-term follow-up of 10 children
Mona I WINGE 1,2 and Magne RØKKUM 1,2
1 Division of Orthopaedic Surgery, Oslo University Hospital, Oslo; 2 Institute of Clinical Medicine, University of Oslo, Oslo, Norway
Background and purpose — The evaluation of metaphyseal angular deformities in children includes indication and timing for corrective osteotomy, and possible need for several operations during growth. Gap-fillers are usually autologous bone grafts, which might cause donor site problems. Calcium phosphate (CaP) bone cement may be a possible alternative.
Patients and methods — We performed 15 corrective osteotomies from 2007 to 2013 in 10 children, ages 5 to 18, with Norian SRS bone cement as a gap-filler, in the distal radius (12), proximal radius (1), and proximal humerus (2). Due to growth arrest and gradually increasing malalignments 3/10 children needed 1–3 additional corrections. Locking plates and screws were used except in 1 case at first surgery, aged 5 (K-wires). 2 children needed additional limb lengthening with external fixator.
Results — All osteotomies healed. Postoperative radiographs and CT scans showed good alignment and gradual transformation of cement into bone. Remodeling was visible intraoperatively in patients needing multiple surgeries. Return to earlier osteotomy sites was unproblematic. No adverse events from using CaP cement were experienced.
Interpretation — CaP cement is an alternative to bone grafts in upper extremity metaphyseal corrective osteotomies in children, and also when greater corrections are necessary or several surgeries indicated during the growth period.
Citation: Acta Orthopaedica 2022; 93: 769–774. DOI http://dx.doi.org/10.2340/17453674.2022.4589.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-01-31. Accepted: 2022-09-06. Published: 2022-09-23.
Correspondence: mwinge@ous-hf.no
The study was designed by MW and MR. Data collection, data analysis, writing and editing of the manuscript was done by MW followed by a data review and manuscript editing by MR.
Acta thanks Yrjänä Nietosvaara and other anonymous reviewers for help with peer review of this study.
Malunions combined with physeal injuries in the upper extremity often require corrective surgery during a child’s growth (1). A bone graft is widely accepted for filling voids in opening wedge osteotomies in children (2,3). However, donor site pain, hematoma, infection, nerve injury, and damage to the iliac crest growth plate may cause considerable morbidity (3).
Norian SRS (Skeletal Repair System, Norian Corporation, Cupertino, CA, USA) CaP bone cement (4) may be an alternative to bone graft (5,6). It has physical and biochemical properties comparable to bone (4,7). It is osteoconductive with gradual resorption and replacement by bone (7). FDA approval was given in 1998 with a recommended site of use at metaphyseal level. It has been used in distal radius fractures and as a gap-filler in corrective surgery of sequelae with promising results (6,8). Cement leakage during vertebroplasty has led to cases with fatal outcomes (9,10). Norian SRS was taken off all markets in 2014, keeping its FDA clearance, and later replaced by Norian Drillable (DePuy Synthes, Johnson & Johnson, USA) (personal communication FDA and Johnson & Johnson). It has also been used in pediatric craniofacial and oncological extremity surgery (11-13). Unacceptably high risks of failure have been noted in large (> 25 cm2) full-thickness calvarial reconstructions, as well as problems of infections and fragmentation of the cement (12-14).
We assessed the long-term clinical and radiological results of using CaP cement as a gap-filler in metaphyseal opening wedge osteotomies in the upper extremity of children.
Patients and methods
We included all children ≤ age 18 who presented consecutively to our clinic from 2006 to 2011 with a deformity in the upper extremity in need of metaphyseal corrective surgery (Table). Partial premature closure was present in all post-traumatic deformities. None were candidates for physeal bar resection.
| Case | Sex | Side | Region | Diagnosis | Surgery at initial injury | Years from injury to surgery | Age at surgery | Amount of distraction (mm) | Fixation method | Plate removal | Follow-up (years) |
| 1 | M | Right | Distal radius | Fracture malunion | Fracture reduction + cast; 2 K-wires | 3 | 1) 9 2) 12 3) 14 |
19.0 AC 18.5 AC+D 10.3 AC+D |
LCP locking plate and screws LCP oblique angled locking plate and screws Long locking radius plate and screws |
Yes | 10 |
| 2 | F | Right | Distal radius | Fracture malunion | K-wires | 3 | 1) 5 2) 6 3) 10 4) 13 |
17.2 AC+D 15.4 AC+D 19.2 AC+D 26 callotasis |
2 K-wires 2 oblique angled locking plates and screws (T and L) Locking distal radius plate and screws External fixator |
Yes | 15 |
| 3 | M | Left | Distal radius | Fracture malunion | Fracture reduction + cast; 1 K-wire | 2 | 18 | 6.7 AC+D | LCP locking plate and screws | No | 9 |
| 4 | M | Left | Distal radius | Fracture malunion | – | 7 | 14 | 24.0 AC+D | 2 locking plates and screws | Yes | 6 |
| 5 | F | Left | Distal radius | Fracture malunion | – | 2 | 11 | 16.4 AC+D | 2 locking plates and screws | No | 5 |
| 6 | F | Left | Proximal radius | Fracture malunion | – | 1 | 6 | 6.1 AC | Locking plate and screws | No | 8 |
| 7 | F | Right | Proximal humerus | Perinatal a | – | 5 | 1) 4 2) 9 3) 12 |
19.6 AC 18.2 AC 84 callotasis |
2 locking plates and screws 2 locking plates and screws External fixator |
Yes | 10 |
| 8 | F | Right | Distal radius | Madelung deformity | – | – | 18 | 10.0 AC | LCP locking plate and screws | Yes | 13 |
| 9 | F | Right | Distal radius | Achondro plasia | – | – | 6 | 11.8 AC | Locking plate and screws | No | 5 |
| 10 | M | Right | Distal radius | Solitary cartilaginous exostosis | – | – | 13 | 10.9 AC | Locking distal radius plate and screws | Yes | 6 |
| AC = angular correction; D = distraction. | |||||||||||
| a plexus injury, epiphysiolysis and infection. | |||||||||||
10 children, 6 girls and 4 boys, median 10.5 (5–18) years were primarily operated on between January 2007 and April 2011 with a metaphyseal upper extremity opening wedge corrective osteotomy. 3 patients underwent 5 secondary opening wedge osteotomies between November 2008 and November 2013. Norian SRS was used as the gap-filler (Figure 1). 2 callotasis limb lengthening procedures were started in January and October 2016.
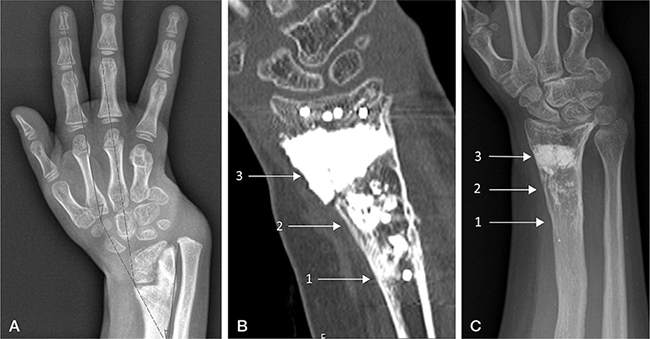
Figure 1. Case 2: (A) Sequela epiphysiolysis distal radius with severe radial deviation at 5 years. (B) CT scan at age 10 after 3rd corrective osteotomy of the distal radius shows three different stages of cement remodeling at 3 levels, depending on time from surgery (arrows). The level of the 1st corrective osteotomy (arrow 1) was most proximal and the 3rd most distal (arrow 3). Most of the CaP cement has remodeled proximally and is barely visible. The cement is fragmented centrally, after the 2nd corrective osteotomy (arrow 2), and much has remodeled into bone, shown as radiolucent broad lines of bone bridges in between radiopaque areas of cement. Distally, at the site of the 3rd correction, the CaP cement is still a uniform radiopaque mass. Initial signs of remodeling are seen in the cortical regions. (C.) Result after distraction lengthening of radius at age 15. The arrows indicate the 3 levels where cement was used, showing the amount of remodeling from initial surgery. Further remodeling has occurred since CT scan done at age 10 and is nearly complete proximally. Ulna plus was –2.1 mm and radial inclination 5.9°.
Preoperative radiological planning included plain radiographs and CT scans of all patients. These detailed the potential growth plate injuries where intraoperative crossing of the epiphysis could be permitted with plates and screws. IV antibiotic prophylaxis was given (cefalotin, Eli Lilly, IN, USA). The metaphyseal osteotomies were performed at point of maximum malalignment. The pasty cement was injected into the void, starting at the bottom and filling the gap to the surface. Complete setting of cement was observed within 10–12 minutes. When angular correction without distraction was needed cortical contact was kept on one side. When combined with distraction in 8 cases, all cortical contact was lost (Table). A cast was applied on all wrists for 8 weeks continued by a removable day splint for an additional 4 weeks during sports activities in 4 cases. The elbow of case 6 was immobilized in a cast for 3 weeks.
A yearly follow-up of patients from first surgery was done for a median 7.5 (5–14) years. No patients were lost to follow-up.
Pre-/postoperatively and at each control we assessed range of motion (ROM) and the presence of pain postoperatively and at last follow-up. Yearly radiographs and CT scans were taken for analysis of alignment during growth and bone bridging, bone healing, and the remodeling of the CaP cement (15). Bone bridging was defined as a line of bone running through the cement and radiological healing as 3 continuous cortical bridges overlapping the osteotomy gap. The images were digitalized and calibrated (PACS—Picture Archiving and Communication System, Sectra, Linköping, Sweden). The distraction in the osteotomy gaps was determined along the cortical border at the site of maximal correction (Table) (6).
Statistics
Given the small sample size we performed no statistical analyses. We present scatterplots with radiographical measurements at 2 timepoints. Data is presented as median (range).
Ethics, funding, and potential conflicts of interest
Written consent was obtained according to the Declaration of Helsinki, and the local ethics committee at Oslo University Hospital, Oslo, Norway approved the study (reference 2012/7042). None of the authors received financial support for this study. The authors declare that they have no conflict of interest.
Results
The patients showed gradual reduction of pain at every checkup. No painkillers were needed after 1–2 weeks, except in Madelung case 8 who experienced new, painful radio-ulnar instability but wished for no further surgery. All were active in their daily life. The median preoperative ROM in the distal radius cases and elbow case was near normal and no patients had reduced ROM after surgery. Shoulder case 7 increased abduction from 30° to 180° and flexion from 30° to 140°. Case 10 developed a forearm compartment syndrome, which at fasciotomy showed no muscle necrosis. Case 4 experienced an extensor tendon rupture 7 years postoperatively after many years of minor extension lag of the 3rd finger.
No signs of inflammation, infection, or other adverse effects potentially related to the CaP cement were seen, including the observations at next surgery. In the 3 cases where multiple corrections were necessary, we observed intraoperatively the macroscopic remodeling of CaP cement (Figure 2), confirming radiographic and CT findings. Cortical bone bridges had created passages through the CaP cement. The sharp borders initially present between bone and CaP cement from the primary corrective surgery had disappeared and remodeled into soft transitions of bony invaginations into the cement. Partial transformation of the cement into bone had occurred.
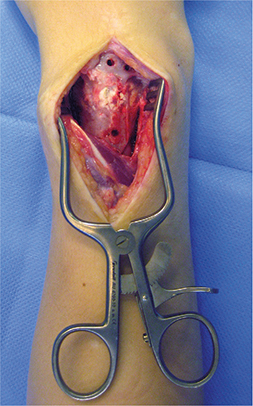
Figure 2. Case 1: Intraoperative findings at time of 2nd corrective surgery. Remodeling of CaP cement with bony ingrowth at level of 1st surgery done 2.8 years earlier. Wide cortical bony bridges are present through the CaP cement. There are signs of resorption of the edges of the cement and replacement by new bone.
6 osteotomies completely healed within 8 weeks. 4 wrists needed 4 extra weeks before continuous cortical bone bridges were visible. Radiographs confirmed the radiopaque nature of the CaP cement. After 2–3 weeks radiographs showed radiolucent lines between the CaP cement and bone, which later filled with bone. CT scans demonstrated the remodeling of cement from an early stage. The radiolucent areas described on radiographs were found to be consistent with new bone formation on CT scans. At a later stage, bone bridging was clearly visible on CT scans, around and through the cement (Figure 1). Signs of healing were first visible at the periphery and later centrally. At different phases of resorption, the CaP cement converts to a gradually fragmented presentation as increasingly wider bone bridges pave through the cement. This continues until the vast majority of cement has remodeled and been replaced by new bone. The first radiological signs of bone bridging through the cement varied from 3 months to 3 years 3 months, dependent on the child’s age.
Scatterplots of 5 radial corrections demonstrate at follow-up comparable results of the operated wrist to the healthy wrist for parameters radial length, radial inclination, and volar angulation. This is not the case, however, for ulna plus at time of follow-up (Figure 3).
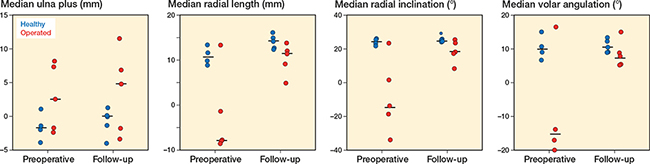
Figure 3. Scatterplots of 4 radiological parameters (ulna plus, radial length, radial inclination, volar angulation) measured preoperatively and at follow-up (median) in 5 distal radial malunions.
Discussion
We demonstrate that CaP cement is a good alternative to bone graft when upper extremity metaphyseal angular corrections are needed in children. The cement was easy to apply, filling defects well, and creating good contact with the cancellous bone surface. The cement’s remodeling properties were visualized intraoperatively during the reoperations. Radiographs and CT scans demonstrated the resorption of the CaP cement with bone bridges paving their way through. Remodeling might be happening at a faster rate in younger patients than in late adolescence, which could be associated with faster turnover of bone and faster fracture healing in the youngest population (16). The slower remodeling time of CaP seems inconsequential as its positive benefits are high mechanical strength and transformation into bone, permitting an unproblematic return to earlier osteotomy sites for new corrective surgery. We experienced 1 case of tendon rupture due to the hardware but no complications from CaP cement at any time. The CaP cement is deemed biocompatible and osteotransductive (17).
A variety of pediatric injuries in the upper extremity are regularly encountered, leading to deformities in need of corrections (18). The main challenge is surgery in children during their growth, especially if the injury happened at a young age. Several corrections might be necessary in the case of physeal injury. The standard management of an angular correction is either an osteotomy and filling of the void with a bone graft, or gradual deformity correction and lengthening with an external fixator (2,19,20). Should a structural graft be needed, bone graft from the iliac crest is still generally preferred but does have its donor site disadvantages (3). Smaller grafts can be taken from the tibia.
Selles performed radial osteotomies in 13 children including grafts in 6 when the defect was > 1 cm (2). The grafts consisted of demineralized bone matrix, autogenic grafts, and allogenic hip-bone grafts. Bone grafts were also used in a series of 3/6 children for correcting distal radius deformities during growth (21). 1 child needed a total of 3 corrective osteotomies. No complications were experienced. Intercalary allografts are an option in the treatment of specific large bone defects, as in pediatric cancer and pathological fracture surgery (22,23).
We performed 8 angular corrections combined with acute lengthening, adding an overcorrection when indicated. We obtained better carpal support after radial lengthening, which was sustained during growth. Distraction osteogenesis was thus not needed at an early age. Axial limb lengthening and humeral symmetry was planned for in adolescence giving good final results. Smaller defects after opening wedge osteotomies in the upper and lower extremity do not necessarily need filling as long as cortical contact is kept on one side (24,25). Secondary loss of radial reduction might occur. There is need for a gap-filler if cortical contact is lost, which was the case in most of our patients (6).
Our study includes corrections in 5 severe distal radius malalignments. We experienced an improvement of alignment in all cases. Continued growth perturbs some radiological results such as ulna plus till last follow-up. This is not reflected in the clinical feedback from patients, as no wish for further surgery rectifying ulnar length at end of growth was required. One can only assume that children adapt to certain upper extremity deformities, present for many years during growth, and not experienced in the same manner by adults. Our main goal was a radial correction resulting in acceptable carpal support from the radius with less focus on optimal radial inclination.
Treatment using external fixators is a good option for older children near the end of growth (26,27). Young children require other considerations due to the considerable remaining growth. A step-by-step correction during growth must be expected. Alternatives to treatment with an external fixator should therefore be considered, as this is a lengthy treatment with added complications (21,27,28). In the older child one can consider ulnar shortening combined with epiphysiodesis if radial alignment is fine. This depends on the final length of the forearm compared with the opposite side. Sometimes the difference in lengths is too obvious, leading to a choice of radial osteotomy instead (21). A good final functional outcome is important and present in all our cases.
Craniofacial surgeons have reported complications such as infections when the cement was used close to mucus membranes and sinuses, leading to the introduction of restrictions for this specific field (12,13). Stable fixations, bleeding control, and awaiting the hardening of cement could reduce complications (13). CaP cement is a useful substitute in the treatment of benign bone tumors with no reported adverse events (11,29).
Gilardino presented long-term follow-up of a pediatric and adult population where CaP cement was used in craniofacial surgery (12). It showed no resorption, suggesting remodeling difficulties in specific areas of use (12). Nakamura describes in a long-term follow-up of mean 6 years incomplete resorption, and wondered if this would impede future surgical reconstructions (11). The area of use in orthopedic surgery has been limited to the metaphysis and is not advised for use in the diaphysis (30). The resorption described is most probably due to the cancellous bone in metaphyseal regions and vascularization of the area (6,15). This could explain the remodeling detected on our CT scans. Stable fixation is recommended in addition to the mechanical properties of the CaP cement (5,6,13).
The main weaknesses of our study are its case series design, and the absence of validated outcome measures and patient-related outcome measures. We present, however, long-term follow-up of a diverse group of upper extremity pediatric cases treated with the same operative technique combined with CaP cement from a young age with 100% follow-up rate. A future cost-effectiveness analysis would be of interest.
Conclusion
CaP bone cement can be an alternative to bone grafting when metaphyseal angular corrections are indicated in the growing child with or without premature growth arrest.
- Salter R B, Harris W R. Injuries involving the epiphyseal plate. J Bone Joint Surg 1963; 45(3): 587-622.
- Selles C A, Mulders M A M, Roukema G R, van der Vlies C H, Cleffken B I, Verhofstad M H J, et al. functional outcomes after corrective osteotomy of symptomatic distal radius malunions in children. J Wrist Surg 2020; 9(2): 136-40.
- Klifto C S, Gandi S D, Sapienza A. Bone graft options in upper-extremity surgery. J Hand Surg Am 2018; 43(8): 755-61.e2.
- Constantz B R, Ison I C, Fulmer M T, Poser R D, Smith S T, VanWagoner M, et al. Skeletal repair by in situ formation of the mineral phase of bone. Science 1995; 267(5205): 1796-9.
- Luchetti R. Corrective osteotomy of malunited distal radius fractures using carbonated hydroxyapatite as an alternative to autogenous bone grafting. J Hand Surg Am 2004; 29(5): 825-34.
- Winge M I, Rokkum M. CaP cement is equivalent to iliac bone graft in filling of large metaphyseal defects: 2 year prospective randomised study on distal radius osteotomies. Injury 2018; 49(3): 636-43.
- Frankenburg E P, Goldstein S A, Bauer T W, Harris S A, Poser R D. Biomechanical and histological evaluation of a calcium phosphate cement. J Bone Joint Surg Am 1998; 80(8): 1112-24.
- Kopylov P, Adalberth K, Jonsson K, Aspenberg P. Norian SRS versus functional treatment in redisplaced distal radial fractures: a randomized study in 20 patients. J Hand Surg Br 2002; 27(6): 538-41.
- Nakano M, Hirano N, Ishihara H, Kawaguchi Y, Matsuura K. Calcium phosphate cement leakage after percutaneous vertebroplasty for osteoporotic vertebral fractures: risk factor analysis for cement leakage. J Neurosurg Spine 2005; 2(1): 27-33.
- Department of justice, USA: Internation medical device maker and four executives charged in connection with unlawful clinical trials [Internet] 2009 [cited June 16, 2009]. Available from: https://www.justice.gov/sites/default/files/usao-edpa/legacy/2011/04/18/synthes_release09.pdf.
- Nakamura T, Matsumine A, Asanuma K, Matsubara T, Sudo A. Treatment of bone defect with calcium phosphate cement subsequent to tumor curettage in pediatric patients. Oncol Lett 2016; 11(1): 247-52.
- Gilardino M S, Cabiling D S, Bartlett S P. Long-term follow-up experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg 2009; 123(3): 983-94.
- Coppey E, Mommaerts M Y. Early complications from the use of calcium phosphate paste in mandibular lengthening surgery: a retrospective study. J Oral Maxillofac Surg 2017; 75(6): 1274.e1-.e10.
- Plum A W, Tatum S A. A comparison between autograft alone, bone cement, and demineralized bone matrix in cranioplasty. Laryngoscope 2015; 125(6): 1322-7.
- Winge M I, Reikeras O, Rokkum M. Calcium phosphate bone cement: a possible alternative to autologous bone graft. A radiological and biomechanical comparison in rat tibial bone. Arch Orthop Trauma Surg 2011; 131(8): 1035-41.
- Stagi S, Cavalli L, Iurato C, Seminara S, Brandi M L, de Martino M. Bone metabolism in children and adolescents: main characteristics of the determinants of peak bone mass. Clin Cases Miner Bone Metab 2013; 10(3): 172-9.
- Winge M I, Johansson C B, Rokkum M. Biopsies from the distal radius after implantation of calcium phosphate cement. J Hand Surg Asia Pacific (accepted); 2022.
- Gauger E M, Casnovsky L L, Gauger E J, Bohn D C, Van Heest A E. Acquired upper extremity growth arrest. Orthopedics 2017; 40(1): e95-e103.
- Al-Sayyad M J. Taylor spatial frame in the treatment of upper extremity conditions. J Pediatr Orthop 2012; 32(2): 169-78.
- Page W T, Szabo R M. Distraction osteogenesis for correction of distal radius deformity after physeal arrest. J Hand Surg Am 2009; 34(4): 617-26.
- Hove L M, Engesaeter L B. Corrective osteotomies after injuries of the distal radial physis in children. J Hand Surg Br 1997; 22(6): 699-704.
- Misaghi A, Jackson T J, Stans A A, Shaughnessy W J, Rose P S, Moran S L, et al. Intercalary allograft reconstruction of the proximal tibia with and without a free fibula flap in pediatric patients. J Pediatr Orthop 2020; 40(9): e833-e8.
- Baldwin P, Li D J, Auston D A, Mir H S, Yoon R S, Koval K J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma 2019; 33(4): 203-13.
- Ozer K, Kiliç A, Sabel A, Ipaktchi K. The role of bone allografts in the treatment of angular malunions of the distal radius. J Hand Surg Am 2011; 36(11): 1804-9.
- Morita M, Kamegaya M, Takahashi D, Kamada H, Tsukagoshi Y, Tomaru Y. Proposal of a new type of innominate osteotomy without the use of bone graft in children: a preliminary study. JB JS Open Access 2019; 4(3): e0016.1-7.
- Meier R, Prommersberger K J, van Griensven M, Lanz U. Surgical correction of deformities of the distal radius due to fractures in pediatric patients. Arch Orthop Trauma Surg 2004; 124(1): 1-9.
- Murase T, Oka K, Moritomo H, Goto A, Sugamoto K, Yoshikawa H. Correction of severe wrist deformity following physeal arrest of the distal radius with the aid of a three-dimensional computer simulation. Arch Orthop Trauma Surg 2009; 129(11): 1465-71.
- Kataoka T, Oka K, Murase T. Rotational corrective osteotomy for malunited distal diaphyseal radius fractures in children and adolescents. J Hand Surg Am 2018; 43(3): 286.e1-.e8.
- Matsumine A, Kusuzaki K, Matsubara T, Okamura A, Okuyama N, Miyazaki S, et al. Calcium phosphate cement in musculoskeletal tumor surgery. J Surg Oncol 2006; 93(3): 212-20.
- Oka K, Murase T, Moritomo H, Goto A, Sugamoto K, Yoshikawa H. Corrective osteotomy using customized hydroxyapatite implants prepared by preoperative computer simulation. Int J Med Robot 2010; 6(2): 186-93.