Salphage: salvage bacteriophage therapy for a recalcitrant Klebsiella pneumoniae prosthetic shoulder infection — a case report
James B DOUB 1, Akira SHISHIDO 1, Uma SRIKUMARAN 2, John HASKOOR 2, Phuong TRAN-NGUYEN 3, Myounghee LEE 3, Silvia WÜRSTLE 4,5, Alina LEE 4,5, Kaitlyn KORTRIGHT 4,5, and Benjamin K CHAN 4,5
1 Division of Clinical Care and Research, Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD; 2 Department of Orthopaedic Surgery, Johns Hopkins University School of Medicine, Baltimore, MD; 3 Department of Pharmacy, University of Maryland Medical Center, Baltimore, MD; 4 Department of Ecology and Evolutionary Biology, Yale University, New Haven, CT; 5 Yale Center for Phage Biology & Therapy, Yale University, New Haven, CT, USA
Citation: Acta Orthopaedica 2022; 93: 756–759. DOI http://dx.doi.org/10.2340/17453674.2022.4579.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-20. Accepted: 2022-08-29. Published: 2022-09-20.
Correspondence: Jdoub@ihv.umaryland.edu
The authors appreciate the help of the research staff at the clinical research unit in the Institute of Human Virology who helped monitor the patient while he was receiving bacteriophage therapy.
JBD and AS wrote the manuscript and then all other authors edited the manuscript. JBD, US, and JH conducted the experimental treatment and clinical management. PT and ML prepared doses of experimental bacteriophages. BKC, KK, AL, and SW isolated, purified, and tested the bacteriophage therapy utilized against the patient clinical isolate.
Acta thanks Michael Hadjiargyrou and Gina Suh for help with peer review of this study.
A healthy 70-year-old male initially presented with an irreparable full-thickness rotator cuff tear treated with a left reverse shoulder arthroplasty (RSA). 3 weeks later a traumatic fall caused a glenoid fracture. This was treated with surgical revision of his RSA and glenoid open reduction and internal fixation. No overt infection was observed, but cultures grew extended-spectrum beta-lactamase (ESBL) Klebsiella pneumoniae (Table 1) and subsequently the patient was treated with 6 weeks of intravenous ertapenem. However, the wound continued to have purulent drainage necessitating further surgical debridement. He then developed recurrent shoulder dislocations requiring revision surgery in which cultures again grew ESBL K. pneumoniae that was treated with 10 weeks of intravenous ertapenem therapy.
After 6 weeks off ertapenem he developed clinical recurrence with a sinus tract and erythema. He then underwent further surgical debridement, which again grew ESBL K. pneumoniae. His isolate continued to not be susceptible to any oral antibiotics (Table 1), thereby negating use of chronic oral suppression therapy. He was therefore treated with 12 weeks of intravenous ertapenem therapy, but 8 weeks after stopping ertapenem recurrence of his infection occurred with re-emergence of sinus tract and erythema. After prolonged discussions regarding alternative therapies, he elected to undergo experimental bacteriophage therapy with combined debridement, antibiotics, irrigation and implant retention surgery (DAIR).
2 obligate lytic bacteriophages KP1 and KP2 were isolated from New Haven wastewater influent on K. pneumoniae ATCC #43816. The ability of these bacteriophages to lyse this patient’s clinical isolate were confirmed by the double agar overlay method where the efficiency of plaquing was 1. In addition, the ability of both bacteriophages to degrade biofilm was examined by growing the patient’s clinical isolate for 72 hours on titanium discs and then exposing the discs to bacteriophage preparations for 24 hours. The discs were then sonicated and bacteria quantified to ensure biofilm activity compared to controls without bacteriophages. Upon confirmation of activity to planktonic and sessile states, both bacteriophages were amplified and purified for in vivo administration at a dose of 1 x 1010 plaque-forming units (PFU) per mL. The final bacteriophage doses created were then tested for endotoxin levels and to ensure sterility of the product (Table 2).
Expanded access was granted by the FDA (IND-27333) and approval by the University of Maryland Institutional Review Board (HP-00094884EA) was obtained. He then underwent DAIR and placement of a single Hickman catheter for repeat intra-articular (IA) bacteriophage therapy (Figure). Operative cultures grew ESBL K. pneumoniae with the same sensitivities (Table 1) and Cutibacterium acnes. Postoperatively, he was started on daily intravenous ertapenem and then, 1 day later, he was started on daily IA bacteriophage therapy. The IA doses comprised 1×1010 PFU/mL diluted in 10 mL of normal saline and infused through the Hickman catheter. The patient received daily IA doses with Klebsiella Phage 1 (KP1) for 2 days and then daily IA doses of Klebsiella Phage 2 (KP2) for 2 days. After IA administration the patient received daily intravenous bacteriophage therapy with KP1 and then KP2 on subsequent days for a total of 2 days of intravenous bacteriophage therapy. This was administered by diluting 1×1010 PFU/mL in 50 mL of normal saline and infusing this over 30 minutes. The patient tolerated the therapy without any adverse reactions and the Hickman catheter was then removed.
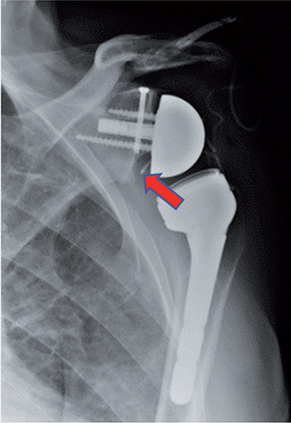
Left shoulder reverse arthroplasty after combined debridement, anti-biotics, irrigation and implant retention surgery, and bacteriophage therapy but prior to removal of the Hickman catheter (red arrow).
He completed 6 weeks of daily intravenous ertapenem and was then started on chronic amoxicillin-clavulanate suppression therapy to suppress C. acnes. Amoxicillin was not used given historical poor intolerance. 6 months later, he had subtle transient swelling of the shoulder after more than usual movements of that shoulder over the prior 2 days. No drainage or frank erythema was seen. This was evaluated with serum C-reactive protein and erythrocyte sedimentation rate, which were within normal limits, and an arthrocentesis in which only 1.5 mL of serosanguinous fluid was aspirated. The swelling resolved without any interventions and despite the lack of symptoms his culture grew K. pneumoniae with the same susceptibilities as seen previously (Table 1). For the next 8 months he continued to have no clinical prosthetic joint infection (PJI) symptoms and C-reactive protein and erythrocyte sedimentation rate have remained within normal limits. Therefore, given the lack of symptoms, ethically we did not conduct another arthrocentesis. Nonetheless 14 months since receiving bacteriophage therapy, the patient is content to be without recurrence of his PJI, with reduced pain and with adequate range of motion of his shoulder to conduct activities he wants to participate in.
Discussion
RSA is an effective option over conventional arthroplasties, especially in patients with rotator cuff pathologies (1,2). However, the incidence of PJI after RSA ranges from 0.5% to 6.7% (3). While the gold standard treatment for chronic hip and knee PJI is 2-stage revision surgery, pooled reinfection rates of chronic shoulder PJI are higher in 2-stage revisions than in 1-stage revisions (4,5). When chronic PJIs recur after revision surgeries, these infections are considered recalcitrant, and management is difficult and not standardized. Deepseated infections in which bacteria are in persistent phenotypic states make these infections difficult to eradicate with conventional antibiotics (6). Our patient had failed surgical interventions, prolonged antibiotics courses and, obstinately, his K. pneumoniae isolate was resistant to all oral antibiotics (Table 1) thus preventing the use of chronic suppression therapy. Moreover, the presence of a sinus tract indicated this patient had a chronic deep-seated infection in his joint that conventional managements could not eradicate. Therefore, we used personalized adjuvant IA and intravenous bacteriophage therapy combined with DAIR to improve his quality of life and prevent recurrence of his PJI. To our knowledge this is the first shoulder PJI treated with bacteriophage therapy.
Bacteriophage therapy is a promising adjuvant agent in the treatment of PJI given this therapeutic has anti-biofilm activity and the ability to self-replicate (7). This is reinforced in several in vitro experiments and other case reports documenting potential effectiveness in PJI (8,9). The advantages of combining bacteriophage therapy with DAIR include (i) removal of planktonic infection, (ii) ensuring the prosthetic is salvageable, (iii) removal of the synovial fluid that harbors plasma protein–bacterial aggregates, (iv) manual debridement of the prosthesis that harbors biofilm, and (v) direct instilment of bacteriophage therapy to surgically debrided biofilm, thereby circumventing the unknown pharmacokinetics of intravenous bacteriophage therapy (10). In this case we also elected to use a single Hickman catheter to give additional IA doses of bacteriophage therapy. This is similar to protocols that use IA antibiotic therapy for recalcitrant PJI (11). However, with bacteriophage therapy much shorter durations of IA therapy are theoretically needed, given that bacteriophages self-replicate when engaged in an infection (7). Moreover, shorter IA courses also mitigate the undesirable ramifications of microbial retrograde translocation into the joint that make Hickman catheter use controversial in PJI (11). Therefore, we used the catheter for 4 days and it was then removed to reduce the risks of bacterial retrograde translocation.
Our protocol discussed here is also unique given we sequentially changed bacteriophage therapy from one bacteriophage to another after several IA doses. This was done given the rapid development of bacteriophage resistance that can occur when a single bacteriophage is used (12). While a bacteriophage cocktail can mitigate this to some extent, there can be bacteriophage competition and inhibition when phages are used in a cocktail (12). Therefore, we elected to use different bacteriophages sequentially to achieve similar benefits of a cocktail but not risk potential inhibition when bacteriophages are used simultaneously. This technique is not readily utilized because the narrow spectrum of activity makes finding multiple bacteriophages with activity to a clinical isolate an arduous undertaking. While we utilized this technique with good clinical success, further translational studies are needed to determine whether cocktail therapies or sequential therapies are more advantageous.
Moreover, our protocol was focused on repeated IA dosing through Hickman catheters to deliver large doses directly to the infected prosthetic. Theoretically this allowed for high concentrations of bacteriophages to be infused into the infected joint, but bacteriophages are not motile and consequently using only IA doses limits this therapeutic to the immediate joint space and surrounding tissues (7,10). Other infection niduses not reached with IA dosing are theoretically also present, especially with chronic PJI where biofilms can be present at the bone–implant interface (10). Therefore, we also gave 2 doses of intravenous bacteriophage therapy to reach areas that were not easily assessable with IA dosing that may have harbored niduses of deep-seated infection. It also must be reinforced that we used bacteriophage therapy as an adjuvant with conventional antibiotics therapies, given the potential synergistic effects with certain types of antibiotics, most notably beta-lactams, which have been documented (13,14). However, more research is needed to better clarify bacteriophage and antibiotic interactions to enhance effectiveness of this therapeutic.
With our protocol we were able to achieve our goal of preventing recurrence of the patient’s sinus tract and PJI symptoms while also improving his quality of life. Interestingly, an arthrocentesis six months after bacteriophage therapy still grew K. pneumoniae from the scant fluid even though he had no clinical symptoms other than subtle transient swelling. Given the lack of symptoms, we continued only to monitor the patient clinically and no recurrence has occurred, which is unusual for this patient based on his infection history. Therefore, our hypothesis is that the bacteriophage therapy induced his bacteria to become less virulent and/or nonpathogenic. To prove this hypothesis assays such as a Caenorhabditis elegans virulence assay would need to be conducted on the bacteria before and after bacteriophage therapy. An alternative hypothesis is that the administered bacteriophages are still present in the joint and have formed a potential steady state of predator–prey dynamics thereby preventing outward symptoms of infection, but never fully eradicating the chronic biofilm infection. This is similar to the interactions bacteriophages and bacteria have in nature in which full eradication of a bacterial colony is evolutionarily undesirable (15). This could be evaluated by testing for active bacteriophages in the joint and is supported by prolonged bacteriophage activity observed in chronic otitis externa patients (16).
Unfortunately, the arthrocentesis culture was not conducted at the University of Maryland Medical Center, thereby not allowing assessment of these hypotheses. In addition, we did not subject the patient to another arthrocentesis given the lack of symptoms, but we plan to conduct the studies mentioned if symptoms recur. This does limit our hypotheses, but other bacteriophage researchers should be cognizant of these to thereby evaluate future patients to improve our understanding of bacteriophage therapy in the treatment of PJI. As well, determining success of bacteriophage therapy in PJI is not standardized, in part because standard-of-care PJI treatments do not ensure clearance of infection with arthrocentesis cultures. Consequently, successful use of bacteriophage therapy in PJI will likely need to be measured in preventing clinical recurrences at prolonged clinical time points. If adjuvant bacteriophage therapy can prevent clinical recurrences at these prolonged time points, this would revolutionize the PJI field by creating a therapy that reduces the debilitating morbidity associated with revision surgeries. However, further research is needed to clarify several aspects of this therapeutic before definitive clinical trials are conducted.
In conclusion, this case reinforces that bacteriophage therapy may be a promising adjuvant therapeutic with surgical interventions in the treatment of recalcitrant PJIs to reduce morbidity and mortality. Additionally, we highlight the potential effectiveness of using Hickman catheters to repeatedly administer large doses of IA bacteriophage therapy directly to the site of infection. However, this case also reinforces our nascent knowledge of bacteriophage therapeutics and suggests the need for more translational research, thereby to effectively utilize these therapeutics and have reproducible outcomes.
Ethics, funding, and potential conflicts of interest
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Expanded access was granted by the FDA (IND-27333) and approval was granted by the University of Maryland Institutional Review Board (HP-00094884EA). No funding was received. JBD has a patent pending with respect to bacteriophage use in prosthetic joint infections.
- Rugg C M, Coughlan M J, Lansdown D A. Reverse total shoulder arthroplasty: biomechanics and indications. Curr Rev Musculoskelet Med 2019; 12(4): 542-53.
- Palsis J A, Simpson K N, Matthews J H, Traven S, Eichinger J K, Friedman R J. Current trends in the use of shoulder arthroplasty in the United States. Orthopedics 2018; 41(3): e416-e423.
- Contreras E S, Frantz T L, Bishop J Y, Cvetanovich G L. Periprosthetic infection after reverse shoulder arthroplasty: a review. Curr Rev Musculoskelet Med 2020; 13(6): 757-68.
- Aïm F, Marion B, Kerroumi Y, Meyssonnier V, Marmor S. One- or two-stage exchange for periprosthetic shoulder infection: systematic review and meta-analysis. Orthop Traumatol Surg Res 2020; 106(1): 5-15.
- Garrigues G E, Zmistowski B, Cooper A M, Green A; ICM Shoulder Group. Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: management of periprosthetic shoulder infection. J Shoulder Elbow Surg 2019; 28(6): S67-S99.
- Del Pozo J L. Biofilm-related disease. Expert Rev Anti Infect Ther 2018; 16: 51-65.
- Abedon S T. Ecology of anti-biofilm agents II: bacteriophage exploitation and biocontrol of biofilm bacteria. Pharmaceuticals (Basel) 2015; 8: 559-89.
- Suh G A, Lodise T P, Tamma P D, Knisely J M, Alexander J, Aslam S et al. Considerations for the use of phage therapy in clinical practice. Antimicrob Agents Chemother 2022; 66(3): e0207121. doi: 10.1128/AAC.02071-21.
- Gibb B P, Hadjiargyrou M. Bacteriophage therapy for bone and joint infections. Bone Joint J 2021; 103-B(2): 234-44. doi: 10.1302/0301620X.103B2.BJJ-2020-0452.R2.
- Doub J B, Ng V, Johnson A, Amoroso A, Kottilil S, Wilson E. Potential use of adjuvant bacteriophage therapy with debridement, antibiotics, and implant retention surgery to treat chronic prosthetic joint infections. OFID 2021; 8(6): ofab277.
- Whiteside L A. Direct intra-articular antibiotic infusion for resistant organisms in the treatment of infection in joint arthroplasty. Semin Arthroplasty 2011; 22(3): 185-8.
- Abedon S T, Danis-Wlodarczyk K M, Wozniak D J. Phage cocktail development for bacteriophage therapy: toward improving spectrum of activity breadth and depth. Pharmaceuticals (Basel) 2021; 14(10): 1019.
- Łusiak-Szelachowska M, Międzybrodzki R, Drulis-Kawa Z, Cater K, Knežević P, Winogradow C, et al. Bacteriophages and antibiotic interactions in clinical practice: what we have learned so far. J Biomed Sci 2022; 29(1): 23. doi:10.1186/s12929-022-00806-1.
- Gu Liu C, Green S I, Min L, Clark J R, Salazar K C, Terwilliger A L, et al. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. mBio 2020; 11(4): e01462-20. doi: 10.1128/mBio.01462-20.
- Naureen Z, Dautaj A, Anpilogov K, Camilleri G, Dhuli K, Tanzi B, et al. Bacteriophages presence in nature and their role in the natural selection of bacterial populations. Acta Biomed 2020; 91(13-S): e2020024.
- Wright A, Hawkins C H, Anggård E E, Harper D R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa: a preliminary report of efficacy. Clin Otolaryngol 2009; 34(4): 349-57.