Denosumab prevents acetabular bone loss around an uncemented cup: analysis of secondary outcomes in a randomized controlled trial
Demostenis KIRITOPOULOS 1, Andreas NYSTRÖM 1, Gösta ULLMARK 2, Jens SÖRENSEN 3, Marianne PETRÉN-MALLMIN 4, Jan MILBRINK 1, Nils P HAILER 1, and Hans MALLMIN 1
1 Department of Surgical Sciences/Section of Orthopedics, Uppsala University; 2 Department of Orthopedics, Gävle Hospital; 3 Department of Surgical Sciences, Section of Nuclear Medicine & PET, Uppsala University; 4 Department of Surgical Sciences/Section of Radiology, Uppsala University, Sweden
Background and purpose — Uncemented total hip arthroplasty (THA) is associated with periprosthetic bone loss. In a secondary outcome analysis from a randomized controlled trial, we studied whether denosumab can prevent loss of acetabular periprosthetic bone mineral density (pBMD) in patients who received a trabecular metal cup during uncemented THA.
Patients and methods — 64 patients (aged 35–65 years) with unilateral osteoarthritis of the hip were randomized to 2 subcutaneous injections with denosumab or placebo, given 1–3 days post-surgery and 6 months post-surgery. Acetabular pBMD was measured in 5 regions of interest (ROIs) by dual-energy X-ray absorptiometry. Serum markers for bone metabolism were analyzed. Periprosthetic osteoblastic activity, measured as standardized uptake values (SUVs) by [18F] positron emission tomography/computed tomography, was evaluated in 32 of the 64 study patients.
Results — After 12 months, patients treated with denosumab had higher pBMD compared with the placebo-treated patients in 4 of 5 ROIs and in sum of ROIs 1–5. After 24 months, the effect on pBMD for patients treated with denosumab declined. Serum markers declined pronouncedly up to 12 months in patients treated with denosumab, but rebounded above baseline levels after 24 months. Patients treated with denosumab had statistically significantly lower SUVs in all ROIs, except ROI 5, after 6 months.
Interpretation — Based on this exploratory analysis of secondary endpoints the application of denosumab seems associated with preserved acetabular pBMD, reduced bone metabolism and attenuated periprosthetic osteoblastic activity. However, given the known rebound affects after discontinuation of denosumab treatment, these effects cannot be expected to persist. If prolonged treatment or shift to other regimes would be beneficial to reduce the risk of cup loosening is yet to be investigated.
Citation: Acta Orthopaedica 2022; 93: 709–720. DOI http://dx.doi.org/10.2340/17453674.2022.4537.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-03-15. Accepted: 2022-08-07. Published: 2022-09-07.
Correspondence: demostenis.kiritopoulos@surgsci.uu.se
Study design: HM, JM, NPH, JS, GU, and MPM. Study conduct: DK, AN, and HM. Data collection: DK, AN, and HM. Data analysis: DK, AN, HM, GU, and MPM. Data interpretation: all authors. DK, AN, and HM take responsibility for the integrity of the data analysis. Drafting of manuscript: DK, HM, and NPH. Revising manuscript content: all authors.
Trial administration, monitoring, data management, and statistical analyses were conducted by personnel at the Uppsala Clinical Research Center, Uppsala, Sweden.
The authors thank Catharina Strömstedt for excellent work as research nurse and Enn Maripuu for programming of the F–PET analysis software tool and support in using it. They also thank all the participants of this study for making it possible.
Acta thanks Hannu T Aro and Lene Bergendal Solberg for help with peer review of this study.
Implant loosening is the most common cause for total hip arthroplasty (THA) revision (1). Periprosthetic bone mineral density (pBMD) loss around the acetabular cup and the femoral stem has been reported (2,3) and is mediated by osteoclast activation, stress shielding, polyethylene wear, implant material, and stiffness (4,5).
Regimes to overcome pBMD loss have included treatment with antiresorptive drugs, but none of these preserved periprosthetic bone in the long term (6,7). Denosumab, a human monoclonal antibody directed against receptor activator of nuclear factor Kappa-B ligand (RANK-L), inhibits osteoclast recruitment and activation (8). Treatment with denosumab depresses bone metabolism markers (9), increases BMD, and attenuates the risk of fractures in patients with osteoporosis (10). Reports on the effects of denosumab treatment on periprosthetic bone are sparse, and we are unaware of studies on its impact on acetabular pBMD (11,12).
Acetabular cups with a trabecular surface are used in primary and revision THA. The dynamics around such implants are unknown, but studies indicate a reduction of pBMD (3,13). Few studies on pBMD around acetabular cups are designed as randomized controlled trials (RCTs), and no studies evaluating the effect of drugs have included [18F]-sodium fluoride positron emission tomography/computed tomography (F-PET) to measure osteoblast activity, periprosthetic standardized uptake value, pSUV (14,15).
We hypothesized that denosumab would inhibit pBMD loss after THA. We have performed an RCT on patients with unilateral osteoarthritis of the hip (OAH) who received an uncemented THA. The primary outcome variable, femoral pBMD, has been published (11). Denosumab prevented periprosthetic bone loss 1 year after insertion of an uncemented THA stem. Denosumab also decreased periprosthetic 18F uptake locally as an indirect measure of bone metabolism and a prompt, systemic decrease in biochemical markers of bone resorption was observed. However, the effect declined after discontinuation of denosumab treatment.
Here, we report the effects of denosumab treatment on acetabular pBMD and pSUV, secondary outcomes of the previously referred RCT.
Patients and methods
Trial design
The study was performed as a double-blind placebo RCT at the University Hospital of Uppsala (11). The effect of denosumab on systemic and local bone metabolism was evaluated by (i) assessing periprosthetic, contralateral hip, and vertebral BMD by dual-energy X-ray absorptiometry (DXA), (ii) measuring biochemical markers of bone turnover, and (iii) performing F-PET to measure pSUV in half of the study population (n = 32). Clinical outcome was assessed using the Harris Hip Score (HHS).
Participants
Patients were recruited from August 2012 to January 2015. All patients 35 to 65 years of age living in the Uppsala region and referred to the Department of Orthopedics, Uppsala University Hospital with unilateral OAH (Kellgren–Lawrence grade 3–4 for the affected hip and 0–1 for the unaffected hip) and with a BMI < 35 were eligible for inclusion (16) (Figure 1 and Table 1, see Supplementary data).
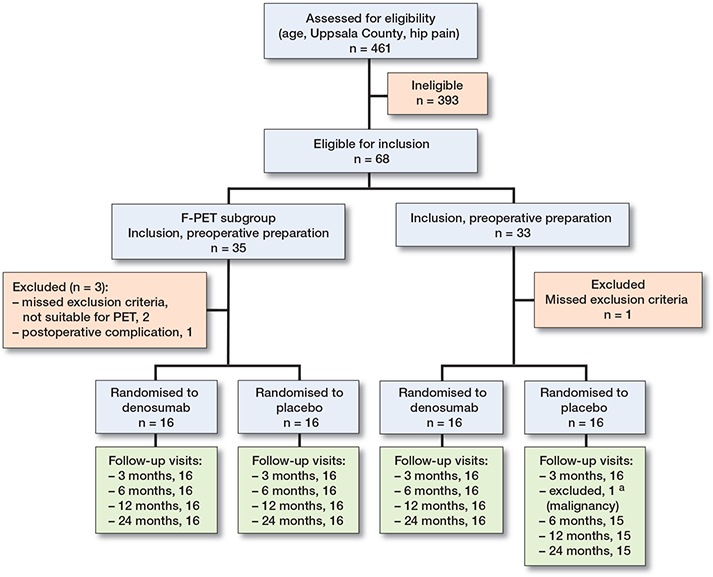
Figure 1. CONSORT flowchart illustrating enrollment and randomization process. Numbers represent patients available for analysis for the secondary outcomes. a HHS and biomarkers at 6 months.
Drugs and randomization
7 to 14 days before surgery, morning fasting blood samples were drawn. At the same time, the patients started a daily oral regimen of calcium (500 mg) and vitamin D3 (800 IE) over a 1-year period.
The patients were randomized in blocks of 4 to a subcutaneous injection of 1 mL containing 60 mg of denosumab or 1 mL of sodium chloride 0.9%. The study drug was given after baseline pBMD assessment along with DXA scans and fasting morning blood samples had been drawn 1–3 days postoperatively. The 2nd and final injection was delivered in the same way 6 months postoperatively. Half of the study population was also evaluated with F-PET, and the study drug was given after F-PET scans had been conducted.
Peri- and postoperative procedures and implants
All patients underwent surgery in a lateral decubitus position with an anterolateral approach using an uncemented CFP stem with a 28-mm CoCrMo head and an uncemented Continuum cup with a trabecular tantalum surface fitted with a highly cross-linked polyethylene elevated liner (see Supplementary data for details).
Bone mineral density
All scans were performed on a Prodigy Advance system (GE Healthcare, Chicago, IL, USA). Preoperatively, the lumbar spine and both proximal femora were scanned. Orthopedic hip implant scans were performed postoperatively and after 3, 6, 12, and 24 months and analyzed for acetabular pBMD in 5 ROIs and the sum of ROIs 1–5 according to Digas (3) (Figure 2). The precision of the periprosthetic acetabular DXA measurements, expressed as a coefficient variation (CV), is 2.99% for ROI 1 and 3.59% for ROI 3 (13).
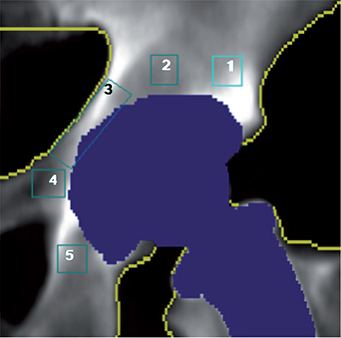
Figure 2. A DXA scan showing the 5 ROIs according to Digas.
Standardized uptake value
The 1st 32 patients were additionally investigated by F-PET to quantify regional mineral formation rates. F-PET scans were performed 7–14 days before surgery and 3 and 6 months postsurgery. The 5 volumes of interest VOIs, corresponding to the 5 ROIs by DXA, were chosen for analysis of acetabular pSUV and the VOIs are hereafter named ROIs (see Supplementary data for details). The inter- and intraobserver agreement of periprosthetic VOI with F-PET had an intraclass correlation coefficient of 0.86 and > 0.95, respectively.
Biochemical markers of bone metabolism
All blood samples were acquired fasting in the morning 7–14 days before surgery, 1–3 days post-surgery, and after 3, 6, 12, and 24 months. Carboxy-terminal telopeptide of type 1 collagen (CTX, β-CrossLaps, Cobas, Roche, Basel, Switzerland) was measured as a bone resorption marker and procollagen type 1 amino-terminal propeptide (P1NP, Cobas, Roche) as a bone formation marker.
Our laboratory is certified according to the international standard ISO 15189:201. The CV was 3% for P1NP and 6% for β-CrossLaps.
Patient-reported outcome measures
The HHS was used to assess patient-reported clinical efficacy of the operation, recorded 7–14 days pre-surgery and then 3, 6, 12, and 24 months post-surgery.
Conventional radiography
Hip and pelvic digital radiographs were obtained preoperatively. The degree of OAH was bilaterally classified according to the Kellgren and Lawrence system postoperatively and after 1 year.
All analyses were performed by investigators blinded to treatment assignment.
Statistics
The sample size calculations were performed for the primary outcome femoral pBMD and are described in detail in our previous publication (11).
Analyses of efficacy outcomes were based on all randomized patients who received the study drug and who had evaluable post-baseline data. Safety analyses were based on all patients who received any amount of study drug or placebo.
Descriptive statistics were used to compare baseline characteristics of the trial participants. Continuous variables were summarized using means (SD) or as medians (min–max). Categorical variables were described using frequencies. Treatment contrasts were expressed as model-based geometric mean ratios (with 95% confidence intervals [CIs]), estimated using analysis of covariance (ANCOVA) with randomized treatment and the baseline value for each corresponding outcome as independent variables. Continuous efficacy variables were transformed using natural logarithms before analysis. For the HHS, a nonparametric ANCOVA (i.e., rank analysis of covariance combined with Mantel–Haenszel statistics) was used. No adjustment for multiplicity was undertaken, and all secondary endpoint analyses should be regarded as exploratory.
A 2-tailed p-value of < 0.05 was considered statistically significant.
Ethics, funding, data sharing, and potential conflicts of interests
The study was approved by the Regional Ethical Review Committee, Uppsala (Dnr 2011/297/2), was performed in compliance with the Declaration of Helsinki, and was registered at ClinicalTrials.gov 2011-001481-18, NCT01630941. All patients gave written informed consent.
The study was funded by Uppsala University, the Regional Research Council of Uppsala–Örebro, Stiftung Endoprothetik (grant no. S 03/10), and Skobranschens Utvecklingsfond. The study did not receive any financial support from the pharmaceutical industry.
An anonymized minimal data set can be shared upon reasonable request.
NPH has received institutional grants and personal fees as lecturer from Waldemar Link GmbH, Heraeus, and Zimmer Biomet. GU has received grants and personal fees as lecturer from Waldemar Link GmbH. The other authors have had no financial relationships with any organization that might have an interest in the submitted work in the past 3 years. None of the authors have had any other relationships or activities that could appear to have influenced the submitted work.
Results
Characteristics of the study population
Of 461 assessed patients, 64 patients were included and randomized to treatment, of whom 32 were additionally investigated by F-PET (Figure 1). All patients received the designated cup. 1 patient diagnosed with rectal cancer chose to withdraw 3 months after enrollment into the study. Baseline characteristics were similar in the 2 treatment groups (Table 2) as well as for the study group investigated by F-PET (Table 3, see Supplementary data). None of the patients had osteoporosis according to the definition of WHO, i.e., a T-score < –2.5 (17).
| Characteristic | Denosumab (n = 32) | Placebo (n = 32) |
| Age | 58 (5) | 59 (5) |
| Male, n (%) | 12 (38) | 13 (41) |
| Body mass index | 27 (4) | 27 (3) |
| Kellgren–Lawrence grading | ||
| unaffected hip | 1 (0–1) | 1 (0–1) |
| affected hip | 3 (3–4) | 3 (3–4) |
| Harris Hip Score | 58 (28–81) | 51 (33–77) |
| CTX (μg/L) | 0.44 (0.19) a | 0.44 (0.18) |
| P1NP (μg/L) | 46 (16) a | 44 (15) |
| Z-score b | ||
| total hip (unaffected hip) | 0.58 (1.13) | 0.65 (0.67) |
| total hip (affected hip) | 0.33 (1.20) | 0.33 (0.91) |
| L1–L4 | 0.91 (1.18) | 0.83 (0.91) |
| a n = 31; because of incorrect handling, blood samples from 1 patient in the denosumab group were unavailable for analysis. b Age- and sex-matched and weight-adjusted comparison with a White/Caucasian US reference population. |
||
Periprosthetic bone mineral density
Denosumab-treated patients had 10% higher pBMD (CI 1.1–1.2) in ROI 1 than placebo-treated patients after 3 months, and 17% (CI 1.1–1.2) higher pBMD after 12 months (Figure 3). Similar increases compared with placebo-treated patients were seen in ROI 2, ROI 3, ROI 4, and the sum of ROIs 1–5 up to 12 months after surgery. After 24 months, the effect on pBMD for patients treated with denosumab declined, although it was still statistically significantly higher in ROI 2 and the sum of ROIs 1–5 compared with patients treated with placebo (Table 4, see Supplementary data).
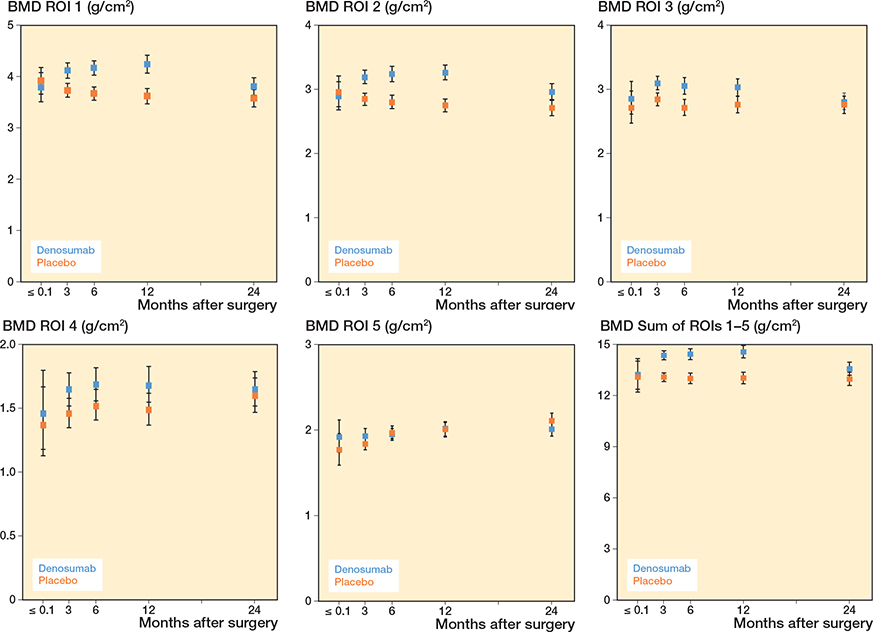
Figure 3. Periprosthetic BMD in ROI 1, ROI 2, ROI 3, ROI 4, ROI 5, and the sum of ROIs 1–5. For visits pre-randomization, descriptive geometric means with 95% confidence intervals are given. For visits post-randomization, model-based geometric means with confidence intervals are given. The drug was administered at 1 to 3 days postoperatively.
Compared with baseline, pBMD increased in all ROIs in patients treated with denosumab up to 12 months but then declined to the baseline level after 24 months in proximal and medial ROI 1, ROI 2, and ROI 3. In the distal ROI 4 and ROI 5 and the sum of ROIs 1–5, pBMD was still higher after 24 months (Table 4, see Supplementary data).
Compared with baseline, pBMD declined in ROI 1 and ROI 2 in patients treated with placebo at 12 and 24 months. In ROI 3 and the sum of ROIs 1–5, pBMD remained at baseline level at 12 and 24 months. In ROI 5 pBMD was higher at 12 and 24 months than baseline, and in ROI 4 pBMD was higher only at 24 months (Table 4, see Supplementary data).
Standardized uptake value
Patients treated with denosumab had reduced acetabular pSUV in all ROIs, including the sum of ROIs 1–5, after 3 and 6 months when compared with patients treated with placebo. However, the reduction in pSUV, varying from –21% to –34%, was statistically significant in ROI 1, ROI 2, ROI 3, ROI 4, and the sum of ROIs 1–5 only after 6 months (Figure 4 and Table 5, see Supplementary data).
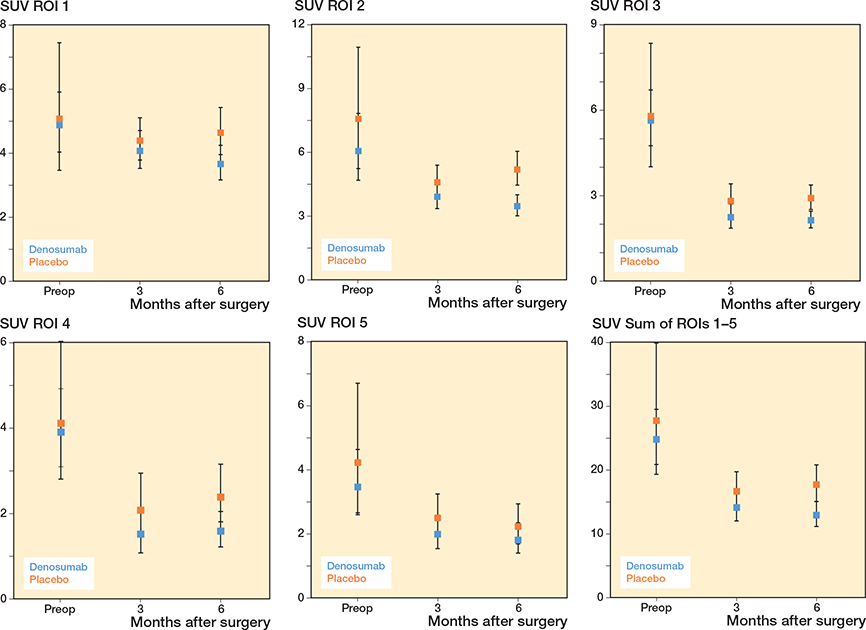
Figure 4. Periprosthetic SUV in ROI 1, ROI 2, ROI 3, ROI 4, ROI 5, and the sum of ROIs 1–5. For visits pre-randomization, descriptive geometric means with 95% confidence intervals are given. For visits post-randomization, model-based geometric means with confidence intervals are given. The drug was administered at 1 to 3 days postoperatively.
Compared with baseline, pSUV was statistically significant reduced by 17–62% in all ROIs in patients treated with denosumab at 3 and 6 months. Compared with baseline, pSUV had less but similar decreases of 34–55% in ROI 2, ROI 3, ROI 4, ROI 5, and the sum of ROIs 1–5 in patients treated with placebo at 3 and 6 months (Table 5, see Supplementary data).
Biochemical markers
A pronounced reduction in blood concentrations of bone resorption and formation markers were registered in patients treated with denosumab after 3, 6, and 12 months. However, after 24 months, both marker levels were above baseline levels (Figures 5 and 6, see Supplementary data).
Patient-reported outcome measures
The placebo-treated patients had slightly higher HHS after 12 months than the patients treated with denosumab (100 vs. 94) (Table 6, see Supplementary data).
Adverse events
Serious adverse events and adverse events were equally distributed between patients treated with denosumab and placebo (Table 7, see Supplementary data).
Discussion
The main findings of this RCT are that 2 doses of denosumab prevent loss of acetabular pBMD after inserting an uncemented acetabular trabecular tantalum metal cup during the 1st year; however, pBMD declines in the 2nd year. Moreover, and not previously reported, acetabular pSUV is potently reduced 6 months after the 1st dose of denosumab.
Acetabular periprosthetic bone mineral density
2 doses of denosumab prevented loss of acetabular pBMD compared with placebo and increased acetabular pBMD compared with baseline. Others have reported a loss of acetabular pBMD in proximal and central periacetabular ROI and increased pBMD in the distal ROI during the 1st year after inserting an uncemented THA cup (3,13,14,18). 1 RCT on the effects of a single IV injection of pamidronate reported a reduced loss of acetabular pBMD compared with placebo after 26 weeks (19). However, the effect disappeared after 2 years, and migration of the acetabular implant was not affected by pamidronate (20).
Only 1 previous RCT has evaluated the pharmacological effects of antiresorptive treatment on acetabular pBMD after a THA, and, to our knowledge, none has reported on the effects of denosumab in this setting (19,20). Yet, several RCTs that evaluated 1-year results of antiresorptive treatment on femoral pBMD indicate that the loss of pBMD can be attenuated or prevented after uncemented THA (7,11,12). However, the effects of antiresorptive treatment on femoral pBMD decline during the 2nd and 3rd year (7,11,12). Thus, the effect of 2 doses of denosumab on acetabular pBMD in the present study was similar to what has been reported for femoral pBMD.
Acetabular periprosthetic standardized uptake value
Few RCTs have investigated F-PET in bone. Increased bone turnover markers, osteoblastic activity by F-PET, and BMD in postmenopausal women with osteopenia have been reported 12 weeks after treatment with daily subcutaneous injections of teriparatide (21). In contrast, we found decreased bone turnover markers and acetabular pSUV but increased pBMD in patients treated with denosumab.
The reduced pSUV in patients treated with denosumab compared with placebo in both acetabular and femoral periprosthetic bone after 3 and 6 months is probably related to the biologic effect of the 1st dose of denosumab, with depressed osteoclast activity and insufficient coupling to osteoblast activity, reflected by the systemically depressed levels of CTX and P1NP (11). An experimental study suggests that uptake of [18F] NaF and increased SUV can be the result of a mechanism other than the activity of the osteoblasts, such as affinity of [18F] NaF to hydroxyapatite (22). However, that hypothesis is challenged by our results on acetabular pSUV as well as on femoral pSUV (11).
Theoretically, reduced bone metabolism, systemically and in periprosthetic bone, could negatively impact osteointegration, fixation, and stability of orthopedic implants.
Effects of antiresorptive treatment have shown that implant stability is achieved, femoral pBMD reached a plateau, and there were excellent patient-reported outcome measures 1 year after an uncemented THA (7,11). These findings indicate successful ingrowth and incorporation for an uncemented stem because of or despite antiresorptive drugs. 1 RCT that applied a 2-dose regime with denosumab 6 months apart and starting 1 month prior to surgery in elderly women reported increased femoral pBMD after 48 weeks that declined after 3 years. Implant stability after 48 weeks for an uncemented femoral stem was not affected by denosumab compared with placebo (12).
No data has been published for the effect of denosumab on cup stability by RSA. We did not perform any cup revisions during the 2-year study, indicating that denosumab is not harmful to the initial stability of an uncemented cup.
Compared with baseline, acetabular pSUV was reduced for both the placebo and denosumab treated patients. This is in contrast to increased levels of femur pSUV that we have reported previously (11).
An explanation for these differences in postoperative findings for pSUV of the acetabulum and proximal femur could be that the proximal part of the hip joint, the acetabulum, is close to bone affected by OAH, whereas the femoral head affected by OAH, the distal part of the OAH-affected hip joint, is resected and replaced (23,24). This implies that only extra-articular proximal femoral regions remain to be investigated for the pSUV after a THA, whereas the acetabular THA component is positioned in the previously affected subchondral bone.
Biochemical markers of bone metabolism
The present and an additional RCT investigating the antiresorptive effect of 2 doses of denosumab given in the first 6 months in connection with an uncemented THA reported distinct depressed CTX levels after 3 months and robust depressed levels at 6 and 12 months accompanied by similarly depressed levels of the bone formation marker P1NP (11,12). These findings could be interpreted as inadequate coupling between the osteoclasts and the osteoblasts, consistent with reports from large osteoporosis studies for denosumab (10). After the withdrawal of denosumab treatment, and in line with findings from osteoporosis studies, a rebound phenomenon with statistically significant above baseline serum levels for both bone resorption and formation markers was reported in the present RCT (25) (Figures 5 and 6, see Supplementary data).
Strength and limitations
The main strength of our study is that, to our knowledge, this is the first RCT that has included a pharmaceutical drug in the study protocol to evaluate the effect on acetabular pBMD and pSUV after a THA. Strict inclusion and exclusion criteria, defined radiological diagnosis of OAH, standardized surgical procedures, implants and postoperative care, carefully conducted case-report formulas, external monitoring, and a minimal number of patients lost to follow-up ensure that our study has excellent internal validity. In addition, the study was performed as an independent academic study without any support from the pharmaceutical industry.
Our study also has some limitations. The number of patients for the study was based on a power analysis for the primary outcome, femoral pBMD but not acetabular pBMD, 1 year after the 1st dose of denosumab or placebo. The secondary outcomes in the present report were planned and specified before the study start and followed according to the clinical trial protocol. However, secondary outcomes, while informative, should be interpreted cautiously.
Moreover, considering the width of the relevant CIs, the differences in effects between the treatment groups could be affected and should also be interpreted with caution.
The possibility to generalize the results to other implants (uncemented or cemented), age groups, anatomical sites, or diagnoses is not immediately evident. This is the 1st RCT to report effects of denosumab on acetabular pBMD, preceded by only 1 RCT that reported the effects of one IV dose of pamidronate (19,20). Thus, the external validity is limited.
Also, the importance of acetabular pBMD as a surrogate variable for implant loosening and unfavorable clinical results remains to be validated.
The positive effect on pBMD reported from RCTs during the 1-year anti-resorptive treatment on femur pBMD has been transient at a later follow-up (7,12). To preserve a sustainable effect on pBMD, prolonged treatment with denosumab or switching to an alternative antiresorptive treatment could be considered but this remains to be determined.
Patients with osteoporosis on long-term treatment with denosumab have reported a rapid loss of gained BMD and have an increased risk of vertebral fractures when treatment is discontinued (26). However, no deleterious clinical effects of discontinuing only 2 doses of denosumab on orthopedic implant stability, revision rate, PROMs, and rates of adverse or serious adverse events have been reported from RCTs (12,27). Although not included in the study protocol, estimation of serum calcium levels could be of importance for safety reasons.
Conclusions
Our findings on this exploratory analysis of secondary endpoints indicate that denosumab seems to attenuate loss of acetabular pBMD, most probably by preventing periprosthetic osteoclast activation. However, this effect almost disappears after discontinuation of treatment. Further research is needed to determine whether inhibition of osteoclast recruitment by RANK-L inhibitors can reduce the need for subsequent acetabular revision surgery.
- Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complicationbased analysis using worldwide arthroplasty registers. J Arthroplasty 2013; 28(8): 1329-32.
- Lazarinis S, Mattsson P, Milbrink J, Mallmin H, Hailer N P. A prospective cohort study on the short collum femoris-preserving (CFP) stem using RSA and DXA: primary stability but no prevention of proximal bone loss in 27 patients followed for 2 years. Acta Orthop 2013; 84(1): 32-9.
- Digas G, Kärrholm J, Thanner J. Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop 2006; 77(2): 218-26.
- Glassman A H, Bobyn J D, Tanzer M. New femoral designs: do they influence stress shielding? Clin Orthop Relat Res 2006; 453: 64-74.
- Howie D W, Neale S D, Martin W, Costi K, Kane T, Stamenkov R, et al. Progression of periacetabular osteolytic lesions. J Bone Joint Surg Am 2012; 94(16): e1171-1176.
- Friedl G, Radl R, Stihsen C, Rehak P, Aigner R, Windhager R. The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty: a randomized, double-blind, controlled trial. J Bone Joint Surg Am 2009; 91(2): 274-81.
- Muren O, Akbarian E, Salemyr M, Bodén H, Eisler T, Stark A, et al. No effect of risedronate on femoral periprosthetic bone loss following total hip arthroplasty: a 4-year follow-up of 61 patients in a double-blind, randomized placebo-controlled trial. Acta Orthop 2015; 86(5): 569-74.
- Delmas P D. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom Off J Int Soc Clin Densitom 2008 Jun; 11(2): 325-38.
- Bone H G, Bolognese M A, Yuen C K, Kendler D L, Wang H, Liu Y, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008; 93(6): 2149-57.
- Cummings S R, San Martin J, McClung M R, Siris E S, Eastell R, Reid I R, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361(8): 756-65.
- Nyström A, Kiritopoulos D, Ullmark G, Sörensen J, Petrén-Mallmin M, Milbrink J, et al. Denosumab prevents early periprosthetic bone loss after uncemented total hip arthroplasty: results from a randomized placebo-controlled clinical trial. J Bone Miner Res Off J Am Soc Bone Miner Res 2020; 35(2): 239-47.
- Aro H T, Nazari-Farsani S, Vuopio M, Löyttyniemi E, Mattila K. Effect of denosumab on femoral periprosthetic BMD and early femoral stem subsidence in postmenopausal women undergoing cementless total hip arthroplasty. JBMR Plus 2019; 3(10): e10217.
- Lazarinis S, Milbrink J, Mattsson P, Mallmin H, Hailer N P. Bone loss around a stable, partly threaded hydroxyapatite-coated cup: a prospective cohort study using RSA and DXA. Hip Int J Clin Exp Res Hip Pathol Ther 2014; 24(2): 155-66.
- Salemyr M, Muren O, Eisler T, Bodén H, Chammout G, Stark A, et al. Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty: a randomised controlled trial. Int Orthop 2015; 39(5): 823-32.
- Laursen M B, Nielsen P T, Søballe K. Bone remodelling around HAcoated acetabular cups: a DEXA study with a 3-year follow-up in a randomised trial. Int Orthop 2007; 31(2): 199-204.
- Kellgren J H, Lawrence J S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957; 16(4): 494-502.
- WHO: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser 1994; 843: 1-129.
- Mueller L A, Kress A, Nowak T, Pfander D, Pitto R P, Forst R, et al. Periacetabular bone changes after uncemented total hip arthroplasty evaluated by quantitative computed tomography. Acta Orthop 2006; 77(3): 380-5.
- Wilkinson J M, Stockley I, Peel N F A, Hamer A J, Elson R A, Barrington N A, et al. Effect of pamidronate in preventing local bone loss after total hip arthroplasty: a randomized, double-blind, controlled trial. J Bone Miner Res 2001; 16(3): 556-64.
- Wilkinson J M, Eagleton A C, Stockley I, Peel N F A, Hamer A J, Eastell R. Effect of pamidronate on bone turnover and implant migration after total hip arthroplasty: a randomized trial. J Orthop Res 2005; 23(1): 1-8.
- Frost M L, Moore A E, Siddique M, Blake G M, Laurent D, Borah B, et al. 18F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: a prospective, randomized, controlled clinical study. J Bone Miner Res Off J Am Soc Bone Miner Res 2013; 28(6): 1337-47.
- Bernhardsson M, Sandberg O, Ressner M, Koziorowski J, Malmquist J, Aspenberg P. Shining dead bone: cause for cautious interpretation of [18F]NaF PET scans. Acta Orthop 2018; 89(1): 124-7.
- Ullmark G, Sörensen J, Maripuu E, Nilsson O. Fingerprint pattern of bone mineralisation on cemented and uncemented femoral stems: analysis by [18F]-fluoride-PET in a randomised clinical trial. Hip Int J Clin Exp Res Hip Pathol Ther 2019; 29(6): 609-17.
- Ullmark G, Sörensen J, Nilsson O, Maripuu E. Bone mineralisation adjacent to cemented and uncemented acetabular cups: analysis by [18F]-fluoride-PET in a randomised clinical trial. Hip Int J Clin Exp Res Hip Pathol Ther 2020; 30(6): 745-51.
- Tsourdi E, Zillikens M C, Meier C, Body J J, Gonzalez Rodriguez E, Anastasilakis A D, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab 2020 Oct 26; dgaa756. doi: 10.1210/clinem/dgaa756. Online ahead of print.
- Cummings S R, Ferrari S, Eastell R, Gilchrist N, Jensen J E B, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res Off J Am Soc Bone Miner Res 2018; 33(2): 190-8.
- Ledin H, Good L, Aspenberg P. Denosumab reduces early migration in total knee replacement. Acta Orthop 2017; 88(3): 255-8.
Supplementary data
Peri- and postoperative procedures and implants
Preoperative digital radiographic planning for implant size, positioning, and inclination was performed before all procedures. Patients underwent surgery in a lateral decubitus position with an anterolateral approach by one of 2 surgeons (JM, NH) in regional spinal anesthesia or general anesthesia. Thereafter, an uncemented femoral neck-preserving stem (CFP, LINK, Hamburg, Germany) with a 28-mm CoCrMo head was implanted in the femur. However, for technical reasons, one patient was provided with an uncemented Cone stem with a 28-mm head. The acetabular implant, a uni-hole Continuum cup (Zimmer, Warsaw, IN, USA), is a press-fit hemispheric cup made of Tivanium, a titanium alloy, to which a surface of Trabecular Metal, a highly porous biomaterial made from elemental tantalum is bonded. The surface offers a high coefficient of friction and scratch fit that reduces micromotion and supposedly allows for rapid bone ingrowth. The acetabular bed was reamed line to line and the implant was anchored in the acetabular bed according to the manufacturer’s manual. The Continuum cup was fitted with a highly cross-linked polyethylene elevated liner with an inner diameter of 28 mm. Operative infection prophylaxis with cefuroxime 1.5 g x 3 (or clindamycin 600 mg x 2 in cases of penicillin allergy) was given intravenously, with the first dose administered < 60 minutes before surgery. Tranexamic acid (10 mg/kg bodyweight) was injected as an intravenous infusion before the start of surgery to reduce blood loss, provided no contraindications were present. Perioperative local infiltration anesthesia consisting of 150 ml of 0.2% ropivacaine and 1 mg adrenaline was administered, but no ketorolac was added to avoid potential effects on bone formation. Non-steroidal anti-inflammatory drugs were not prescribed, and patients were instructed not to use them after discharge. 0.4 ml enoxaparin (100 mg/ml) as thromboprophylaxis was given subcutaneously daily for 4 weeks postoperatively. The average operating time was 54 minutes (range 33–75).
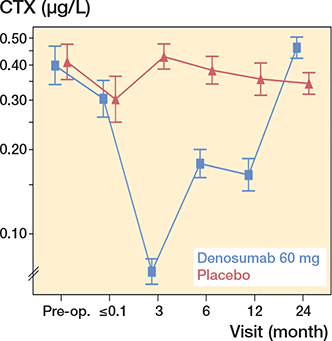
Figure 5. CTX-Crosslaps. For visits pre-randomization, descriptive geometric means with confidence intervals are given. For visits postrandomization, model-based geometric means with confidence intervals are provided. The drug was administered 1–3 days postoperatively.
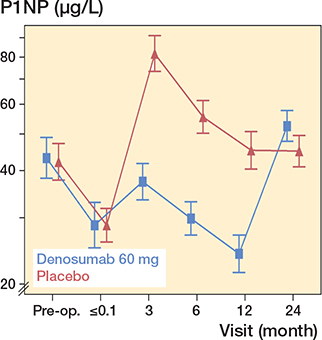
Figure 6. P1NP. Y-axis is truncated for better visualization. For visits pre-randomization, descriptive geometric means with confidence intervals are given. For visits post-randomization, model-based, geometric means with confidence intervals are shown. The drug was administered 1–3 days postoperatively.
| Characteristic | Denosumab (n = 16) | Placebo (n = 16) |
| Age | 58 (5) | 59 (4) |
| Male, n (%) | 8 (50) | 5 (31) |
| Body mass index | 26 (4) | 26 (4) |
| Kellgren–Lawrence grading | ||
| unaffected hip | 1 (0–1) | 1 (0–1) |
| affected hip | 3 (3–4) | 3 (3–4) |
| Harris Hip Score | 60 (57–65) | 48 (41–58) |
| EQ-VAS, median (IQR) | 60 (40–66) | 40 (24–60) |
| CTX (μg/L) | 0.39 (0.14) | 0.48 (0.22) |
| P1NP (μg/L) | 41 (12) | 46 (17) |
| Z-score a | ||
| total hip (unaffected hip) | 0.26 (1.07) | 0.74 (0.73) |
| total hip (affected hip) | 0.02 (1.04) | 0.32 (1.08) |
| L1–L4 | 0.48 (1.03) | 0.79 (0.90) |
| a Age- and sex-matched and weight-adjusted comparison with a White/Caucasian US reference population. None of the patients were osteoporotic according to the criteria of the World Health Association. | ||
| Visit | Treatment group | n | Median | Median change from baseline | p-value a | p-value b |
| 1–3 days | Denosumab 60 mg | 32 | 58.0 | |||
| Placebo | 32 | 51.0 | ||||
| 3 months | Denosumab 60 mg | 32 | 81.0 | 22.5 | < 0.0001 | 0.1 |
| Placebo | 32 | 86.0 | 33.0 | < 0.0001 | ||
| 6 months | Denosumab 60 mg | 32 | 87.0 | 32.0 | < 0.0001 | 0.07 |
| Placebo | 32 | 92.0 | 38.0 | < 0.0001 | ||
| 12 months | Denosumab 60 mg | 32 | 93.5 | 36.0 | < 0.0001 | 0.01 |
| Placebo | 31 | 100 | 42.0 | < 0.0001 | ||
| 24 months | Denosumab 60 mg | 32 | 97.0 | 38.0 | < 0.0001 | 0.2 |
| Placebo | 31 | 100 | 42.0 | < 0.0001 | ||
| a Change from baseline. b Treatment difference |
||||||