Implant surface culture may be a useful adjunct to standard tissue sampling culture for identification of pathogens accounting for fracture-device-related infection: a within-person randomized agreement study of 42 patients
Nan JIANG 1, Yan-jun HU 1, Qing-rong LIN 1, Peng CHEN 2, Hao-yang WAN 2, Si-ying HE 2, Paul STOODLEY 3,4, and Bin YU 1,2
1 Division of Orthopaedics & Traumatology, Department of Orthopaedics, Southern Medical University Nanfang Hospital, Guangzhou, PR China; 2 Guangdong Provincial Key Laboratory of Bone and Cartilage Regenerative Medicine, Southern Medical University Nanfang Hospital, Guangzhou, PR China; 3 Departments of Microbial Infection and Immunity and Orthopaedics, The Ohio State University Wexner Medical Center, Columbus, OH, USA; 4 National Centre for Advanced Tribology at Southampton (nCATS) and National Biofilm Innovation Centre (NBIC), Department of Mechanical Engineering, University of Southampton, Southampton, UK
Background and purpose: Identification of pathogens causing fracture-device-related infection (FDRI) is always a challenge as the positive rate of standard tissue sampling culture (TSC) remains unsatisfactory. This study evaluates the efficiency of implant surface culture (ISC) as an adjunct to standard TSC for identification of FDRI-associated microorganisms.
Patients and methods: Between November 2020 and March 2022, patients diagnosed with FDRI defined by the International Fracture-Related Infection (FRI) Consensus Group, and indicated for implant removal, underwent both methods for bacteria detection. The test order of ISC and TSC was randomly selected for each patient included, as a withinperson randomized design. For ISC, the recovered implants were gently covered with tryptic soy agar after rinsing with normal saline twice, and then incubated at 37℃ 5% CO2 for up to 14 days. For TSC, 5 specimens were sampled and sent to the Clinical Laboratory of Southern Medical University Nanfang Hospital, Guangzhou, for culture and identification.
Results: 42 consecutive patients were included, with a mean age of 46 years. The most frequent infection site and implant type were the tibia (21 cases) and plates with screws (30 cases), respectively. Altogether 21 patients were found with positive outcomes by both methods, and the identified pathogens were consistent. ISC found an additional 15 patients showing positive results, which were negative by TSC. Furthermore, the mean culture time of ISC was shorter than that of TSC (1.5 days vs. 3.2 days).
Interpretation: ISC may be a useful adjunct to TSC for detection of bacteria causing FDRI, with a relatively higher positive rate and a shorter culture time.
Citation: Acta Orthopaedica 2022; 93: 703–708. DOI http://dx.doi.org/10.2340/17453674.2022.4530.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-10. Accepted: 2022-07-28. Published: 2022-09-07.
Correspondence: yubin@smu.edu.cn
NJ and YJH contributed equally to this study. PS and BY were co-corresponding authors. Study design: PS, BY. Conduct of experiment: PC, HYW, SYH. Participant screening and inclusion: NJ, YJH, QRL. Data acquisition: NJ, YJH, PC. Statistical analysis: NJ, HYW. Analysis and interpretation of data: NJ, YJH, BY. Paper drafting: NJ, BY. Paper revision: NJ, YJH, QRL, HYW, PC, SYH, PS, BY.
The authors are grateful for funding support from NIH R01, National Natural Science Foundation of China, and Guangdong Basic and Applied Basic Research Foundation. They would also like to thank the working staff, Dr Jing Chen from the Department of Clinical Laboratory of Southern Medical University Nanfang Hospital, for her help in pathogen identification.
Acta thanks Martin Buttaro and Charles Vogely for help with peer review of this study.
Fracture-device-related infection (FDRI) is defined as infection of the osseous tissue contacting an implant with or without infection of the surrounding soft tissue, resulting from contamination of pathogens and/or compromised immunity of the host (1). Diagnosis is sometimes difficult due to the non-specific symptoms, and treatment is often complex, with a high risk of infection recurrence (2,3). In addition, FDRI also brings a series of adverse events, such as prolonged length of hospital stay, multiple surgeries, limb amputation, side effects of antibiotic medications, and socioeconomic issues (4).
To increase the cure rate, one of the key points is how to correctly and effectively identify the related pathogens. Currently, intraoperative tissue sampling culture (TSC) remains the gold standard for bacteria identification (5). However, the positive rate of culture is not high (6). In order to increase the positive rate, different adjunctive strategies had been introduced, such as sonicate fluid culture (7) and intramedullary tissue culture (8).
Recently, a study reported using a novel “agar candle dip” method to map the bacterial biofilms on recovered orthopedic implants (9), which showed promise in growing bacteria directly from the implants. These findings suggest that culturing directly from implant surfaces may be an effective method for bacteria detection as there may exist bacterial biofilms attached to the implant surfaces. Therefore, we evaluated the efficiency of this method, here referred to as “implant surface culture” (ISC), which is more accurately descriptive, as an adjunct to standard TSC for identification of FDRI-related microorganisms.
Patients and methods
Study design, setting, inclusion, and exclusion criteria
This study, designed as a within-person randomized agreement study (10,11), was conducted following a prespecified protocol (see Supplementary data) in the Southern Medical University Nanfang Hospital, a tertiary healthcare center providing specialist treatment to patients with musculoskeletal infections. Included patients were those diagnosed with FDRI, who had had removal of the implants, and complied with the protocol. FDRI patients who underwent debridement, antibiotic therapy at the time of surgery, implant retention (DAIR) surgery, or nonoperative treatment or violated the protocol were not included. FDRI diagnosis was diagnosed according to the criteria outlined by the International Fracture-Related Infection (FRI) Consensus Group (12,13). FDRI was established based on any of the following confirmatory criteria, including a sinus tract and wound breakdown to the bone or the implant, pus in the fracture site, visible microorganisms on histological analysis, and presence of over 5 NP/HPF (14,15) on histology.
ISC and TSC procedures
All patients received routine tests after admission, and antibiotics were stopped for at least 2 weeks prior to surgery. During surgery, empirical intravenous cephalosporins or alternatively clindamycin were administered only after implants had been removed, and multiple specimens had been collected and sent for culture and histology. ISC and TSC were conducted independently, thus the test order of the 2 methods did not influence the outcomes.
For ISC, the removed implants were first rinsed with normal saline twice to wash out the residual blood, tissue, and potential planktonic bacteria, and were placed in aseptic culture plates in the operation room. The explants were then transported to the laboratory and processed within 2 hours. Their surfaces were gently covered with cooled but still molten tryptic soy agar (TSA) and incubated at 37℃ containing 5% CO2. The recovered implants were inspected every day for bacterial outgrowth, and if necessary sterile TSA was carefully supplemented in order not let the surface dry out. If bacterial colonies were found, at least 3 different sites were swabbed and sent to the Department of Clinical Laboratory of our hospital for pathogen identification. Such a procedure was continued for 14 days as suggested (16), or until there was evidence of bacterial colony growth. The implants were then discarded as medical waste.
For TSC, an experienced orthopedic surgeon sampled at least 5 different sites suspected of infection, usually at the implant–bone interface, using the “no-touch-technique” as recommended (13). The “No-touch technique” refers to separate, unused surgical instruments being used for each sample obtained, to avoid cross-contamination. Specimens were transported individually using sterile containers and processed within 2 hours by the Department of Clinical Laboratory of our hospital. The specimens were first homogenized separately in 10 mL of saline solution using glass beads, and were then inoculated into blood culture bottles (BACTEC Lytic/10 Anaerobic/F bottle and BACTEC Plus Aerobic/F bottle, Becton Dickinson, Franklin Lakes, NJ, USA) for incubation for at least 7 days. Any identified bacterial colonies were collected and sent for further identification.
Statistics
Statistical analysis was conducted using the Statistical Package for the Social Sciences software (version 17.0, SPSS Inc., Chicago, IL, USA). McNemar’s test and a paired t-test with 95% confidence interval (CI) were used to compare the positive rate and culture time between the 2 methods, respectively. All reported values were 2-sided with a p-value of < 0.05 considered statistically significant.
Ethics, data sharing, funding, and potential conflicts of interest
All patients signed the informed consent form, and this study was approved by the Medical Ethical Committee of the Southern Medical University Nanfang Hospital (NFEC-2020-075). Primary outcomes of the included patients are listed in Table 1; additional anonymized data can be shared on reasonable request. Research was funded by grants from NIH R01 (grant number: GM124436), National Natural Science Foundation of China (grant number: 82172197), and Guangdong Basic and Applied Basic Research Foundation (grant number: 2022A1515012385). There is no conflict of interest to declare.
Results
Participant flow, demographics, infection site, and implant type of the included patients
50 patients were considered for inclusion initially, and 42 were included finally. The test order of TSC and ISC was randomly performed, and each group had data of 42 patients analyzed (Figure 1). There were 33 males and 9 females. The mean age of the patients was 46 years (SD 15), with males and females aged 44 years (SD 14) and 54 years (SD 17), respectively. The most frequent infection site was the tibia (21 cases), followed by femur (12 cases), calcaneus (5 cases), humerus (1 case), patella (1 case), fibula (1 case), and clavicle (1 case). 30 patients had a plate and screws, with an intramedullary nail in 7 cases, Kirschner wire and steel wire in 4 cases, and a cannulated screw in 1 case.
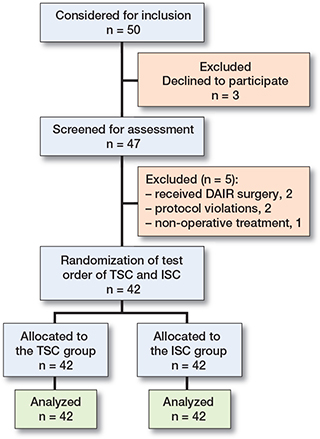
Figure 1. Flowchart of the FDRI patients included in this study.
Culture outcome and culture time by TSC and ISC
Among the 42 patients, 21 patients were found to have positive cultures by the 2 methods, and the detected pathogens were consistent between methods. However, ISC identified an additional 15 patients with positive culture outcomes, which were negative by the TSC method. Thus, the detection rate following ISC increased significantly (p = 0.001). Furthermore, the mean culture time of ISC was much shorter than that by TSC (1.5 days [SD 1.1] vs. 3.2 days [SD 0.7], p < 0.001) (CI of the difference –2.2 to –1.1 days) among the 21 patients. The detailed outcomes are listed in the Table, and Figure 2 displays 4 FDRI patients with positive outcomes using the ISC method.
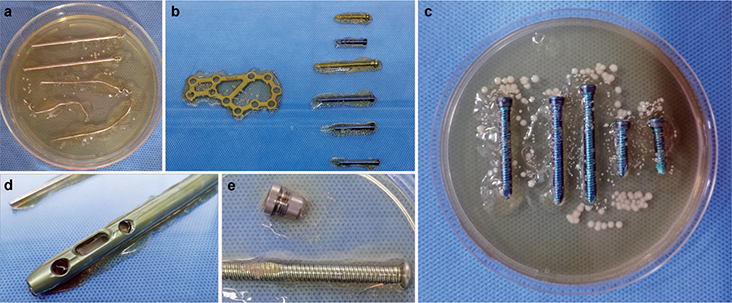
Figure 2. Presentations of 4 FDRI patients showed positive culture outcomes using the ISC method. a. Case no. 6: A 34-year-old male was diagnosed with patellar FDRI, with ISC of Kirschner wire and steel wire showing S. aureus colonies. The TSC outcome also revealed S. aureus infection but with a longer culture time (3 days vs. 1 day). b. Case no. 13: A 51-year-old male was diagnosed with calcaneal FDRI, with ISC of plate and screws displaying S. aureus colonies, which was supported by the TSC result. c. Case no. 14: A 63-year-old male was diagnosed with tibial FDRI, and the ISC outcome showed a mass of S. aureus colonies on and surrounding the screws, which was inconsistent with the TSC outcome. d,e. Case no. 29: A 53-year-old male was diagnosed with femoral FDRI, and ISC of the intramedullary and tail cap showed S. aureus colonies. However, the TSC outcome was negative.
Pathogen type
Among the 36 patients with positive cultures by ISC, 33 cases were identified with monomicrobial infection. Staphylococcus aureus (20 cases), Staphylococcus epidermidis (3 cases), and Enterococcus faecalis (3 cases) were the top 3 detected bacteria species (Table). There were 2 cases showing negative culture by ISC that were positive by TSC, one being Streptococcus dysgalactiae, the other Streptococcus intermedius.
Discussion
Surgery is one of the most effective ways to treat FDRI. For surgical treatment, aside from radical debridement, sensitive antibiotics use is another indispensable strategy to treat such infections. However, the optimal antibiotic therapy relies on correct and effective identification of FDRI associated pathogens. Nevertheless, to date, the positive rate of TSC is less than expected from other diagnostic criteria (6,17), despite growing investigations focusing on improving diagnostics by various sampling and culturing procedures of tissue samples (18-20). Here, we analyzed the efficiency of ISC as an adjunct to TSC for detection of FDRI-related pathogens, with reference to a previous study focusing on mapping bacterial biofilms on recovered orthopedic implants (9). Our results showed that, compared with the TSC, ISC was able to detect additional pathogens that failed to be identified by TSC, and also had a shorter time to culture. In addition, the culture outcomes were inconsistent with each other among patients with a positive culture between the two methods. These data suggest that ISC may be a useful adjunct to TSC for identification of FDRIrelated microorganisms.
Currently, TSC is still the mainstay to identify FDRI-associated pathogen, though the sensitivity of TSC remains unsatisfactory, ranging from 43% (21) to 89% (22), with most of the reported outcomes around 60% (23). It is known that TSC outcome is influenced by multiple factors, such as recent surgical intervention and antibiotic use, specimen selections, culture condition, and even the number of tissue samples for culture (18). In order to increase the positive rate of TSC, several recommendations have been proposed by the AO Foundation, the European Bone and Joint Infection Society (EBJIS), the Orthopaedic Trauma Association, and the PRO-IMPLANT Foundation, such as at least 2 weeks free of antibiotics, collection of 5 or more deep tissue or fluid samples, and administration of antibiotics immediately after sampling obtained (13).
Aside from TSC, new emerging techniques have recently been developed, also aimed at increasing the detection rate of FDRI-associated microorganisms. Based on a retrospective analysis of 230 cases, Bellova et al. (7) found that sonicate fluid culture might be a useful adjunct in diagnosis of FDRI, especially for low-grade infections. Outcomes of an updated meta-analysis revealed that traditional tissue sampling was more sensitive while sonication fluid sampling was more specific, and, thus, the authors suggested the integration of both methods to obtain optimal results (23). Aside from sonication fluid culture, a recent study (8) compared the efficiency of intramedullary tissue cultures from the Reamer–Irrigator–Aspirator (RIA) system with standard tissue culture. Although similar results were found between the 2 strategies, RIA-system cultures were able to display additional relevant pathogens that were not identified by traditional tissue culture. Therefore, the authors concluded that such a novel method could be used as an adjunct to standard tissue cultures. However, RIA does not allow easy acquisition of multiple independent samples, something that will increase the accuracy of tissue culture by avoiding potential false-positive results. In general, compared with TSC, such emerging pathogen identification strategies display advantages. However, their efficiency should be evaluated by future studies.
The satisfying outcome of the aforementioned “agar candle dip” method (9) implies that culture on explant components may be a useful strategy to identify FDRI-related pathogens because of the possibility of bacterial biofilms attached to the implant surfaces. We believe that ISC primarily has 3 advantages compared with TSC. First, whole-implant surface culture may reduce the risk of specimen selection bias. One of the important factors that affects TSC results is the selection of specimens during surgery, which largely relies on the surgeon’s experience. Thus, selection bias cannot be avoided. Also, biofilms are known to be heterogenous, often occurring as small aggregates of 10s to 100s of microns (24-26) and swabbing discrete locations may miss biofilm. Second, the bacterial biofilms attached to the explant surfaces may be less influenced by systematic antibiotic use and thus return a higher positive on culture. Although antibiotics were stopped for at least 2 weeks before surgery in our study, the detection rate of TSC was not as high as expected from other diagnostic criteria. Third, if biofilm is present it is allowed to grow from its in-situ environment without disturbing it with methods such as sonication scraping or swabbing. However, ISC also has several drawbacks. First, the implants need to be specially handled in the operation room and the implants take up more room in a clinical microbiology culture room. Additionally, pouring or supplementation of the TSA agar means that the implant was exposed for a relatively long period, which may increase the risk of contamination. It is notable that 4 patients with definite diagnosis of FDRI by another diagnostic criterion showed negative outcomes for both methods, which may be associated with several possible factors, such as special bacteria type or special requirements of culture conditions. We used TSA agar to cover the explant surface, but whether this is the optimal culture medium requires further investigation. There were still 2 cases showing negative outcomes following the ISC method but with positive outcomes by TSC; thus, whether ICS can be used in all FDRI cases or only specific patient group needs to be further evaluated as it requires specialized handling and training.
With regards to the difference and benefits between ISC and sonication, sonication is designed to remove biofilm bacteria from hardware into the surrounding media for subsequent culture. However, this necessarily requires that the biofilm is physically disrupted and it is not known how this may affect culture viability. Also, although it is very useful in improving culture recovery from whole implants, it does not provide information on how the biofilm may be distributed. The ISC method has the potential to identify particular components, materials, and surface features that may be more susceptible to biofilm formation. However, further studies are required to assess the importance of such mapping to current and future infection management.
Our study provides a new strategy for identification of FDRI-related pathogens. One limitation of the method rests with the fact that it cannot be conducted where implant hardware is not removed. The current study also has several limitations. First, the outcomes were based on an analysis of 42 patients, and the sample size was limited. To obtain more reliable conclusions, more participants should be included. Second, we conducted only preliminary comparisons between ISC and TSC, and to better evaluate ISC efficiency a control group should be considered to calculate its sensitivity and specificity. Third, our study did not trace the treatment efficacy, especially for those with TSC showing a negative result while ISC revealed a positive outcome. We believe that a positive culture outcome may have an influence on efficacy, which needs to be certified by future studies.
In summary, we found that ISC may be a useful adjunct to TSC for detection of FDRI-related microorganisms, identifying additional pathogens with a relatively shorter culture time.
- Jiang N, Wang B W, Chai Y M, Wu X B, Tang P F, Zhang Y Z, et al. Chinese expert consensus on diagnosis and treatment of infection after fracture fixation. Injury 2019; 50(11): 1952-8. doi: 10.1016/j.injury.2019.08.002.
- Baertl S, Metsemakers W J, Morgenstern M, Alt V, Richards R G, Moriarty T F, et al. Fracture-related infection. Bone Joint Res 2021; 10(6): 351-3. doi: 10.1302/2046-3758.106.BJR-2021-0167.R1.
- Bezstarosti H, Van Lieshout E M M, Voskamp L W, Kortram K, Obremskey W, McNally M A, et al. Insights into treatment and outcome of fracture-related infection: a systematic literature review. Arch Orthop Trauma Surg 2019; 139(1): 61-72. doi: 10.1007/s00402-018-3048-0.
- Walter N, Rupp M, Hierl K, Pfeifer C, Kerschbaum M, Hinterberger T, et al. Long-term patient-related quality of life after fracture-related infections of the long bones. Bone Joint Res 2021; 10(5): 321-7. doi: 10.1302/2046-3758.105.BJR-2020-0532.
- Post J C, Preston R A, Aul J J, Larkins-Pettigrew M, Rydquist-White J, Anderson K W, et al. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA 1995; 273(20): 1598-604.
- Wang B, Xiao X, Zhang J, Han W, Hersi S A, Tang X. Epidemiology and microbiology of fracture-related infection: a multicenter study in Northeast China. J Orthop Surg Res 2021; 16(1): 490. doi: 10.1186/s13018-021-02629-6.
- Bellova P, Knop-Hammad V, Königshausen M, Schildhauer T A, Gessmann J, Baecker H. Sonication in the diagnosis of fracture-related infections (FRI): a retrospective study on 230 retrieved implants. J Orthop Surg Res 2021; 16(1): 310. doi: 10.1186/s13018-021-02460-z.
- Onsea J, Pallay J, Depypere M, Moriarty T F, Van Lieshout E M M, Obremskey W T, et al. Intramedullary tissue cultures from the Reamer-Irrigator-Aspirator system for diagnosing fracture-related infection. J Orthop Res 2021; 39(2): 281-90. doi: 10.1002/jor.24816.
- Moley J P, McGrath M S, Granger J F, Sullivan A C, Stoodley P, Dusane D H. Mapping bacterial biofilms on recovered orthopaedic implants by a novel agar candle dip method. APMIS 2019; 127(3): 123-30. doi: 10.1111/apm.12923.
- Kottner J, Audigé L, Brorson S, Donner A, Gajewski B J, Hróbjartsson A, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol 2011; 64(1): 96-106. doi: 10.1016/j.jclinepi.2010.03.002.
- Pandis N, Chung B, Scherer R W, Elbourne D, Altman D G. CONSORT 2010 statement: extension checklist for reporting within person randomised trials. BMJ 2017; 357: j2835. doi: 10.1136/bmj.j2835.
- McNally M, Govaert G, Dudareva M, Morgenstern M, Metsemakers W J. Definition and diagnosis of fracture-related infection. EFORT Open Rev 2020; 5(10): 614-19. doi: 10.1302/2058-5241.5.190072.
- Govaert GAM, Kuehl R, Atkins BL, Trampuz A, Morgenstern M, Obremskey WT, et al. Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma 2020; 34(1): 8-17. doi: 10.1097/BOT.0000000000001614.
- McNally M, Ferguson J, Kugan R, Stubbs D. Ilizarov treatment protocols in the management of infected nonunion of the tibia. J Orthop Trauma 2017; 31(Suppl. 5): S47-S54. doi: 10.1097/BOT.0000000000000987.
- Morgenstern M, Athanasou N A, Ferguson J Y, Metsemakers W J, Atkins B L, McNally M A. The value of quantitative histology in the diagnosis of fracture-related infection. Bone Joint J 2018; 100-B(7): 966-72. doi: 10.1302/0301-620X.100B7.BJJ-2018-0052.R1.
- Steinmetz S, Wernly D, Moerenhout K, Trampuz A, Borens O. Infection after fracture fixation. EFORT Open Rev 2019; 4(7): 468-75. doi: 10.1302/2058-5241.4.180093.
- Zhao X Q, Wan H Y, Qin H J, Jiang N, Yu B. Interleukin-6 versus common inflammatory biomarkers for diagnosing fracture-related infection: utility and potential influencing factors. J Immunol Res 2021; 2021: 1461638. doi: 10.1155/2021/1461638.
- Dudareva M, Barrett L K, Morgenstern M, Atkins B L, Brent A J, McNally M A. Providing an evidence base for tissue sampling and culture interpretation in suspected fracture-related infection. J Bone Joint Surg Am 2021; 103(11): 977-83. doi: 10.2106/JBJS.20.00409.
- Morgenstern M, Kuhl R, Eckardt H, Acklin Y, Stanic B, Garcia M, et al. Diagnostic challenges and future perspectives in fracture-related infection. Injury 2018; 49(Suppl. 1): S83-S90. doi: 10.1016/S0020-1383(18)30310-3.
- Hellebrekers P, Rentenaar R J, McNally M A, Hietbrink F, Houwert R M, Leenen L P H, et al. Getting it right first time: the importance of a structured tissue sampling protocol for diagnosing fracture-related infections. Injury 2019; 50(10): 1649-55. doi: 10.1016/j.injury.2019.05.014.
- Banousi A, Evangelopoulos D S, Stylianakis A, Fandridis E, Chatziioannou S, Sipsas N V, et al. A comparative study of heterogeneous antibiotic resistance of microbial populations in conventional periprosthetic tissue cultures and sonication fluid cultures of orthopaedics explanted prostheses. Eur J Orthop Surg Traumatol 2020; 30(7): 1307-18. doi: 10.1007/s00590-020-02704-4.
- Finelli C A, da Silva C B, Murca M A, dos Reis F B, Miki N, Fernandes H A, et al. Microbiological diagnosis of intramedullary nailing infection: comparison of bacterial growth between tissue sampling and sonication fluid cultures. Int Orthop 2021; 45(3): 565-73. doi: 10.1007/s00264-020-04771-y.
- Ahmed E A, Almutairi M K, Alkaseb A T. Accuracy of tissue and sonication fluid sampling for the diagnosis of fracture-related infection: diagnostic meta-analysis. Cureus 2021; 13(5): e14925. doi: 10.7759/cureus.14925.
- McConoughey S J, Howlin R, Granger J F, Manring M M, Calhoun J H, Shirtliff M, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol 2014; 9(8): 987-1007. doi: 10.2217/fmb.14.64.
- Stoodley P, Nistico L, Johnson S, Lasko L A, Baratz M, Gahlot V, et al. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty: a case report. J Bone Joint Surg Am 2008; 90(8): 1751-8. doi: 10.2106/JBJS.G.00838.
- Palmer M P, Altman D T, Altman G T, Sewecke J J, Saltarski C, Nistico L, et al. Bacterial identification and visualization of bacterial biofilms adjacent to fracture sites after internal fixation. Genet Test Mol Biomarkers 2022; 26(2): 70-80. doi: 10.1089/gtmb.2019.0225.
Supplementary data
Protocol
After admission (before surgery):
Suspected of having FDRI by clinical signs and symptoms, imaging tests, or serological tests.
Screened for eligibility and informed consent signed.
Antibiotics have been stopped for at least for 2 weeks prior to surgery.
Surgery plans to remove the implants.
During surgery:
At least 3 different sites suspected of infection should be collected and sent for histology.
Empirical intravenous antibiotics can be used only after implants have been removed, and multiple specimens have been collected and sent for culture and histology.
ISC protocol:
Implant types include but not limited to plate, screw, intramedullary, Kirschner wire, and steel wire.
Rinse the removed implants with normal saline twice.
Put the explants on an aseptic culture plate.
Transfer the explants to the laboratory and dispose within 2 hours after implant removal.
Cover the surface of the explant surfaces gently with cooled but still molten tryptic soy agar (TSA).
Incubate the plate at 37℃ containing 5% CO2.
Inspect the explants every day for bacterial outgrowth and supplement TSA to the surfaces when necessary.
In the case of bacterial outgrowth, at least three different sites of colonies should be swabbed aseptically and sent to the Clinical Laboratory Department for microorganism identification.
Observe the explants for 14 days and if there is no evidence of bacterial outgrowth after 2 weeks, discard the explants harmlessly as medical waste.
Record the culture outcomes and culture time.
TSC protocol:
Sample at least 5 different sites suspected of infection, and implant–bone interface should be the superior site.
Use separate, unused surgical instruments for each sample collection.
Transport the specimens individually using sterile containers and disposal within 2 hours by the Clinical Laboratory Department.
Homogenize the specimens separately in 10 mL of saline solution using glass beads.
Inoculate the homogenized specimens into blood culture bottles (BACTEC Lytic/10 Anaerobic/F bottle and BACTEC Plus Aerobic/F bottle) for at least 7 days, and extend the culture time to 14 days when necessary.
Collect bacterial colonies observed for identification.
Record the culture outcomes and culture time.