Arthroplasty registries at a glance: an initiative of the International Society of Arthroplasty Registries (ISAR) to facilitate access, understanding, and reporting of registry data from an international perspective
Anne LÜBBEKE 1,2, Lotje A HOOGERVORST 3, Perla J MARANG-VAN DE MHEEN 3,4, Heather A PRENTICE 5, Ola ROLFSON 6, Rob G H H NELISSEN 3, Arnd STEINBRÜCK 7, Gearoid MCGAURAN 8, Christophe BAREA 1, Kajsa ERIKSON 6, Alma B PEDERSEN 9,a, Martyn PORTER 10,11,a; and the ISAR group
1 Division of Orthopaedics and Trauma Surgery, Geneva University Hospitals and University of Geneva, Switzerland; 2 Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, UK; 3 Department of Orthopaedics, Leiden University Medical Center, The Netherlands; 4 Safety & Security Science, Delft University of Technology, The Netherlands; 5 Medical Device Surveillance & Assessment, Kaiser Permanente, San Diego, CA, USA; 6 Department of Orthopedics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden and the Swedish Arthroplasty Register, Gothenburg, Sweden; 7 German Arthroplasty Registry (EPRD), Berlin, Germany; 8 Medical Devices Department, Health Products Regulatory Authority, Dublin, Ireland; 9 Department of Clinical Epidemiology, Aarhus University Hospital, DK and Department of Clinical Medicine, Aarhus University, DK; 10 Emeritus Consultant Orthopaedic Surgeon, Wrightington Hospital, UK; 11 Bristol University, UK
a Equal contributions as last authors
Background and purpose — The amount of information publicly available from arthroplasty registries is large but could be used more effectively. This project aims to improve the knowledge concerning existing registries to facilitate access, transparency, harmonization, and reporting.
Methods — Within the International Society of Arthroplasty Registries (ISAR) we aimed at developing, testing, adopting, and making publicly available a short, standardized registry description with items considered relevant for stakeholders using a cross-sectional study survey. Items were chosen based on a literature review and expert advice, selected by 9 ISAR working group members, tested iteratively in 3 registries, and commented upon by 4 external experts. All 29 ISAR member registries as of July 2023 were invited to participate in the project.
Results — Included items covered general descriptive information regarding registries, information related to governance, outcomes, data quality, data access, and registry production. The template was adopted, completed, and made publicly available by 25 of the 29 registries. Of those, 2/3 were national registries. 23 captured both hip and knee arthroplasties and 10 captured shoulder arthroplasties. Most registries had public reporting of data quality, methods, and results. Data was accessible in all but 2 registries, mainly as aggregated data. Important items relevant to registry quality for researchers to consistently indicate in scientific papers include scope, inclusion criteria, outcomes definitions, coverage/completeness, and validation processes.
Conclusion — This ISAR initiative implemented a short, standardized description to facilitate appropriate use of orthopedic registry data worldwide relevant for a diverse group of stakeholders including researchers, industry, public health and regulatory agencies.
Citation: Acta Orthopaedica 2025; 96: 116–126. DOI: https://doi.org/10.2340/17453674.2024.42706.
Copyright: © 2025 The Author(s). Published by MJS Publishing – Medical Journals Sweden, on behalf of the Nordic Orthopedic Federation. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Submitted: 2024-09-24. Accepted: 2024-12-09. Published: 2025-01-24.
Correspondence: anne.lubbekewolff@hug.ch
The authors would like to thank Tom Melvin, Petra Schnell-Inderst, and Robert Geertsma for their comments on earlier versions of the template and Uwe Otte for the realization of the figures and tables.
Handling co-editor: Bart A Swierstra
Acta thanks Ian Harris, Wierd P Zijlstra, and an anonymous reviewer for help with peer review of this manuscript.
Arthroplasty registries have existed since the 1970s [1]. Their value for stakeholders is well described and includes improving patients’ outcomes and quality of care as well as facilitating research [2-6]. Registries provide information on real-world outcomes on a large scale (e.g., at national level), which is increasingly used by other stakeholders, e.g., for post-market surveillance in regulatory decision-making [7-9] or in scientific publications [10].
The usefulness and usability of registry data depend largely on understanding how the data was obtained, why it was recorded, the quality of data analysis, and its users’ ability to interpret the results [11]. Annual reports and peer-reviewed publications from registries have shown differences regarding incidence and indication for surgery, patient characteristics, implants and fixation methods used, and implant survival. These differences could partly be due to factors such as variations in data collection methods, definitions, and data quality, highlighting the need for harmonization and transparency [12]. In 2005, the International Society of Arthroplasty Registries (ISAR) was established with the mission of “improving outcomes for individuals receiving joint replacement surgery worldwide” [13].
To enhance and facilitate appropriate use of registry data among stakeholders it is important to provide knowledge concerning registries that is publicly available, short, and harmonized across registries internationally. ISAR takes here the initiative to provide “Arthroplasty registries at a glance” and thereby to contribute to better and more effective use including consistent reporting of registry data.
Thus, this project aims to:
- develop a template for a short, focused, harmonized description of an arthroplasty registry, capturing the key characteristics needed to interpret and use their data;
- test the template’s content with ISAR participating registries and selected stakeholders;
- apply the template within the ISAR participating registries; and
- provide a use case: guidance for researchers regarding scientific reporting from registries.
Methods
Study design
This is a cross-sectional survey reporting on the key characteristics of each arthroplasty registry and the STROBE guidelines were followed. We describe the development, testing, and implementation of a short, standardized registry description in English. All ISAR member registries as of July 2023 were invited to participate in the initiative.
Development and testing of the template
A list of items to be considered for inclusion in the template was identified based on a literature review, previous experience with harmonization in international registry collaboration, and expert advice [11-12,14-17]. The list of items and their definitions was discussed during several ISAR workgroup meetings between March 2021 and March 2023. ISAR workgroup members (n = 9; with combined expertise in orthopedic surgery, arthroplasty registry lead, medical device regulation, and epidemiology), had the opportunity to exclude and include items on the list. The items that were agreed upon by all ISAR workgroup members formed the first draft of the ISAR registries template. After the ISAR registries template draft was made, it was tested by 3 registries (1 national, 1 regional, and 1 hospital-based) for ease of use and understanding and modified accordingly. In the next step the template was reviewed by medical device regulators (n = 2) and regulatory science researchers (n = 2). A revised version, which integrated their feedback, was again discussed during an ISAR workgroup meeting, where the final version of the template was agreed upon (see Supplementary data).
Data collection using the template and application
Data were collected from the registries using the online application Microsoft Forms (Microsoft Corp, Redmond, WA, USA) and transformed into a CSV file for final descriptive analysis. The completed template was sent back to each registry in a reader-friendly version to be made available by the registry on their website and/or in their annual report.
Use case: Guidance for scientific reporting of registry data
As an example of how information collected by the template can be used for a specific stakeholder group, in this case researchers, items were selected that should be included in scientific reporting of studies from arthroplasty registries. We showed how these linked to the requirements of the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) [18] by developing an extension to the statement. RECORD itself is an extension of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [19].
Results
Template description
The template included general information on the registry, information on input important to interpret the data, and information on the outputs produced by the registry. More specifically, the following elements (Supplementary data) were considered important and included in the template:
- general descriptive information (when, where, what, whom to contact);
- registry input information:
- information on data ownership, funding, and consent;
- definition of main outcomes;
- data quality (coverage, completeness, validation processes, response rates of patient-reported outcome measurements [PROMs]);
- data access (data linkage and data sharing);
- registry outputs (outcomes reported, reports and/or publications).
Data collection using the template
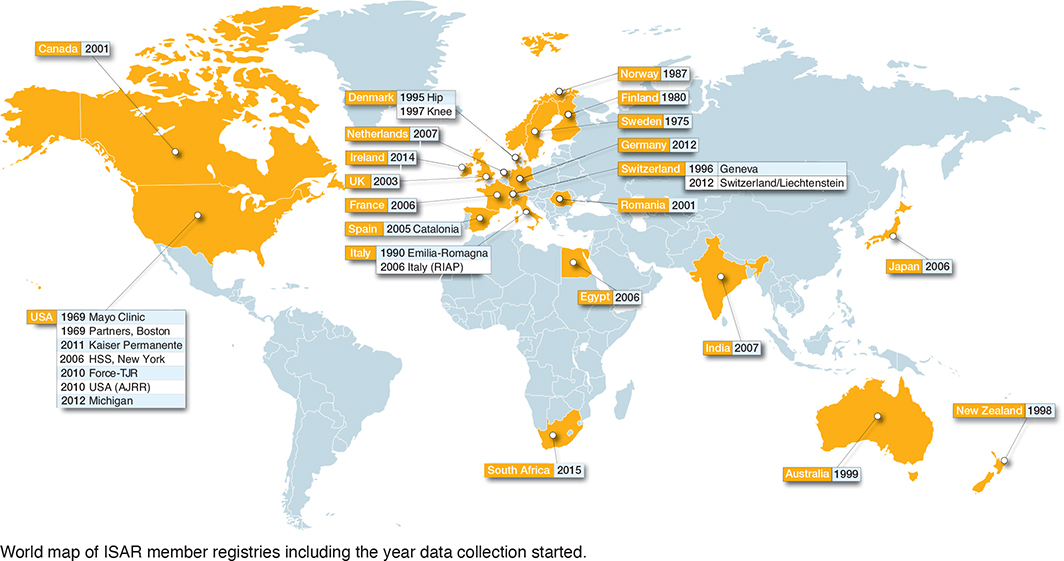
29 registries were identified as members of ISAR in 2023 (Figure). The first established registry was an institution-based registry from the United States of America (USA): the Mayo Clinic registry. The most recently established registry was the South African provider-based registry “JointCare” that started in 2015. Of the 29 eligible registries, 25 registries completed the ISAR template and were included in the analysis. Most registries were national (18 out of 25), and the remainder were regional (4), hospital-based (2), or provider-based (1) (Figure and Table 1). Data was owned either by a public authority (10 out of 25), a healthcare provider/institution (9 out of 25), or by the relevant national orthopedic society (6 out of 25). Most registries reported that they captured data on both hip and knee arthroplasties (23 out of 25), followed by shoulder arthroplasties (10), and a few registries captured data on ankle, elbow, spine, and hand implants (Table 1). 3 registries reported capturing all arthroplasty implants: the Australian, New Zealand, and Norwegian registries.
Table 1. Scope of registries and types of joint arthroplasties covered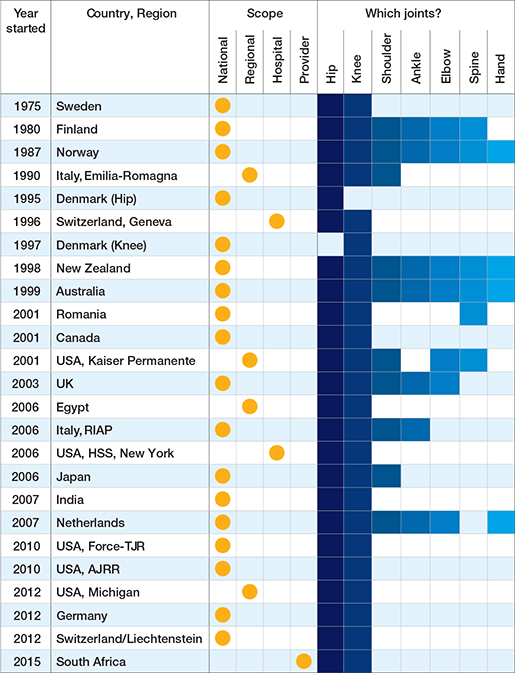
Hospital coverage (the number of participating hospitals relative to the total number of eligible hospitals) ranged from 12% in 1 registry to 100% in 14 registries and was unknown for 1 registry (Table 2). In general, hospital coverage increased with the number of years the registry had existed. Completeness of primary hip/knee procedures captured by the registry was ≥ 95% for 17 and ≥ 80% for 19 registries. Validation of completeness against an external data source was performed by 23 registries. Regarding outcomes reported by registries, revision for any cause was the most frequently reported outcome (n = 23) followed by PROs at n = 15. Specific reasons for revision were captured by 22 registries, and Unique Device Identifiers (UDIs) by 10 registries. Implant outlier identification procedures (i.e., procedures to identify implants with significantly higher risks than other comparable implants) were implemented by approximately half of the registries (n = 13). Of those 13, 8 reported the outlier implants publicly. Sharing registry data for research purposes with external parties was mostly possible, for anonymized patient-level data under specific conditions as well as for aggregated data (19 and 23, respectively) (Table 2). Publicly available annual reports were produced by 21 registries (Table 3). The completed individual registries’ templates are available as Supplementary data.
Table 2. Overview of coverage, procedure completeness, outcomes captured, and data sharing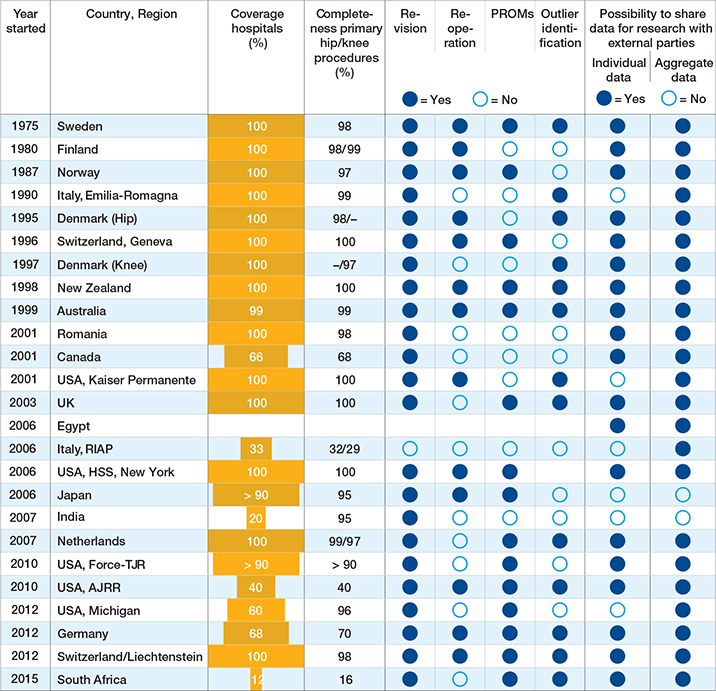
| Country/Region | Registry name | Annual report website |
| Sweden | Swedish Arthroplasty Register | sar.registercentrum.se/about-the-register/annual-reports/p/SJW4-ZGyo |
| Finland | Finnish Arthroplasty Register | thl.fi/far |
| Norway | Norwegian Arthroplasty Register (NAR) | helse-bergen.no/nrl |
| Italy, Emilia-Romagna | Register of Orthopaedic Prosthetic Implants (RIPO) | ripo.cineca.it/authzssl/Reports.html |
| Denmark | Danish Hip Arthroplasty Register | dhr.dk |
| Switzerland, Geneva | Geneva Arthroplasty Registry (GAR) | Available upon request |
| Denmark | Danish Knee Arthroplasty Registry | www.sundhed.dk/sundhedsfaglig/kvalitet/kliniske-kvalitetsdatabaser/planlagt-kirugi/knaealloplastikregister/ |
| New Zealand | New Zealand Joint Registry | www.nzoa.org.nz/nzoa-joint-registry |
| Australia | Australian Orthopaedic Association National Joint Replacement Registry | aoanjrr.sahmri.com/annual-reports-2022 |
| Romania | Romanian Arthroplasty Register | www.rne.ro |
| Canada | Canadian Joint Replacement Registry | www.cihi.ca/en/cjrr-annual-report-hip-and-knee-replacements-in-canada |
| USA, Kaiser Permanente | Kaiser Permanente Medical Device Surveillance and Assessment/National Implant Registries | annualreport.kpimplantregistries.org/annual-report/ |
| UK | National Joint Registry (NJR) | reports.njrcentre.org.uk/ |
| Egypt | Egyptian Community Arthroplasty Registry | None currently available |
| Italy | Italian Arthroplasty Registry (RIAP) | riap.iss.it/riap/en/activities/reports/ |
| USA, HHS, New York | Hospital for Special Surgery | None currently available |
| Japan | Japanese Orthopaedic Association National Registry/Japan Arthroplasty Register | https://www.joanr.org/ and https://jsra.info/ |
| India | Indian Society of Hip & Knee Surgeons | www.ishks.com |
| Netherlands | Dutch Arthroplasty Register (LROI) | www.lroi-report.nl |
| USA, Force-TJR | Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR) | Available upon request |
| USA, AJRR | American Joint Replacement Registry | aaos.org/registries |
| USA, Michigan | Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI) |
marcqi.org/marcqi-registry-reports-marcqi-annual-reports/ |
| Germany | German Arthroplasty Registry (EPRD) | www.eprd.de/en/downloads/reports |
| Switzerland/Liechtenstein | SIRIS—Swiss National Joint Registry Hip & Knee | www.siris-implant.ch/ |
| South Africa | JointCare Registry | www.joint-care.co.za |
Use of the template by researchers
As data from arthroplasty registries are used extensively by researchers, they are important for this group of stakeholders. Table 4 (see Appendix) shows the items that should consistently be reported—as an extension of the STROBE and RECORD checklists—in publications of arthroplasty-based research studies to better understand the data quality and interpret strengths and limitations of study findings.
Discussion
We developed a template for a short, focused, harmonized description of an arthroplasty registry, capturing the key characteristics needed to interpret and use their data. After testing its content, which was based on input from diverse registry stakeholder groups, in 3 different types of registries, the template was filled in and adopted by the vast majority of the registries.
We also provided a use case in the form of a list of items that should be reported in scientific publications based on arthroplasty registries. The items include the registry’s scope, inclusion criteria, outcomes definitions, coverage/completeness, and validation processes.
Taken together, the cross-sectionally obtained information from the templates shows that ISAR member registries monitor the outcomes of arthroplasty procedures in all continents, but with a larger concentration in Europe and North America, and that the monitoring focuses mainly on risks but in some cases also on benefits as measured with use of patient-reported outcomes. 2/3 of the registries operate on a national level, and half of them have been active for more than 2 decades. In the majority, data quality with respect to coverage and completeness is high, methods and results are reported transparently, and aggregated or individual data is accessible. Overall, the amount of publicly available information produced for stakeholders is extensive, but this information is underutilized by interested parties, especially in the regulatory field [20], and reporting of registry information in scientific publications is inconsistent.
This initiative to standardize information across ISAR member registries intends to increase the discoverability, accessibility, interpretability, and usability of registry data, by creating and implementing a short, standardized template to describe registries, thereby increasing their more effective use. For stakeholders who are interested in a specific arthroplasty implant—such as regulators, clinicians, notified bodies, and industry personnel—this template can help them to more easily identify all registries that collect data on that implant as well as outcomes. Moreover, this initiative has the objective to strengthen individual registries’ visibility and aims, and to further harmonize data input and output and increase quality and usability of the registries’ work. For researchers using registry data, the benefit is to facilitate consistent reporting within the RECORD checklist. The completed templates will be made available by each registry on their websites and/or at the beginning of their annual reports, and on the ISAR website (https://www.isarhome.org/). The templates will be updated annually.
Prior publications have either described general requirements with respect to the structure, quality, analysis, and use of medical device registries [11,15,16] or they have focused on specific medical specialties and areas, such as the scope, content, and quality of orthopedic and cardiovascular registries [12,21], the use of PROs in arthroplasty [22], or benchmarking and outlier identification in arthroplasty registries [23].
Strengths and limitations
In our work, for the first time, we developed, tested, and implemented a short-form template (“Arthroplasty registries at a glance”) in a specific society (ISAR) bringing together a particular group of arthroplasty registries. The current initiative covers the registries that were members of ISAR and agreed to participate in 2023. Participation rate of the members was high (25 out of 29). Nevertheless, in addition to any changes that may be needed in the existing template, it is essential that continued efforts are made, coordinated by ISAR, to ensure that new registries will be included and encouraged to adopt the template. Moreover, registries covering the spine tend to be more diverse and are often run by more than 1 medical society, thus these results may give only a partial view of existing spine registries worldwide. For the template to cover a more diverse range of medical device registries, some modifications will be necessary such as adding items regarding patient inclusion and exclusion criteria and adapting the main outcomes. Finally, while we included all ISAR members at a certain point in time there are a few other registries (n = 6) we are aware of that are not included in this work for reasons related to early phase of registry creation or pending ISAR membership.
Conclusion
We developed, tested, and implemented a template for a short, standardized description of ISAR member registries. The included items cover descriptive information and main registry input and output including outcome measures, information related to governance, data quality, data access, and registry production, which were identified as relevant elements for a diverse group of stakeholders. We also showed, as an extension to the RECORD checklist, how these items can be used in scientific reporting of studies using registry data. The template could be the step forward to improve harmonization, quality, interpretability, and usability of registry data, thereby allowing for a more effective and appropriate use by interested parties.
Supplementary data
Conflict of interest statements, STROBE checklist, ISAR template, list of ISAR members, and complete short descriptions of the 25 registries that participated are available as supplementary data on the article page, doi: 10.2340/17453674.2024.42706
- Malchau H, Garellick G, Berry D, Harris W H, Robertson O, Kärrholm J, et al. Arthroplasty implant registries over the past five decades: development, current, and future impact. J Orthop Res 2018; 36(9): 2319-30. doi: 10.1002/jor.24014.
- Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand 2000; 71(2): 111-21. doi: 10.1080/000164700317413067.
- Graves S. The value of arthroplasty registry data. Acta Orthop 2010; 81 (1): 8-9. doi: 10.3109/17453671003667184
- Galea V P, Rojanasopondist P, Matuszak S J, Connelly J W, Bragdon C R, Paxton L, et al. The benefits of national and regional arthroplasty registries. Instr Course Lect 2019; 68: 681-94. PMID: 32032065.
- Okafor C E, Nghiem S, Byrnes J. Are joint replacement registries associated with burden of revision changes? A real-world panel data regression analysis. BMJ Open 2023; 13(1): e063472. doi: 10.1136/bmjopen-2022-063472.
- Prentice H A, Harris J E, Sucher K, Fasig B H, Navarro J A, Okike K M, et al. Improvements in quality, safety and costs associated with use of implant registries within a health system. Jt Comm J Qual Patient Saf 2024; 50(6): 404-15. doi: 10.1016/j.jcjq.2024.01.011.
- Pijls B. The value of hip and knee arthroplasty registries. Expert Rev Med Devices 2023; 20(12): 1005-8. doi: 10.1080/17434440.2023.2282747.
- No author. Available from: https://www.ema.europa.eu/en/documents/work-programme/european-collaboration-between-regulators-and-health-technology-assessment-bodies-joint-work-plan-2021-2023-between-ema-and-european-hta-bodies-facilitated-through-eunethta21_en.pdf
- US Food and Drug Administration. Use of real-world evidence to support regulatory decision-making for medical devices. August 31, 2017. Available from: https://www.fda.gov/media/99447/download?attachment
- Romanini E, Schettini I, Torre M, Venosa M, Tarantino A, Calvisi V, et al. The rise of registry-based research: a bibliometric analysis. Acta Orthop 2021; 92 (5): 628-32. doi: 10.1080/17453674.2021.1937459.
- Gliklich R E, Dreyer N A, Leavy M B, editors. Registries for evaluating patient outcomes: a user’s guide [Internet]. 4th ed. Chapter 13: Analysis, interpretation, and reporting of registry data to evaluate outcomes. Rockville, MD: Agency for Healthcare Research and Quality (US2020 Sep. Report No.: 19(20)-EHC020.AHRQ Methods for Effective Health Care. PMID: 33001604. Bookshelf ID: NBK562575.
- Hoogervorst L A, Geurkink T H, Lübbeke A, Buccheri S, Schoones J W, Torre M, et al. Quality and utility of European cardiovascular and orthopaedic registries for the regulatory evaluation of medical device safety and performance across the implant lifecycle: a systematic review. Int J Health Policy Manag 2023; 12: 7648. doi: 10.34172/ijhpm.2023.7648.
- International Society of Arthroplasty Registries (ISAR). Available from: https://www.isarhome.org/home
- Mäkelä K, Furnes O, Hallan G, Fenstad A M, Rolfson O, Kärrholm J, et al. The benefits of collaboration: the Nordic Arthroplasty Register Association. EFORT Open Rev 2019; 4(6): 391-400. doi: 10.1302/2058-5241.4.180058.
- IMDRF Registry Working Group. Patient registry: essential principles; 2 October 2015. Available from: https://www.imdrf.org/sites/default/files/2021-09/imdrf-cons-essential-principles-151124.pdf.
- Niederländer C S, Kriza C, Kolominsky-Rabas P. Quality criteria for medical device registries: best practice approaches for improving patient safety – a systematic review of international experiences. Expert Rev Med Devices 2017; 14(1): 49-64. doi: 10.1080/17434440.2017.1268911.
- Quality Framework and Guidelines for OECD Statistical Activities. OECD; 2011. Available from: https://www.oecd.org/sdd/qualityframeworkforoecdstatisticalactivities.htm
- Benchimol E I, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med 2015; 12(10): e1001885. doi: 10.1371/journal.pmed.1001885
- von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18(6): 800-4. doi: 10.1097/ede.0b013e3181577654.
- Zippel C, Bohnet-Joschko S. Post market surveillance in the German medical device sector: current state and future perspectives. Health Policy 2017; 121(8): 880-6. doi: 10.1016/j.healthpol.2017.06.005.
- Lübbeke A, Silman A J, Barea C, Prieto-Alhambra D, Carr A J. Mapping existing hip and knee replacement registries in Europe. Health Policy 2018; 122(5): 548-57. doi: 10.1016/j.healthpol.2018.03.010.
- Wilson I, Franklin P D, Lübbeke A, Lyman S, Overgaard S, Rolfson O, et al. Orthopaedic registries with patient-reported outcome measures. EFORT Open Rev 2019; 4(6): 357-67. doi: 10.1302/2058-5241.4.180080.
- De Steiger R N, Hallstrom B R, Lübbeke A, Paxton E W, van Steenbergen L N, Wilkinson M. Identification of implant outliers in joint replacement registries. EFORT Open Rev 2023; 8(1): 11-17. doi: 10.1530/eor-22-0058.