Safety notices and registry outlier data measure different aspects of safety and performance of total knee implants: a comparative study of safety notices and register outliers
Lotje A HOOGERVORST 1,a, Yijun REN 2,a, Tom MELVIN 3, Ashley A STRATTON-POWELL 3, Anne LÜBBEKE 4,5, Robert E GEERTSMA 6, Alan G FRASER 7, Rob G H H NELISSEN 1, Enrico G CAIANI 2,8,b, Perla J MARANG-VAN DE MHEEN 1,9,b
1 Department of Orthopaedics, Leiden University Medical Center, Leiden, the Netherlands; 2 Department of Electronics, Information and Biomedical Engineering, Politecnico di Milano, Milan, Italy; 3 School of Medicine, Trinity College Dublin, Dublin, Ireland; 4 Division of Orthopaedic Surgery and Traumatology, Geneva University Hospitals and University of Geneva, Geneva, Switzerland; 5 Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK; 6 Centre for Health Protection, National Institute for Public Health and the Environment, Bilthoven, the Netherlands; 7 Department of Cardiology, University Hospital of Wales, Heath Park Way, Cardiff, UK; 8 Istituto Auxologico Italiano IRCCS, Milan, Italy; 9 Safety & Security Science and Centre for Safety in Healthcare, Delft University of Technology, Delft, the Netherlands
a Shared first authorship
b Shared last authorship
Background and purpose — Safety notices for medical devices such as total knee arthroplasty (TKA) implants may indicate problems in their design or performance that require corrective action to prevent patient harm. Safety notices are often published on national Ministries of Health or regulatory agencies websites. It is unknown whether problems triggering safety notices identify the same implants as those identified by registries as “outlier.” We aimed to assess the extent to which safety notices and outlier identification in registries signal the same or different TKA implants.
Methods — The CORE-MD tool, an automated web scraper tool, was used to collect safety notices related to TKA implants on 13 national Ministries of Health websites and regulatory agencies. Safety notices were defined according to the Medical Device Regulation (MDR) as “a communication sent by a manufacturer to users or customers in relation to a field safety corrective action.” Identified TKA outliers, defined as having a significantly higher revision risk than other comparable TKA implants, were extracted from registry reports.
Results — 787 safety notices for 38 TKA implants and 35 TKA outliers were identified, together identifying 47 unique TKA implants. 26 (55%) TKA implants had safety notices and were also outliers, 12 (26%) TKA implants had only safety notices, and 9 (19%) were outliers only. TKA implants with safety notices only had similar types of problems to TKA outliers with safety notices, with “Manufacturing/Packaging/Shipping” problems being most frequent (44%). Cumulative revision risks (1/5/10 years) were lower for TKA implants with safety notices only than for TKA outliers with safety notices.
Conclusion — 55% of the TKA with a safety notice were identified as outliers in the registry, whereas around 25% of TKA outliers are not the subject of publicly released safety notices, with safety notices pointing to TKA implants not identified by registries as potentially having a higher risk of failure. This suggests that safety notices and registry outlier data measure different aspects of safety and performance.
Citation: Acta Orthopaedica 2024; 95: 667–676. DOI: https://doi.org/10.2340/17453674.2024.42361.
Copyright: © 2024 The Author(s). Published by MJS Publishing – Medical Journals Sweden, on behalf of the Nordic Orthopedic Federation. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits sharing, adapting, and using the material for any purpose, including commercial use, with the condition of providing full attribution to the original publication.
Submitted: 2024-05-04. Accepted: 2024-10-23. Published: 2024-11-25.
Correspondence: l.a.hoogervorst@lumc.nl
LH, YR: data collection, statistical analysis, interpretation of data, writing the original draft of the manuscript. AAS-P, AGF, AL, EGC, REG, RGHHN, and TM: critical revision of the manuscript for important intellectual content. PJM-vdM: interpretation of data, critical revision of the manuscript for important intellectual content.
Handling co-editors: Keijo Mäkelä and Robin Christensen
Acta thanks Gary Hooper and Einar Andreas Sivertsens for help with peer review of this manuscript.
Medical devices are subject to post-market surveillance (PMS) where manufacturers have to collect and review data on experience with their devices [1]. Once collected, these data must be analyzed by the manufacturer to evaluate if any corrective or preventive actions are needed. If action is required to prevent patient harm, a safety notice must be issued [2]. Safety notices can be published on the websites of manufacturers, Ministries of Health, and regulatory agencies. Safety notices may include a recall, amended instructions for use, adverse events, or additional information concerning the device. From a safety and performance perspective, total knee arthroplasty (TKA) implants are of interest, as together with total hip implants they are the most used arthroplasty implants.
Safety notices are relevant for clinicians and hospitals as they may guide implant selection. Safety notices can be issued for a wide variety of implant-related issues (e.g., packaging and labeling), which are not always associated with the safety or performance of a TKA implant. On the other hand, several arthroplasty registries have procedures in place to identify TKA implants with outlier performance (i.e., a significantly higher revision risk than other comparable implants) [3], defined solely based on revision risk [4,5]. Safety notices, however, may also refer to signals based on other outcomes (e.g., poor patient satisfaction scores), meaning that safety notices and outlier identification may reflect different aspects of patient safety. Furthermore, safety notices may be issued based on other data sources such as peer-reviewed publications. Hence, it is unknown whether problems triggering safety notices identify the same TKA implants as those identified by registries as outliers.
We aimed to assess the extent to which safety notices and outliers identified by registries signal the same or different TKA implants, and to explore possible reasons for any discrepancies.
Methods
Study design
This study focused on the agreement between 2 real-world data sources that are intended to signal problems related to medical devices, and more specifically to assess whether TKA implants for which safety notices were published on the websites of Ministries of Health and regulatory agencies were the same as the TKA implants identified and publicly reported by registries as outliers. Only TKA implants currently used on the market were included. The study was conducted according the to the STARD guidelines [6].
Data collection of safety notices reporting TKA implants
The Coordinating Research and Evidence for Medical Devices (CORE-MD) PMS tool [7], an automated web scraper tool, was used to identify TKA implants with safety notices on the websites of Ministries of Health and regulatory agencies. 13 countries were included in the CORE-MD tool and were therefore assessed in the current study: Australia, Czechia, Denmark, France, Germany, Greece, Ireland, Italy, Portugal, Spain, Sweden, the United States of America (USA), and the Netherlands. Note that all historical and publicly available safety notices were retrieved for each country with their respective last update (Supplementary Table 1).
Details of the applied methodology in the CORE-MD PMS tool have been published [7]. Briefly, the tool screens the website of each Ministry of Health and regulatory agency to collect all safety information, including safety notices, alerts, and recalls. We refer to safety notices to indicate the collective safety information found on these websites.
To include only safety notices for TKA implants currently on the market, a list of all TKA implants from the latest annual reports from the following national and regional registries was constructed: American Joint Replacement Registry (AJRR) [8], Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) [9], Dutch Arthroplasty Register (LROI) [10], Emilia-Romagna Register (RIPO) [11], German Arthroplasty Registry (EPRD) [12], Swiss National Hip & Knee Joint Registry (SIRIS) [13], and the National Joint Registry for England, Wales, Northern Ireland, the Isle of Man and Guernsey (NJR) [14], and up-to-date registry-website data from the Finnish Arthroplasty Register (FAR) [15]. Note that some countries included in the CORE-MD PMS tool to identify safety notices are not used to construct the list of TKA implants currently on the market as they lack a(n) (active) regional or national arthroplasty registry capturing data on TKA implants [3]. We assumed that safety notices would identify problems that relate to the implant itself rather than reflecting, e.g., limited experience by surgeons or patient case-mix, and thereby that the problems highlighted in these countries would reflect problems elsewhere.
The brand name of each TKA implant on this list was used as input for the CORE-MD PMS tool, to extract all associated safety notices for further analysis. Based on the extended safety notice text, the described adverse event was linked to an International Medical Device Regulators Forum (IMDRF) medical device problem code [16]. These IMDRF codes have a hierarchical alphanumerical coding structure, including a letter (i.e., referring to the Annex A in our case) followed by numerical codes at different levels of detail [16,17]. Level 1 terms are represented by the first 2 digits, referring to 27 different medical device problems (Table 1). Level 2 and 3 terms are described by the digits 3 to 4 and 5 to 6 respectively, representing a more detailed description of the problem under 1 of the overarching 27 groups. In this study only the Level 1 terms were used, as they are already detailed enough to distinguish different device problems. All safety notices related to TKA implants were independently classified to an IMDRF code by 2 researchers (LH and YR); possible discrepancies in coding were resolved by discussion. To determine interobserver variability, the Cohen’s kappa (κ) was calculated. Kappa values were categorized into 6 levels: (i) κ ≤ 0 (no agreement); (ii) κ = 0.01–0.20 (none to slight); (iii) κ = 0.21–0.40 (fair); (iv) κ = 0.41–0.60 (moderate); (v) κ = 0.61–0.80 (substantial), and (vi) κ = 0.81–1.00 (almost perfect) (18). Analysis was performed using Python (version 3.11.5; https://www.python.org/downloads/release/python-3115/).
Data collection of registries reporting TKA outliers
Outlier TKA implants currently on the market were identified by European registries publicly reporting on TKA outliers, as found in a recent systematic review [3] and non-European registries as listed on the website of the AOANJRR [19]. All available registries’ annual reports and websites were screened, and any reported TKA outlier was extracted. For all extracted TKA outliers, it was assessed whether they were reported in the latest annual reports and up-to-date website, representing TKA implants currently on the market in these registries. If the TKA outlier was not reported in the latest available registry data (i.e., not implanted in the past year in the included registries), the outlier was considered an off-market implant and excluded from further analysis.
TKA outlier definitions differed between these registries (AOANJRR: “The revision rate (per 100 component years) exceeds twice that for the group and the Poisson probability of observing that number of revisions, given the rate of the group is significant (P < 0.05)”; NJR: “having a more than twice the prosthesis time incident rate when compared to the group, allowing for confidence intervals”; SIRIS: “Revision rates of more than twice compared to the relevant group”; and the definition of an outlier was not reported for the SAR) [20].
For all TKA outliers, the year of first identification and its cumulative revision risks (1/5/10 years), including standard errors (SE) and/or 95% confidence intervals (CI), were extracted. If only the 95% CI was provided, the SE was calculated by subtracting the upper and lower bound of the 95% CI and dividing it by 3.92 [21].
Statistics
First, the overlap between TKA implants with safety notices and TKA outliers was determined by comparing the brand name reported in both safety notices and registry data. 3 groups were distinguished: (i) TKA implants with safety notices but not identified as an outlier (“safety notices only”); (ii) TKA implants with both safety notices and identified as an outlier (“both”); (iii) TKA implants without safety notices but identified as an outlier (“outlier only”). The percentage of TKA implants in each of these groups was related to the number of unique TKA implants identified by both data sources.
Second, to prevent camouflage (i.e., multiple compatible construct combinations existing within 1 implant brand name [22]), the overlap between TKA implants with safety notices and TKA outliers across different variants/subtypes under the same brand name was analyzed. We considered possible subtypes with the same brand name by: (i) fixation (e.g., cemented versus uncemented); (ii) stability (e.g., cruciate retaining versus hinged), and (iii) mobility (e.g., fixed versus mobile).
Third, to explore possible reasons for not signaling the same TKA implants we examined: (i) differences in the frequency of IMDRF codes (Table 1) between the “safety notices only” and “both” groups, and (ii) whether the “safety notices only” group had lower cumulative revision risks (and thus seemingly better performance) than the “both” group, which may explain why they were not detected as TKA outliers. Random effects models were used to calculate the pooled cumulative revision risks (1/5/10 years) across all registries reporting on the specific TKA implant, for the “safety notices only” and “both” groups.
The metafor package in R-statistics (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) was used for analyses.
Ethics, registration, data sharing, use of AI, funding, and disclosures
According to Dutch law, no institutional approval was required. This work was supported by the European Union’s Horizon 2020 Research and Innovation 41 Programme (grant number 965246) and was part of the CORE-MD project. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2024.42361
Results
TKA implants with safety notices
The CORE-MD PMS tool retrieved 104,638 safety notices from 13 Ministries of Health and regulatory agencies websites, of which 1,327 safety notices were considered relevant as they matched with a specific TKA implant included in the latest annual registry reports. For the selected 1,327 safety notices, 540 safety notices were excluded because they were not related to a TKA implant (i.e., associated with surgical protocols) thus resulting in 787 safety notices included for further analysis (Figure and Supplementary Table 1). These 787 safety notices were relevant to 38 unique TKA implant brand names. Most safety notices originated from the USA and were associated with the Nexgen (Zimmer Biomet) (n = 243, 31%) (Table 2).
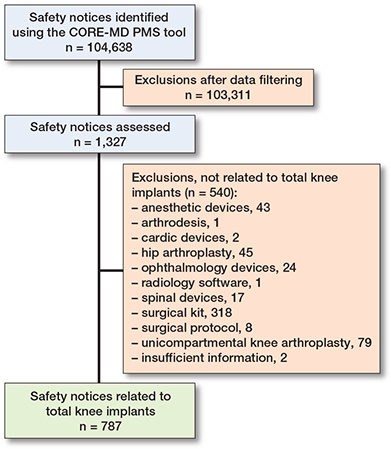
Flowchart showing the selection process of TKA implants with safety notices.
Outlier TKA implants
4 national registries (AOANJRR, NJR, Swedish Arthroplasty Register [SAR], and SIRIS) publicly reported TKA outliers while others might report them on a secure website [3]. After removing duplicate TKA outlier brand names (i.e., the same brand name was mentioned in multiple annual reports) and off-market TKA outliers, 35 unique TKA outlier brand names were included for further analysis (Table 3). Supplementary Table 2 gives more detailed information on specific subtypes within a brand that were identified as outlier along with their performance.
Overlap between TKA outliers and TKA implants with safety notices
Combining the brand names of the 38 TKA implants with a safety notice and the 35 TKA outliers resulted in 47 unique TKA implant brand names, of which 26 (55%) were in the “both” group, 12 (26%) in the “safety notices only” group, and 9 (19%) in the “outlier only” group (Tables 3 and 4). Thus, safety notices did not signal 9 (26%) of the 35 TKA outliers and registries did not signal 12 (32%) of the 38 TKA implants that had safety notices.
Considering the 26 TKA implants in the “both” group, 7 (27%) TKA implants did not have any information in the safety notice regarding fixation, 10 (38%) had no information regarding stability, and 15 (57%) no information regarding mobility, which would be needed to determine whether the exact same TKA implant was concerned (white color, Table 4). Focusing on fixation, 4 out of 26 (15%) TKA implants could be matched to the cemented subtype and 6 (23%) to the uncemented subtype (Table 4). With regard to stability, 2 out of 26 (8%) related to the cruciate retaining, 2 (8%) to the hinged, and 8 (31%) to the posterior stabilized subtype. For mobility, 1 (4%) signaled the fixed, 1 (4%) the mobile, and 5 (19%) the rotating subtype. However, 14 (54%) cemented and 3 (12%) uncemented TKA implants did not relate to the same fixation subtype (Table 4). Similarly, 6 (23%) cruciate retaining, 2 (8%) hinged, and 7 (27%) posterior stabilized TKA implants did not have the same stability and 3 (12%) fixed, 5 (19%) mobile, and 2 (8%) rotating TKA implants did not relate to the same mobility subtype.
Revision rates, timing of safety concerns, and implant problems
For the “both” group, the median 1/5/10-year cumulative revision risks were 1.6% (range: 0.9–9.5), 6.3 (range: 3.6–23.8), and 8.2% (range: 5.6–23.8), respectively, compared with 0.6% (range: 0.3–1.1), 2.3% (range: 1.4–3.7), and 3.8% (range: 3.1–5.1), for the “safety notices only” group (Table 5).
| Implant | Date of first safety notice | Identified as outlier | Year first identified as outlier and registry reporting | Pooled cumulative revision risk (CI) for specified implant brand name | ||
| 1-year | 5-year | 10-year | ||||
| Active Knee | 21/10/2016 | Yes | 2016 (AOANJRR) | 1.1 (0.9–1.4) a | 5.0 (4.6–5.6) a | 8.8 (8.1–9.5) a |
| Advance | 11/7/2016 | Yes | 2013 (AOANJRR) | 2.0 (1.3–3.1) a | 6.4 (5.0–8.2) a | 8.1 (6.4–10.2) a |
| AGC Anatomic | 21/7/2015 | Yes | 2014 (SAR) | – | – | – |
| Attune | 29/6/2015 | Yes | 2023 (AOANJRR) | 1.8 (1.0–3.0) a | – | – |
| Balansys | 29/1/2014 | No | – | 0.9 (0.5–1.2) a,d,e,f | 3.1 (2.3–3.9) a,c,d,e,f | 5.1 (2.2–8.1) a,d |
| Columbus | 17/1/2008 | Yes | 2009 (AOANJRR) | 1.2 (0.9–1.5) a | 4.4 (3.7–5.3) a | 7.3 (6.0–8.8) a |
| Duracon | 20/9/2007 | Yes | 2004 (SAR) | – | – | – |
| EFK | 15/4/2014 | No | – | 0.6 (0.1–1.2) f | 1.7 (0.5–3.0) f | – |
| Endo–Model | 16/4/2012 | Yes | 2019 (NJR) | 1.3 (0.8–2.2) b | 4.8 (3.7–6.3) b | 7.0 (5.3–9.2) b |
| Evolution | 17/2/2015 | No | – | 0.7 (0.3–1.1) a,b,f,g | 2.8 (2.1–3.5) a,b,g | – |
| Gemini | 7/9/2010 | Yes | 2007 (AOANJRR) | 9.5 (2.5–33.0) a | 23.8 (10.7–48.1) a | 23.8 (10.7–48.1) a |
| Genesis | 9/5/2006 | Yes | 2004 (AOANJRR), | 1.0 (0.7–1.3) a,b | 3.6 (3.2–4.1) a,b | 5.6 (4.8–6.3) a,b |
| 2018 (SAR), 2021 (NJR) | ||||||
| GMK Sphere | 3/7/2017 | No | – | 1.1 (0.9–1.4) a,b,e,f,g | 3.7 (2.9–4.5) a,b,e,g | 4.3 (2.4–6.1) a |
| Innex | 25/7/2005 | No | – | 0.9 (0.5–1.3) d,e,f | 2.8 (2.0–3.6) c,d,e,f | 3.5 (2.4–4.6) d |
| iTotal | 23/7/2012 | No | – | 0.4 (0.2–0.9) e | 3.5 (2.5–5.0) e | – |
| Journey | 3/1/2014 | Yes | 2009 (AOANJRR), 2018 (SAR), | 1.6 (0.1–3.1) a,b,e | 6.3 (1.8–10.8) a,b,e | 11.0 (9.9–12.2) a |
| 2019 (SIRIS), 2014 (NJR) | ||||||
| Kinemax | 14/5/2015 | Yes | 2006 (SAR) | – | – | – |
| K-mod | 19/5/2021 | No | – | – | – | – |
| LCS | 2/12/2005 | Yes | 2012 (AOANJRR), 2021 (NJR) | 0.9 (0.2–1.6) a,b | 5.6 (1.8–9.5) a,b | 7.7 (2.5–12.8) a,b |
| Legion | 22/8/2009 | Yes | 2017 (AOANJRR), 2019 (SAR) | 3.3 (2.3–4.6) a | 6.3 (4.8–8.3) a | 9.9 (7.5–13.0) a |
| METS Smiles | 17/8/2016 | Yes | 2018 (NJR) | – | – | – |
| MRK | 31/12/2021 | No | – | 0.3 (0.0–0.6) a,b,d | 1.8 (1.2–2.3) a,b,d | 3.1 (1.6–4.6) a,b |
| Multigen | 12/5/2021 | No | – | – | – | – |
| Mutars | 3/4/2013 | Yes | 2023 (AOANJRR) | 6.5 (4.2–9.9) a | – | – |
| Natural-knee | 7/11/2019 | No | – | 0.4 (0.2–0.7) a,d,f,g | 1.7 (1.2–2.1) a,d,f,g | 3.2 (2.4–3.9) a,d |
| Nexgen | 13/9/2004 | Yes | 2018 (AOANJRR), 2015 (SAR) | 2.4 (1.9–3.2) a | 5.0 (4.2–6.1) a | 6.9 (5.1–9.2) a |
| Noiles | 2/3/2014 | Yes | 2018 (NJR) | – | – | – |
| Optetrak | 1/6/2006 | Yes | 2007 (AOANJRR) | 1.0 (0.0–2.1) a | 10.3 (4.1–16.4) a | 13.7 (7.0–20.4) a |
| Persona | 21/11/2012 | Yes | 2021 (SAR) | – | – | – |
| PFC Sigma | 2/12/2005 | Yes | 2018 (AOANJRR), 2012 (SAR) | 2.2 (1.1–4.6) a | 7.1 (4.7–10.5) a | 7.4 (5.0–10.9) a |
| Physica | 18/4/2019 | Yes | 2019 (SIRIS) | 1.7 (1.3–2.3) e | 6.8 (5.9–7.9) e | – |
| Saiph | 25/3/2022 | No | – | 0.6 (0,3–1,0) b | 1,4 (0,9–2,0) b | – |
| Score | 4/10/2019 | Yes | 2013 (AOANJRR) | 1.5 (0.8–2.2) a | 6.5 (5.5–7.6) a | 11.1 (9.3–12.8) a |
| Scorpio | 26/8/2005 | Yes | 2014 (AOANJRR) | 1.2 (0.7–2.0) a | 6.1 (4.9–7.7) a | 7.4 (6.0–9.2) a |
| TC-plus | 10/6/2008 | Yes | 2008 (AOANJRR) | 1.6 (0.2–10.7) a | 8.4 (3.6–19.1) a | 14.4 (7.4–26.9) a |
| Triathlon | 7/2/2007 | Yes | 2021 (SAR) | – | – | – |
| Unity | 30/9/2021 | No | – | 0.4 (0.1–0.9) a,b,f | 1.5 (0.7–2.3) b,f | – |
| Vanguard | 17/11/2016 | Yes | 2012 (AOANJRR), 2009 (SAR) | 1.9 (1.2–2.6) a | 5.9 (4.7–7.1) a | 8.2 (6.8–9.5) a |
| a Based on revision risks as reported by the AOANJRR; | ||||||
| b based on revision risks as reported by the NJR; | ||||||
| c based on revision risks as reported by the RIPO; | ||||||
| d based on revision risks as reported by the LROI; | ||||||
| e based on revision risks as reported by the SIRIS; | ||||||
| f based on revision risks as reported by the EPRD; | ||||||
| g based on revision risks as reported by the AJRR. | ||||||
| TKA = total knee arthroplasty; CI = confidence intervals; AOANJRR = Australian Orthopaedic Association National Joint Replacement Registry; SIRIS = Swiss National Hip & Knee Joint Registry; SAR = Swedish Arthroplasty Register; NJR = National Joint Registry for England; Wales, Northern Ireland, the Isle of Man and Guernsey. | ||||||
When comparing the dates of the first issuance of safety notices with the dates when the implant was first identified as outlier by registries, no specific data source consistently published safety signals earlier (Table 5).
For the 26 TKA implants in the “both” group, 728 safety notices were issued with the most frequently reported problem being related to “A02–Manufacturing, Packaging or Shipping” (43%), followed by “A23–Use of Device” (16%) (Table 6). The most frequent type of problem found was similar for the 12 TKA implants in the “safety notices only” group (n = 59 safety notices): “A02–Manufacturing, Packaging or Shipping” (44%) (Table 6). Focusing on differences between the 2 groups, safety notices related to “A05–Mechanical Problem” (6%) and “A17–Compatibility Problem” (8%), respectively, were reported only for the “both” group (Table 6) but not encountered for the “safety notices only” group (Table 6). The interobserver agreement to classify safety notices according to the IMDRF codes among the 2 observers was substantial (κ = 0.79; CI 0.76–0.82).
Discussion
Our study is the first to assess the extent of overlap in TKA implants for which safety notices were issued and that were identified as outliers in registry data. We aimed to assess the extent to which safety notices and outlier identification in registries signal the same or different TKA implants. We found that approximately half (55%) of the TKA implants were identified by both safety notices and registries outlier identification procedures, but a quarter of the TKA outliers did not have any publicly released safety notices on the websites of Ministries of Health or regulatory agencies. In addition, there were implant problems identified by safety notices that did not manifest in an outlier status. TKA implants with both safety notices and an outlier status had higher cumulative revision risks (1/5/10 years) than TKA implants with safety notices only.
A recent review that assessed the current state of medical device safety signal detection stated that a global dataset of medical devices should be created using automatic reports from national/regional databases [23]. In the absence of such a global dataset, the CORE-MD PMS tool was developed recently [7]. Our results add that such a global dataset of safety notices may still not identify a quarter of TKA implants with statistically significant poor performance (i.e., TKA outliers). Additionally, a published safety notice by itself does not constitute a sufficient and necessary condition for being identified as a TKA outlier (the “safety notices only” group). We identified that certain IMDRF codes “A05–Mechanical Problem” and “A17–Compatibility Problem” were not encountered in the “safety notices only” group, suggesting that these are more closely related to poorer implant performance. This observation could result in a helpful indication to highlight a higher risk for certain TKA implants with such IMRDF codes identified in safety notices to become an outlier, thus warranting closer scrutiny of these TKA implants.
This multi-registry analysis examined the content of safety notice text, which does not typically include information needed to identify specific variants/subtypes of TKA implants, characterized by fixation, stability, and mobility. Such a lack of information causes camouflage (i.e., multiple implant subtypes exist under the same implant brand name) [22] making it difficult or even impossible to link the correct TKA implants with safety notices to registry data, or to combine data from different real-world data sources. This information is, however, important for action to be taken, as illustrated by a recent study showing good performance for the Nexgen system but significantly higher revision risks for specific combinations with the Nexgen LPS Flex (see also Supplementary Table 2) [24]. In addition, registries often publicly report only TKA implants’ brand names without listing more detailed information (e.g., fixation, stabilization, and mobility) to identify which specific subtype of an implant is concerned. Product codes and unique device identifiers (UDIs), which would be needed to deal with such camouflage, were also not reported in safety notices or publicly by registries, except for the American medical device recall database. Accordingly, we highly recommend minimal reporting requirements for manufacturers with respect to safety notices and also for registries when reporting outliers, including: full brand name, fixation, mobility, stability, and product codes or UDIs.
Arthroplasty registries currently only identify TKA outliers based on revision risks, which may take several years (at least 1) before sufficient numbers are available to detect performance problems [3,4]. Using revision risk may seem a relatively straightforward endpoint (the occurrence of revision surgery), but surgeon, implant, and patient factors determine whether an implant is revised. Moreover, between-registry variation exists regarding definitions and reasons for revision [3,25] although all included registries identifying TKA outliers defined revision as “the replacement/removal/addition of one or more prosthetic components”. But, for instance, revisions due to infection are excluded from the all-cause revision risk in the Swedish registry [26,27]. In contrast, the NJR also includes revision due to infection if no prosthetic component was exchanged, which can result in specific TKA implants being identified as an outlier in the NJR but not in other registries. Interestingly, the number of TKA outliers publicly reported by the AOANJRR is much higher when compared with other registries publicly reporting on outliers. Part of the explanation may be related to the definition, such as the minimum number of implants required for the publication and analysis of implant-specific revision rates, which is much lower in the AOANJRR (500 procedures compared with 2,500 procedures required in the NJR). These heterogeneities highlight the importance of an international agreement on definitions and outcomes, as well as time-points and methodology used for measuring outcomes within registries.
Some safety notices may be released based on implant-related problems causing clinical performance issues relating to a specific TKA implant but also on a case-by-case analysis (i.e., no minimum number of implants at risk is required), meaning that safety notices may provide an earlier signal of a possible performance problem than registries [28]. Accordingly, registries could use this as a signal to analyze specific TKA implants with released safety notices to detect potential adverse trends in performance earlier. However, when considering the timing of safety notices and outlier data being published, none of the data sources consistently released safety signals earlier than any other, highlighting the importance of a multifaced approach combining these 2 data sources. While this provides relevant information and includes all TKA implants for which safety concerns were reported in safety notices or reported as an outlier, it does not answer the question as to what percentage of TKA implants did not have any safety concerns reported. It would seem rather infeasible to estimate this percentage based on all TKA implants currently on the market in all countries examined in the present study. Creating a random sample of TKA implants would be a more feasible alternative to provide such information as a next step.
Limitations
First, the CORE-MD PMS tool searched for safety notices published on the websites of Ministries of Health and regulatory agencies, but we may have missed safety notices if these were reported only on manufacturers’ websites, which would have underestimated the number of TKA outliers with safety notices. Second, both TKA outliers and TKA implants not identified as a TKA outlier had a relatively similar distribution of IMDRF-problem types, suggesting that the IMDRF code may not be sufficient to distinguish between these 2 groups. However, only the Level 1 IMDRF codes were used due to the large number of safety notices to be manually classified, so there may be differences in distribution when Level 2 or 3 problem terms were used. On the other hand, one could argue that such differences in these more detailed problem-type descriptions would not likely entail clinically relevant differences in problems. Third, other factors such as surgeon or hospital performance are known to influence revisions, which may skew the revision risks data. Nonetheless, as we used data from 4 national registries consisting of a large number of TKA outliers, the impact on our results is likely to have been small. Fourth, safety notices were collected from websites in more countries than those for which registry outlier identification data were available, which might have underestimated the number of TKA outliers and explain part of the “safety notices only” group. On the other hand, assuming that safety notices point to a problem with the implant itself, we would expect any performance issue to be similar across countries and thereby picked up by other registries as well. Finally, our analysis does not exclude possible duplicates of the same safety notices published in different countries or for different models/lots within the country. This is because different countries use diverse formats and criteria to issue safety notices: some countries issue separate safety notices for each model (e.g., the USA, resulting in a high number of safety notices from the USA), while others publish only 1 safety notice with multiple models. However, the safety notices would still signal the same TKA implant, which was used as the unit of analysis in the present study, so excluding duplicate safety notices would not have changed our results.
Conclusion
We found that approximately half (55%) of the TKA implants were identified by both safety notices and registries outlier identification procedures, whereas around 25% of TKA outliers are not the subject of publicly released safety notices, highlighting the potential of adopting a multifaceted approach, integrating various real-world data sources and methods to combine information to enhance medical device safety signal detection.
Supplementary data
Supplementary Tables 1–2 are available as Suppldata on the article page, doi: 10.2340/17453674.2024.42361
- Medical Device Regulation (MDR). MDR – Article 83 – Post-market surveillance system of the manufacturer. Available from: https://www.medical-device-regulation.eu/tag/mdr-article-83-post-market-surveillance-system-of-the-manufacturer/ (last accessed February 23, 2024).
- Medical Device Regulation (MDR). MDR – Article 87 – Reporting of serious incidents and field safety corrective actions. Available from: https://www.medical-device-regulation.eu/2019/07/16/mdr-article-87-reporting-of-serious-incidents-and-field-safety-corrective-actions/ (last accessed February 23, 2024).
- Hoogervorst L A, Geurkink T H, Lübbeke A, Buccheri S, Schoones J W, Torre M, et al. Quality and utility of European cardiovascular and orthopaedic registries for the regulatory evaluation of medical device safety and performance across the implant lifecycle: a systematic review. Int J Health Policy Manag 2023; 12: 7648. doi: 10.34172/ijhpm.2023.7648.
- de Steiger R N, Miller L N, Davidson D C, Philip R, Graves S E. Joint registry approach for identification of outlier prostheses. Acta Orthop 2013; 84(4): 348-52. doi: 10.3109/17453674.2013.831320.
- Puijk R, Sierevelt I N, Pijls B G C W, Spekenbrink-Spooren A, Nolte P A. Increased risk of aseptic loosening for posterior stabilized compared with posterior cruciate-retaining uncemented total knee replacements: a cohort study of 13,667 knees from the Dutch Arthroplasty Registry. Acta Orthop 2023; 94: 600-6. doi: 10.2340/17453674.2023.33283.
- Enhancing the QUAlity and Transparency Of health Research. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Available from : https://www.equator-network.org/wp-content/uploads/2015/03/STARD-2015-checklist.pdf (last accessed November 5, 2024).
- Ren Y, Bertoldi M, Fraser A G, Caiani E G. Validation of CORE-MD PMS support tool: a novel strategy for aggregating information from notices of failures to support medical devices’ post-market surveillance. Ther Innov Regul Sci 2023; 57(3): 589-602. doi: 10.1007/s43441-022-00493-y.
- American Joint Replacement Registry (AJRR). The American Joint Replacement Registry Annual Report 2023. Available: https://www.aaos.org/registries/publications/ajrr-annual-report/ (last accessed November 6, 2024).
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Other Registries Worldwide. Available from: https://aoanjrr.sahmri.com/registries (last accessed November 5, 2024).
- Dutch Arthroplasty Register (LROI). Online LROI annual report 2023. Available from: https://www.lroi-report.nl/app/uploads/2023/10/PDF-LROI-annual-report-2023-1.pdf (last accessed November 5, 2024).
- Regional Register of Orthopaedic Prosthetic Implantology (RIPO) overall data hip, knee and shoulder arthroplasty in Emilia-Romagna region (Italy) 2000–2020. Available from: https://ripo.cineca.it/authzssl/pdf/Annual%20report%202020%20Regione%20Emilia%20-%20Romagna.pdf (last accessed November 5, 2024).
- Endoprothesenregister Deutschland (EPRD). Annual Report 2022. Available from : https://www.eprd.de/en/downloads/reports (last accessed November 5, 2024).
- Schweizerisches implantat-register registre suisse des implants (SIRIS). Swiss National Hip & Knee Joint Registry Report 2023. Available from: https://www.swiss-medtech.ch/sites/default/files/2023-11/231117_SIRIS-Report-2023_Final_online.pdf (last accessed November 5, 2024).
- National Joint Registry (NJR). 20th Annual Report. Available from: https://reports.njrcentre.org.uk/ (last accessed November 5, 2024).
- Finnish Arthroplasty register (FAR). Available from: https://www.thl.fi/far/#index. (data until March 2021).
- International Medical Device Regulators Forum (IMDRF). IMDRF terminologies for categorized adverse event reporting (AER): terms, terminology structure and codes. Available from: https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-200318-ae-terminologies-n43.pdf (last accessed February 23, 2024).
- International Medical Device Regulators Forum (IMDRF). Annex A: Medical device problem. Available from: https://www.imdrf.org/working-groups/adverse-event-terminology/annex-medical-device-problem (last accessed February 23, 2024).
- McHugh M L. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012; 22(3): 276-82. PMID: 23092060
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Other Registries Worldwide. Available from: https://aoanjrr.sahmri.com/registries (last accessed February 23, 2024).
- de Steiger R N, Hallstrom B R, Lübbeke A, Paxton E W, van Steenbergen L N, Wilkinson M. Identification of implant outliers in joint replacement registries. EFORT Open Rev 2023; 8(1): 11-17. doi: 10.1530/EOR-22-0058.
- Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. doi: 10.1186/1471-2288-5-13.
- Phillips J R A, Tucker K. Implant brand portfolios, the potential for camouflage of data, and the role of the Orthopaedic Data Evaluation Panel in total knee arthroplasty. Bone Joint J 2021; 103-b(10): 1555-60. doi: 10.1302/0301-620X.103B10.BJJ-2021-0284.R1.
- Pane J, Verhamme K M C, Villegas D, Gamez L, Rebollo I, Sturkenboom M C J M. Challenges associated with the safety signal detection process for medical devices. Med Devices (Auckl) 2021; 14: 43-57. doi: 10.2147/MDER.S278868.
- Wilton T, Skinner J A, Haddad F S. Camouflage uncovered: what should happen next? Bone Joint J 2023; 105-b(3): 221-6. doi: 10.1302/0301-620X.105B3.BJJ-2023-0145.
- van Schie P, Hasan S, van Bodegom-Vos L, Schoones J W, Nelissen R G H H, Marang-van de Mheen P J. International comparison of variation in performance between hospitals for THA and TKA: is it even possible? A systematic review including 33 studies and 8 arthroplasty register reports. EFORT Open Rev 2022; 7(4): 247-63. doi: 10.1530/EOR-21-0084.
- Hoogervorst L A, van Tilburg M M, Lübbeke A, Wilton T, Nelissen R G H H, Marang-van de Mheen P J. Validating Orthopaedic Data Evaluation Panel (ODEP) ratings across 9 orthopaedic registries: total hip implants with an ODEP rating perform better than those without an ODEP rating. J Bone Joint Surg Am 2024; 106(17): 1583-93. doi: 10.2106/JBJS.23.00793.
- Robertsson O, Lidgren L, Sundberg M, W-Dahl A. The Swedish Knee Arthroplasty Register: annual report 2020. Available from: https://www.myknee.se/pdf/SVK_2020_Eng_1.0.pdf (last accessed November 5, 2024).
- Medicines & Healthcare products Regulatory Agency (MHRA). First generation JOURNEY BCS Knee System: higher than expected risk of revision. Available from: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=103076#:~:text=In%20June%202018%2C%20Smith%20%26%20Nephew,main%20cause%20of%20device%20failure (last accessed November 5, 2024).