Total joint arthroplasty for thumb carpometacarpal joint osteoarthritis: a systematic review and meta-analysis of randomized controlled trials
Rasmus LIUKKONEN 1, Venla-Linnea KARJALAINEN 1, Reetta KVIST 2, Matias VAAJALA 1, Ville PONKILAINEN 2, and Teemu KARJALAINEN 2,3
1 Faculty of Medicine and Health Technology, Tampere University, Tampere; 2 Central Finland Central Hospital Nova, Jyväskylä; 3 Department of Hand and Microsurgery, Tampere University Hospital Tampere, Finland
Background and purpose — Thumb carpometacarpal (CMC) joint osteoarthritis (OA) is increasingly treated with total joint arthroplasty (TJA). We aimed to perform a systematic review and meta-analysis of the benefits and harms of the TJA for thumb CMC OA compared with other treatment strategies.
Patients and methods — We performed a systematic search on MEDLINE and CENTRAL databases on August 2, 2023. We included randomized controlled trials investigating the effect of TJA in people with thumb CMC joint OA regardless of the stage or etiology of the disease or comparator. The outcomes were pooled with a random effect meta-analysis.
Results — We identified 4 studies randomizing 420 participants to TJA or trapeziectomy. At 3 months, TJA’s benefits for pain may exceed the clinically important difference. However, after 1-year follow-up TJA does not improve pain compared with trapeziectomy (mean difference 0.53 points on a 0 to 10 scale; 95% confidence interval [CI] 0.26–0.81). Furthermore, it provides a transient benefit in hand function at 3 months (measured with Disabilities of Arm, Shoulder, and Hand questionnaire, scale 0–100, lower is better) compared with trapeziectomy with or without ligament reconstruction tendon interposition. The benefit in function diminished to a clinically unimportant level at 1-year follow-up (4.4 points better; CI 0.42–8.4).
Conclusion — Transient benefit in hand function for TJA implies that it could be a preferable option over trapeziectomy for people who consider fast postoperative recovery important. However, current evidence fails to inform us if TJA carries long-term higher risks of revisions compared with trapeziectomy.
Citation: Acta Orthopaedica 2024; 95: 325–332. DOI: https://doi.org/10.2340/17453674.2024.40816.
Copyright: © 2024 The Author(s). Published by MJS Publishing – Medical Journals Sweden, on behalf of the Nordic Orthopedic Federation. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits sharing, adapting, and using the material for any purpose, including commercial use, with the condition of providing full attribution to the original publication.
Submitted: 2024-01-23. Accepted: 2024-04-13. Published: 2024-06-18.
Correspondence: teemukarjalainen@me.com
All authors: interpretation of results, manuscript editing, and approval of the final version. RL: data collection, statistical analyses, writing of the first version of the manuscript. VLK: data collection, statistical analyses. RK and MV: data collection. VP and TK: study planning, project supervision.
Handling co-editors: Li Felländer-Tsai and Robin Christensen
Acta thanks Maria Wilcke and Malin Zimmerman for help with peer review of this manuscript.
Osteoarthritis (OA) of the carpometacarpal (CMC) joint of the thumb is a common condition affecting the hands of middle-aged and elderly people. The prevalence of this condition is around 15% within the female population, and around 7% within the male population [1].
For people with persistent symptoms after nonoperative treatment, several surgical strategies have been proposed. Traditionally, the most common procedure to treat CMC OA is a trapeziectomy with or without ligament reconstruction tendon interposition (LRTI) arthroplasty [2-5]. Arthrodesis has also been used, but it seems to carry a higher risk of adverse effects without benefits [6]. Various prosthetic implants have also been introduced, including total joint arthroplasties (TJAs) of several designs [5,7]. Purported benefits of TJA include avoidance of metacarpal collapse typical of trapeziectomy [8], but, on the other hand, the implant increases primary costs and involves a risk of loosening or dislocation [9,10]. The risk of reoperation might be higher for TJA, as in a 10-year follow-up, where the implant survival was estimated to be around 90% [11].
To date, the decision to perform trapeziectomy or TJA has largely depended on surgeons’ preferences [5]. Recent randomized controlled trials (RCT) have compared trapeziectomy and TJA in the treatment of CMC OA, but none of them has been sufficiently large to conclude on the benefit of 1 procedure over the other [12-15]. Thus, we aimed to perform a systematic review and meta-analysis of the benefits and harms of the TJA for thumb CMC OA compared with other treatment strategies.
Methods
We adhered to the PRISMA guidelines throughout the study [16].
Identification of studies
We included all randomized, quasi-randomized controlled trials, or studies that compared thumb CMC TJA with any surgical, non-surgical, or placebo treatment in participants with CMC joint OA, regardless of the stage or etiology of the disease. Searches were conducted in MEDLINE and CENTRAL databases on August 2, 2023. The protocol defined comparative observational studies as potentially eligible for inclusion. However, because several RCTs were identified, and non-randomized trials are at high risk of bias, we decided to include only RCTs in the analyses.
Study selection
2 authors independently screened the titles and abstracts and reviewed the full texts of potentially eligible studies. Disagreements were resolved through discussion.
Outcome measures
The main outcome is pain. In cases where multiple pain outcomes were reported in the same trial, we prioritized the Visual Analogue Scale (VAS), which yields a score of 0 to 10, with a higher score indicating more pain. If various pain outcomes were reported, we prioritized pain with activity or overall pain instead of rest pain.
Secondary outcomes were hand or thumb function, as measured by a validated patient reported outcome measure (PROM) or any other self-reported measure; grip strength; pinch strength; overall improvement or satisfaction; Health-Related Quality of Life (HRQoL); adverse events; reoperation; and return to work. The Disability of the Arm, Shoulder, and Hand Questionnaire (DASH) was prioritized as the PROM, as it was the most used measure.
Data collection and handling
The predefined time points were 3 months, 1 year, and 2 to 3 years. Since none of the studies followed participants for longer than 2 years, we collected and analyzed 2-year data.
All data was extracted to a pretested pro forma. This included the name of the first author, year of publication, number of participants, intervention and control treatments, and outcomes at each time point. Adverse events, reoperation, and satisfaction were collected at the latest available follow-up. We preferred data from the intention-to-treat analysis, but if not reported, we used per-protocol or as-treated data.
Risk of bias and certainty of evidence
2 authors independently assessed the risk of bias based on the Cochrane risk of bias tool 1.0 [17]. Disagreements were resolved through discussion and, if necessary, by consulting a third author. We evaluated the certainty of evidence using the GRADEpro tool [18]. We downgraded the certainty reflecting our confidence in the treatment effect based on the following factors: (i) risk of bias (downgraded if included studies were at high or unclear risk of bias), (ii) inconsistency (downgraded if the studies in the meta-analyses were inconsistent without apparent explanation), (iii) imprecision of the estimates (considered precise when the 95% confidence intervals (CIs) excluded or supported clinically significant benefits), (iv) indirectness of the evidence (present when surrogate outcomes were used). We did not assess publication bias due to the low number of studies available for each meta-analysis.
Statistics
For continuous outcomes, we extracted the mean and standard deviation (SD) values. If the mean was not available, we approximated it with the median. When SD was unavailable, we calculated it based on the interquartile range (IQR). For binary outcomes, we extracted events and the total number of participants at each time point. When total numbers were not available at each time point, we used the number of participants randomized to each group.
All meta-analyses were stratified by intervention. We used inverse variance weighting with a random effects model in all meta-analyses. For continuous outcomes, the treatment effect was expressed as mean difference (MD) with CIs. For binary outcomes, we expressed the treatment effect as relative risk (RR) with CIs. When different measures were used for one outcome domain, we used standardized mean difference (SMD) for pooling and then back-translated the SMD to the original scale using typical SD. If studies reported both binary and continuous measures for an outcome, we transformed the odds ratio [19] to SMD and used SMD as the summary measure.
We estimated whether the benefit was patient-important based on the CI of the estimate and the minimal clinically important difference (MCID) value of the corresponding outcome measure. The heterogeneity of the meta-analysis was determined through visual inspection of the forest plots and I2 statistics. We used the packages meta and metafor in R (4.3.0) with the software RStudio (2023.03.0+386) for the meta-analyses [20-23].
Ethics, registration, funding, and disclosures
Due to the study setting, no institutional review board approval was required. This systematic review was prospectively registered in the PROSPERO database (CRD42023451019). No funding was received for this study. The authors have nothing to disclose. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2024.40816
Results
Literature search and study selection
The search yielded 881 records, of which 849 were excluded in the initial screening process. In addition, 1 potential study was identified by citation searching [15]. After reading 33 full texts, we included 4 eligible studies (Figure 1).
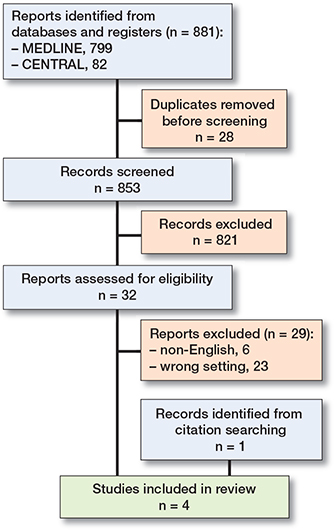
Figure 1. PRISMA flowchart of the study selection process. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The studies were published in 2019 and 2023 and are summarized in Table 1. 3 studies [12,13,15] compared TJA with trapeziectomy and LRTI, and 1 study [14] compared TJA with trapeziectomy without LRTI. We did not identify any studies that compared TJA with a nonoperative treatment or placebo.
Pain
At 3 months and 1 year, 2 studies [13,15] measured pain using VAS, and 1 [14] used the Michigan Hand Outcomes Questionnaire. We considered 1.5 points to represent MCID in VAS [24].
At 3 months, TJA seemed to provide a clinically relevant benefit in terms of pain, but the evidence is very uncertain (downgraded due to risk of bias, inconsistency, and imprecision). The SMD was –1.6 (CI –3.6 to 0.5; 3 studies; 352 participants; I2 = 98%). This translates to 2.0 (CI –0.7 to 4.7) VAS points better for TJA compared with trapeziectomy (Table 2, Figure 2).
| Outcome, time frame | Measurement instruments and relative effects a | Effect estimates | Certainty of evidence b (reasons) | Plain text summary | |
| Trapeziectomy (absolute score or risk) | TJA (mean difference or RR) | ||||
| Pain, 3 months | VAS | SMD –1.6 (CI –3.6 to 0.5) | Very low (inconsistency, imprecision) | Uncertainty about the effect | |
| 3 studies | MCID: 1.5 points | Translates to | |||
| 352 patients | 2.0 (CI –0.7 to 4.7) VAS points better | ||||
| Pain, 1 year | VAS | SMD –0.4 (CI –0.6 to –0.2) | Moderate | TJA does not provide important pain reduction at 1 year | |
| 3 studies | MCID: 1.5 points | Translates to | |||
| 349 patients | 0.5 (CI 0.3 to 0.8) VAS points better | ||||
| Function, 3 months | DASH | 39 | 18 (CI 15 to 21) points better | Moderate | TJA improves function at 3 months |
| 4 studies | MCID: 11 points | ||||
| 385 patients | |||||
| Function, 1 year | DASH | 16 | 4.4 (CI 0.4 to 8.4) points better | Moderate | TJA probably improves function at 1 year |
| 4 studies | MCID: 11 points | ||||
| 380 patients | |||||
| Grip strength, 3 months | – | 16 kg | 3.7 (CI 1.7 to 5.8) kg better | Low (imprecision) | TJA may improve grip strength at 3 months |
| 3 studies | MCID: 5–6.5 kg | ||||
| 227 patients | |||||
| Grip strength, 1 year | – | 23 kg | 2.1 (CI –4.0 to 8.1) kg better | Very low, (inconsistency imprecision) | Uncertainty about the effect at 1 year |
| 3 studies | MCID: 5–6.5 kg | ||||
| 226 patients | |||||
| Pinch strength, 3 months | – | 3.8 kg | 1.5 (CI 0.7 to 2.4) kg better | Low (imprecision) | TJA may improve pinch strength at 3 months |
| 3 studies | MCID: 0.35 kg | ||||
| 228 patients | |||||
| Pinch strength, 1 year | – | 4.8 kg | 0.9 (CI 0.2 to 1.6) kg better | Very low (inconsistency, imprecision) | TJA may improve pinch strength at 1 year |
| 3 studies | MCID: 0.35 kg | ||||
| 226 patients | |||||
| Global satisfaction | SMD –0.42 (CI –0.89 to 0.06) | Very low (inconsistency, imprecision) | Uncertainty about the effect | ||
| 4 studies | |||||
| 395 patients | |||||
| Reoperation | 6/208 | RR 2.0 (CI 0.11 to 36) | Very low (inconsistency, very serious imprecision) | Uncertainty about the effect | |
| 4 studies | |||||
| 406 patients | |||||
| (de Jong et al. excluded) | 0/177 | RR 9.1 (CI 1.2 to 70) | Moderate | Probably higher risk of reoperation after TJA | |
| 3 studies | |||||
| 344 patients | |||||
| Adverse events | 36/122 | RR 0.9 (CI 0.6 to 1.4) | Low (imprecision) | Uncertainty whether TJA causes fewer adverse events | |
| 3 studies | |||||
| 238 patients | |||||
| Return to work | 7 weeks | 6 weeks | Uncertainty about the effect | ||
| 1 study | |||||
| Quality of life, 3 months | No data | ||||
| Quality of life, 1 year | No data | ||||
| a VAS = visual analog pain scale (0–10, lower is better); MCID = minimal clinically important difference; DASH = Disability of the Arm, Shoulder, and Hand Questionnaire, scale 0–100, lower is better. | |||||
| b Evidence was downgraded 1 step for all estimates because all studies were in high risk of bias. | |||||
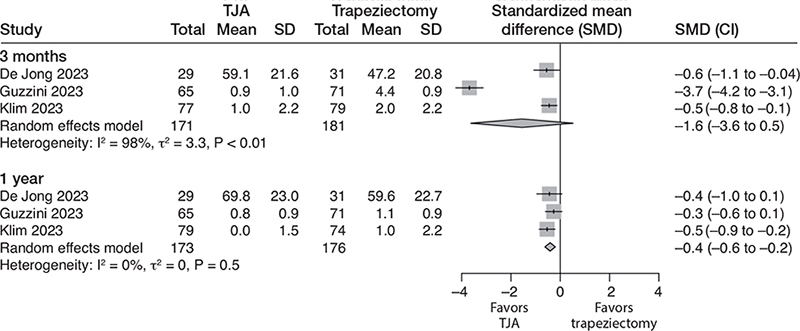
Figure 2. Forest plot for pain. Results are reported separately after 3-month and 1-year follow-up. TJA = total joint arthroplasty, SD = standard deviation, CI = confidence interval.
At 1 year, the benefits of TJA are not clinically important, as indicated by moderate certainty evidence (downgraded due to risk of bias). The SMD was –0.4 (CI –0.2 to –0.6; 3 studies; 349 participants; I2 = 0%), translating to a VAS of 0.5 (CI 0.3–0.8) point better with TJA.
At 2 years, one study [15] found no clinically important benefit for TJA. The mean VAS score was 1.1 in the trapeziectomy group and 0.4 (CI 0.1–0.7) points better with TJA (1 study; 136 participants). The certainty of the evidence was downgraded to low due to the risk of bias and imprecision (only 1 study with a low number of participants).
PROMs
All 4 studies measured PROMs using DASH or QuickDASH (0–100, lower is better; MCID of 10.8 points in DASH; MCID of 11 in QuickDASH [25]) at 3 months and 1 year. At 3 months, moderate certainty evidence (downgraded for risk of bias) indicates that the TJA improves hand function. The mean DASH score was 39 points with trapeziectomy and 18 (CI 15–21) points better with TJA (4 studies; 385 participants; I2 = 0%) (Figure 3).
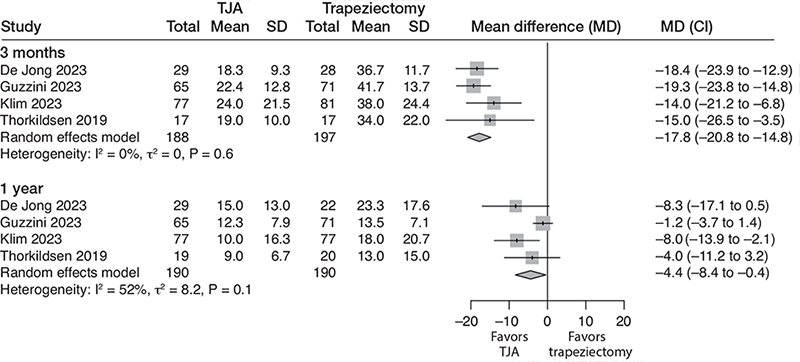
Figure 3. Forest plot for DASH score. Results are reported separately after 3-month and 1-year follow-up. DASH = Disabilities of the Arm, Shoulder, and Hand. See legend to Figure 2 for further abbreviations.
At 1 year, moderate certainty evidence (downgraded for risk of bias) indicates that there is no clinically relevant benefit for TJA. The mean DASH score was 16 with trapeziectomy and 4.4 (CI 0.4–8.4) points better with TJA (4 studies; 380 participants; I2 = 52%).
At 2 years, data from 2 studies [12,15] indicated that TJA did not improve hand function compared with trapeziectomy. The mean DASH score was 16 with trapeziectomy and 3.8 (CI–3.4 to 11) points better with TJA (2 studies; 172 participants; I2 = 59%). The certainty of the evidence was low due to the risk of bias and imprecision.
Grip strength
3 studies [12,14,15] measured grip strength (kg) at 3 months and 1 year. At 3 months, low certainty evidence (downgraded for risk of bias and imprecision) indicates no clinically relevant difference between TJA and trapeziectomy (MCID of 5–6.5 kg in grip strength [26]). Mean grip strength was 16 kg with trapeziectomy and 3.7 (CI 1.7–5.8) kg better with TJA (3 studies; 227 participants; I2 = 0%).
At 1 year, we are uncertain of the effect (very low certainty). The mean grip strength was 23 kg with trapeziectomy and 2.1 (CI –4.0 to 8.1) kg better with TJA (3 studies; 226 participants; I2 = 83%). The certainty of the evidence was downgraded due to the risk of bias, inconsistency, and imprecision.
At 2 years, low certainty evidence (downgraded due to risk of bias and imprecision) indicates that TJA seems to improve grip strength compared with trapeziectomy with or without LRTI. The mean grip strength was 25 kg with trapeziectomy and 6.3 (CI 3.7–8.9) kg better with TJA (2 studies; 172 participants; I2 = 0%).
Pinch strength
3 studies [12,14,15] measured pinch strength (kg) at 3 months and 1 year. Low certainty evidence (downgraded due to risk of bias and inconsistency) suggests that the benefits of TJA compared with trapeziectomy are greater than the MCID of 0.35 kg in pinch strength [27]. Mean pinch strength was 3.8 kg with trapeziectomy and 1.5 (CI 0.7–2.4) kg better with TJA (3 studies; 228 participants; I2 = 82%).
At 1 year, mean pinch strength was 4.8 kg with trapeziectomy and 0.9 (CI 0.2–1.6) kg better with TJA (3 studies; 226 participants; I2 = 73%). The certainty of the evidence was downgraded to very low due to the risk of bias, imprecision, and inconsistency.
At 2 years, 2 studies [12,15] measured pinch strength. Moderate certainty evidence (downgraded due to risk of bias) indicates that the TJA provides a clinically significant benefit in pinch strength over trapeziectomy. Mean pinch strength was 5.2 kg in the trapeziectomy group and 1.4 (1.2–1.7) kg better with TJA (2 studies; 172 participants; I2 = 0%).
Satisfaction
2 studies [14,15] reported satisfaction using a numeric rating scale that yields scores from 0 to 10. Thorkildsen and Røkkum [12] reported satisfaction as a binary outcome. Klim et al. [13] reported satisfaction as a scale with 4 steps from “very satisfied” to “dissatisfied.” To allow pooling, we collected it as a binary outcome with “very satisfied” or “satisfied” indicating satisfaction and transformed the effect to SMD. The SMD was –0.42 (CI –0.9 to 0.1; 4 studies; 395 participants; I2 = 63%), favoring TJA. The certainty of the evidence was downgraded to very low due to the risk of bias, inconsistency, and imprecision.
Reoperation
All 4 studies reported reoperation rates. In the TJA group, 9/198 (5%) were reoperated, while 6/208 (3%) were reoperated in the trapeziectomy group. This corresponds to a risk ratio of 2.0 (CI 0.11–36; 4 studies; 406 participants; I2 = 73%) in favor of trapeziectomy. The certainty of evidence was downgraded to very low due to the risk of bias, inconsistency, and very serious imprecision (the CIs were consistent with large benefits in either direction; Figure 4).
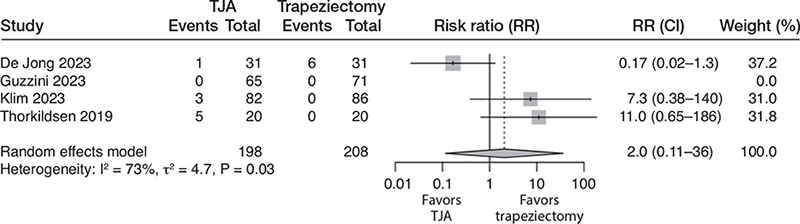
Figure 4. Forest plot for risk of reoperation. See legend to Figure 2 for abbreviations.
Due to great inconsistency and imprecision in reoperation, we additionally report the pooled results excluding the study by de Jong et al. In the remaining studies, 8/167 (5%) were reoperated in the TJA group and 0/177 (0%) in the trapeziectomy group. The pooled risk ratio was 9.1 (CI 1.2–70; 3 studies; 344 participants; I2 = 0%) in favor of trapeziectomy. In this analysis, the certainty of the evidence was downgraded to moderate due to the risk of bias.
Adverse events
3 studies [12,14,15] reported adverse events. There were 33/116 (28%) adverse events in the TJA group and 36/122 (30%) in the trapeziectomy group. There was no difference in pooled risk ratio, which was 0.9 (CI 0.6–1.4; 3 studies; 238 participants; I2 = 4%). The certainty of the evidence was downgraded to low due to the risk of bias and imprecision (Figure 5).
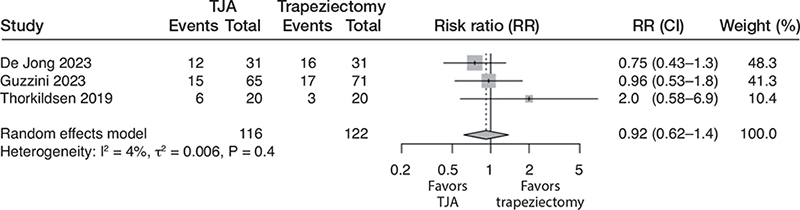
Figure 5. Forest plot for adverse events. See legend to Figure 2 for abbreviations.
Return to work
Only 1 study [14] reported a return to work so a meta-analysis was not performed. The study found no statistically significant difference between the treatments in return to work. The median time off from work in the TJA group was 6 weeks (IQR 1–10) compared with 7 (IQR 4.5–9) in the trapeziectomy group .
Health-related quality of life (HRQoL)
No studies measured generic HRQoL. 1 study [13] reported the Hospital Anxiety and Depression Scale (HADS) questionnaire as quality of life. At 3 months, the median HADS score (0–42, lower is better) was 8 (IQR 4–14) with trapeziectomy and 5 (IQR 1–12) with TJA (P = 0.2). At 1 year the score was 8 (IQR 2–16) in the trapeziectomy group and 5 (IQR 1–12) in the TJA group (P = 0.2).
Discussion
We aimed to perform a systematic review and meta-analysis to estimate the benefits and harms of TJA for thumb CMC OA compared with other treatment strategies.
We found that pain relief is comparable between TJA and trapeziectomy at 1 year. TJA, however, seems to provide clinically relevant benefits in hand function compared with trapeziectomy in the short term. This benefit diminishes by 1-year follow-up to a clinically negligible level, but grip and pinch strength may still be higher with TJA compared with trapeziectomy, suggesting possible long-term benefits over trapeziectomy. We found no evidence of harms for TJA compared with trapeziectomy but our confidence at both risk for reoperation and adverse effect is constrained by imprecise estimates and disparities among study findings.
Concerning pain, our conclusions diverge from prior reviews, likely due to an exclusive focus on RCTs and our utilization of a systematic (GRADE) approach to evaluate the certainty of evidence [28,29]. Furthermore, the analyses in the previous reviews were not stratified by the duration of the follow-up, making direct comparison unfeasible. Regarding function, our findings are in line with the previous reviews [28,29]. However, the previous reviews included mostly observational studies, rendering the conclusions less reliable. As 3 of the 4 RCTs in this review have been published recently, they were not included in the previous reviews.
TJA has been associated with a higher risk of complication and reoperations, limiting its popularity [28,29]. Froschauer et al. reported only a 60% 5-year survival rate [30]. Recent observational studies, however, suggest a 5-year survival rate up to 96% [31]. We considered the evidence for the reoperation rate to be too uncertain to draw firm conclusions because the included studies were at risk of bias, reported inconsistent findings, and the risk estimates were imprecise. By removing one outlier study, the reoperation rate was lower with trapeziectomy than with TJA. Inconsistency may relate to a threshold to intervene and is likely not a reliable outcome in unblinded studies. After a failed TJA, trapeziectomy is still a viable option, but after a failed trapeziectomy, reoperations are considered unreliable at improving symptoms [32].
Because the primary costs of TJA are higher than trapeziectomy, the benefits and harms of these procedures should be evaluated carefully before making clinical guidelines. As many of the estimates were of low or very low certainty, large, blinded trials are needed. The better functional outcomes after TJA, and the possibility of lower pain, might imply shorter intervals between operation and returning to work, although this review could not confirm this due to lack of data. Therefore, the working-age population may be a population of particular interest in future trials.
Limitations
We identified no studies comparing TJA with nonoperative treatment, no treatment, or placebo treatment. Therefore we were not able to answer the question as to whether TJA is better than nonoperative treatments. The evidence comes from trials that were at high risk of bias and sometimes inconsistent, limiting our certainty to the estimates. This inconsistency may relate to different prosthesis designs, but current data is too limited for stratified analyses. Furthermore, many of the estimates were imprecise, indicating a lack of information for some of the outcomes such as risk of adverse effects and reoperation. We searched the databases systematically but were unable to assess the publication as since too few studies were included. Studies with unfavorable results may have been left unpublished, biasing the pooled estimates in this study. One main limitation of the present study is that there are no high-quality studies on the long-term outcomes between TJA and trapeziectomy.
Conclusion
Transient benefit in hand function for TJA implies that it could be a preferable option over trapeziectomy for people who consider fast postoperative recovery important. However, current evidence fails to inform us whether TJA carries long-term higher risks of revisions compared with trapeziectomy, which might impact decision-making. Further rigorous blinded trials with long-term follow-up are warranted.
- Haara M M, Heliövaara M, Kröger H, Arokoski J P A, Manninen P, Kärkkäinen A, et al. Osteoarthritis in the carpometacarpal joint of the thumb: prevalence and associations with disability and mortality. J Bone Joint Surg Am 2004; 86: 1452-7. doi: 10.2106/00004623-200407000-00013.
- Epping W, Noack G. [Surgical treatment of the saddle joint arthrosis]. Handchir Mikrochir Plast Chir 1983; 15: 168-76. PMID: 6629152.
- Wittemann M, Demir E, Sauerbier M, Germann G. [The Epping resection-suspension arthroplasty procedure. A standard procedure in the operative treatment of trapeziometacarpal osteoarthrosis?]. Handchir Mikrochir Plast Chir 2002; 34: 49-58. doi: 10.1055/s-2002-22108.
- Gervis W H. Osteo-arthritis of the trapezio-metacarpal joint treated by excision of the trapezium. Proc R Soc Med 1947; 40: 492. PMID: 19993599.
- Martou G, Veltri K, Thoma A. Surgical treatment of osteoarthritis of the carpometacarpal joint of the thumb: a systematic review. Plast Reconstruct Surg 2004; 114: 421. doi: 10.1097/01.PRS.0000131989.86319.B1.
- Vermeulen G M, Brink S M, Slijper H, Feitz R, Moojen T M, Hovius S E R, et al. Trapeziometacarpal arthrodesis or trapeziectomy with ligament reconstruction in primary trapeziometacarpal osteoarthritis: a randomized controlled trial. J Bone Joint Surg 2014; 96: 726-33. doi: 0.2106/JBJS.L.01344.
- Vitale M A, Taylor F, Ross M, Moran S L. Trapezium prosthetic arthroplasty (silicone, Artelon, metal, and pyrocarbon). Hand Clin 2013; 29: 37-55. doi: 10.1016/j.hcl.2012.08.020.
- Saab M, Chick G. Trapeziectomy for trapeziometacarpal osteoarthritis. Bone Joint Open 2021; 2: 141-9. doi: 10.1302/2633-1462.23.BJO-2020-0188.R1.
- Bellemère P, Lussiez B. Thumb carpometacarpal implant arthroplasty. Hand Clinics 2022; 38: 217-30. doi: 10.1016/j.hcl.2021.12.006.
- Huang K, Hollevoet N, Giddins G. Thumb carpometacarpal joint total arthroplasty: a systematic review. J Hand Surg Eur 2015; 40: 338-50. doi: 10.1177/1753193414563243.
- Duerinckx J, Verstreken F. Total joint replacement for osteoarthritis of the carpometacarpal joint of the thumb: why and how? EFORT Open Rev 2022; 7: 349-55. doi: 10.1530/EOR-22-0027.
- Thorkildsen R D, Røkkum M. Trapeziectomy with LRTI or joint replacement for CMC1 arthritis, a randomised controlled trial. J Plast Surg Hand Surg 2019; 53: 361-9. doi: 10.1080/2000656X.2019.1635490.
- Klim S M, Glehr R, Graef A, Amerstorfer F, Leithner A, Glehr M. Total joint arthroplasty versus resection-interposition arthroplasty for thumb carpometacarpal arthritis: a randomized controlled trial. Acta Orthop 2023; 94: 224-9. doi: 10.2340/17453674.2023.11919.
- de Jong T R, Bonhof-Jansen E E D J, Brink S M, de Wildt R P, van Uchelen J H, Werker P M N. Total joint arthroplasty versus trapeziectomy in the treatment of trapeziometacarpal joint arthritis: a randomized controlled trial. J Hand Surg Eur 2023: 17531934231185245. doi: 10.1177/17531934231185245.
- Guzzini M, Arioli L, Annibaldi A, Pecchia S, Latini F, Ferretti A. Interposition arthroplasty versus dual cup mobility prosthesis in treatment of trapeziometacarpal joint osteoarthritis: a prospective randomized study. Hand (N Y) 2023: 15589447231185584. doi: 10.1177/15589447231185584.
- Page M J, McKenzie J E, Bossuyt P M, Boutron I, Hoffmann T C, Mulrow C D, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021: n71. doi: 10.1136/bmj.n71.
- Higgins J P T, Altman D G, Sterne J A C. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Hoboken, NJ: Wiley; 2011.
- GRADE Working Group. GRADEpro guideline development tool [software]. McMaster University and Evidence Prime; 2021.
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000; 19: 3127-31. doi: 10.1002/1097-0258(20001130)19:22<3127: : AID-SIM784>3.0.CO; 2-M.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010; 36: 1-48. doi: 10.18637/jss.v036.i03.
- Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health 2019; 22: 153-60. doi: 10.1136/ebmental-2019-300117.
- R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
- RStudio Team. RStudio: Integrated development environment for R. Vienna: R Foundation for Statistical Computing; 2020.
- Randall D J, Zhang Y, Li H, Hubbard J C, Kazmers N H. Establishing the minimal clinically important difference and substantial clinical benefit for the pain visual analog scale in a postoperative hand surgery population. J Hand Surg Am 2022; 47: 645-53. doi: 10.1016/j.jhsa.2022.03.009.
- Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther 2014; 44: 30-9. doi: 10.2519/jospt.2014.4893.
- Bohannon R W. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci 2019; 31: 75-8. doi: 10.1589/jpts.31.75.
- Villafañe J H, Valdes K, Bertozzi L, Negrini S. Minimal clinically important difference of grip and pinch strength in women with thumb carpometacarpal osteoarthritis when compared to healthy subjects. Rehabil Nurs 2017; 42: 139-45. doi: 10.1002/rnj.196.
- Raj S, Clay R, Ramji S, Shaunak R, Dadrewalla A, Sinha V, et al. Trapeziectomy versus joint replacement for first carpometacarpal (CMC 1) joint osteoarthritis: a systematic review and meta-analysis. Eur J Orthop Surg Traumatol 2022; 32: 1001-21. doi: 10.1007/s00590-021-03070-5.
- Qureshi M K, Halim U A, Khaled A S, Roche S J, Arshad M S. Trapeziectomy with ligament reconstruction and tendon interposition versus trapeziometacarpal joint replacement for thumb carpometacarpal osteoarthritis: a systematic review and meta-analysis. J Wrist Surg 2021; 11: 272-8. doi: 10.1055/s-0041-1731818.
- Froschauer S M, Holzbauer M, Hager D, Schnelzer R, Kwasny O, Duscher D. Elektra prosthesis versus resection-suspension arthroplasty for thumb carpometacarpal osteoarthritis: a long-term cohort study. J Hand Surg Eur 2020; 45: 452-7. doi: 10.1177/1753193419873230.
- Cebrian-Gomez R, Lizaur-Utrilla A, Sebastia-Forcada E, Lopez-Prats F A. Outcomes of cementless joint prosthesis versus tendon interposition for trapeziometacarpal osteoarthritis: a prospective study. J Hand Surg Eur 2019; 44: 151-8. doi: 10.1177/1753193418787151.
- Herren D B, Fuchs N, Schindele S, Marks M. Outcomes and recommendations for revision of thumb carpometacarpal resection arthroplasty. J Hand Surg Eur 2021; 46: 1101-7. doi: 10.1177/17531934211050559.