Major lower extremity amputations – risk of re-amputation, time to re-amputation, and risk factors: a nationwide cohort study from Denmark
Anna Trier Heiberg BRIX 1,2, Katrine Hass RUBIN 2,3, Tine NYMARK 1,2, Hagen SCHMAL 1,4, and Martin LINDBERG-LARSEN 1,2
1 Department of Orthopedic Surgery and Traumatology, Odense University Hospital, Odense; 2 Department of Clinical Research, University of Southern Denmark, Odense; 3 Research Unit OPEN, Odense University Hospital and University of Southern Denmark, Odense, Denmark; 4 Department of Orthopedics and Traumatology, University Medical Center Freiburg, Germany
Background and purpose — Re-amputation after lower extremity amputation is frequent. The primary aim of our study was to investigate cumulative re-amputation risk after transtibial amputation (TTA), knee disarticulation (KD), and transfemoral amputation (TFA) and secondarily to investigate time to re-amputation, and risk factors.
Methods — This observational cohort study was based on data from the Danish Nationwide Health registers. The population included first-time major lower extremity amputations (MLEA) performed in patients ≥ 50 years between 2010 and 2021. Both left and right sided MLEA from the same patient were included as index procedures.
Results — 11,743 index MLEAs on 10,052 patients were included. The overall cumulative risks for re-amputation were 29% (95% confidence interval [CI] 27–30), 30% (CI 26–35), and 11% (CI 10–12) for TTA, KD, and TFA, respectively. 58% of re-amputations were performed within 30 days after index MLEA. Risk factors for re-amputation within 30 days were dyslipidemia (hazard ratio [HR] 1.2, CI 1.0–1.3), renal insufficiency (HR 1.2, CI 1.1–1.4), and prior vascular surgery (HR 1.3, CI 1.2–1.5).
Conclusion — The risk of re-amputation was more than twice as high after TTA (29%) and KD (30%) compared with TFA (11%). Most re-amputations were conducted within 30 days of the index MLEA. Dyslipidemia, renal insufficiency, and prior vascular surgery were associated with higher risk of re-amputation.
Citation: Acta Orthopaedica 2024; 95: 86–91. DOI https://doi.org/10.2340/17453674.2024.39963.
Copyright: © 2024 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-09-03. Accepted: 2024-01-04. Published: 2024-02-02.
Correspondence: anna.trier.heiberg.brix@rsyd.dk
ATHB, KHR, and MLL designed the study protocol, and had access to the database. ATHB conducted the data management and analysis. ATHB drafted the manuscript, first revised critically by MLL, then KHR, TN, and HS thereafter. All authors approved the final manuscript.
The authors acknowledge OPEN Registry & Statistics for their insightful data management and statistics advice.
Handling co-editors: Bart A Swierstra and Philippe Wagner
Acta thanks Peter F M Choong and Hedvig Örneholm for help with peer review of this manuscript.
Major lower extremity amputation (MLEA) is defined as amputation above the ankle, and is divided into hip disarticulation, transfemoral amputation (TFA), knee disarticulation (KD), and transtibial amputation (TTA). Deciding on the optimal amputation level for the individual patient may be very difficult, lack consensus and is influenced by many factors. The paradigm for MLEAs has been to salvage as much of the lower extremity as possible. Limb-preserving amputations aim to gain the best functional outcome for the amputee, even though the risk of re-amputation may be higher for below-knee amputations [1-4]. Despite the paradigm of limb salvage, the most common surgical strategy in Denmark for MLEAs is TFA, with a decreasing trend in the performed number of KDs and TTAs (personal communication, Brix ATH, 2023).
The risk of re-amputation after MLEA has been reported as between 2% and 25%, depending on the index level and postoperative period investigated [3,4,6,7]. Risk factors for re-amputation after MLEA are smoking, high age, low transcutaneous oximetry, diabetes, renal insufficiency, and prior revascularization [1,3,4,6-8].
Insight into re-amputation risk is important in the shared decision-making between patients and surgeons before choosing the initial amputation level. Furthermore, it is important to know current nationwide status and development over time to improve future treatment pathways. Hence, the primary aim of our study was to investigate re-amputation risk after TTA, KD, and TFA, and secondarily to investigate time to re-amputation and risk factors for re-amputation.
Methods
This is an observational cohort study with data from the Danish Nationwide Health registers. The RECORD guide-lines for reporting routinely collected observational data were followed [9].
Data sources
Data on all first-time MLEAs from January 1, 2010 to December 31, 2021 was obtained from the Danish National Patient Register (DNPR). The data completeness of DNPR is > 99%, and it holds data on all Danish hospitalizations, including ICD-10 diagnoses and NOMESCO surgical procedures from 1977 to the present [10]. All Danish citizens are assigned a unique social security number, which makes identification and linkage of events in registers possible [11]. The Charlson Comorbidity Index (CCI) is calculated with data from the DNPR in a 10-year timeframe, according to Quan et al. [12].
In Denmark, all reimbursed prescriptions for medication from 1995 are collected in the Danish National Prescription Database (DNPD) and classified by Anatomical Therapeutic Chemical (ATC) codes [13]. For selected comorbidities, data from the DNPD was used to ensure correct diagnosis, as DNPR contains only diagnoses acquired in a hospital and not from the patient’s general practitioner [13-15]. Data from the Danish Civil Registration System was used to classify marital status and registration of death [11].
Study population
The population includes first-time MLEA performed in patients ≥ 50 years old in Denmark from January 1, 2010, to December 31, 2021. The index procedure was defined as the first MLEA on right or left leg as both sides from the same patients could be included as index procedures. Patients with a sarcoma diagnosis related to the index procedure were excluded. If more than 1 MLEA was conducted on the same day on the same extremity, the most distal level was registered as index level, and the next procedure as the first re-amputation. To follow the events, all procedures must have a site registered, and only complete courses with the site registered on all procedures were included in the main analysis (Figure 1). Patients with revisions or hip disarticulation as the only index procedure were excluded.
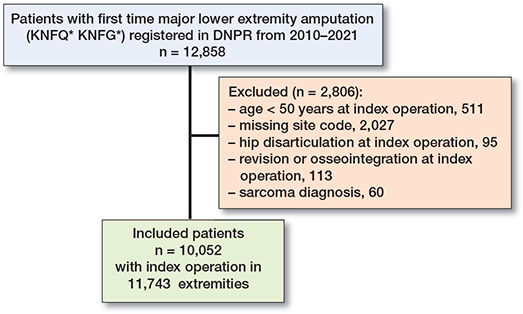
Figure 1. Overview of the study population. DNPR = Danish National Patient Register.
The progression of events should follow the logical consistency of amputation. For a complete overview of data management, see Supplementary data.
To ensure that only first-time MLEAs performed between 2010 and 2021 were included in the study population, a washout was conducted. All patients with procedure codes for amputation on thigh/hip (KNFQ*) and amputation on lower leg/knee (KNGQ*) from 1996–2009 were excluded from the dataset before analysis. The procedure codes for MLEA procedures in the DNPR are not yet validated [10]. Due to applied data protection rules, the Danish Health Data Authority conducted the washout.
Definition of re-amputation
We defined a “re-amputation” as the next amputation-related surgery on the same extremity. The re-amputation could therefore be a soft tissue revision at the same level, either with or without bone shortening, or an amputation at a higher level. The specific procedure codes for re-amputations are listed in Supplementary data.
Definition of comorbidities
The definition of the comorbidity variables diabetes, peripheral arterial disease (PAD), cardiovascular disease (CVD), hypertension, dyslipidemia, and renal insufficiency was based on ICD10 codes from the DNPR. For some of the comorbidity variables (diabetes, hypertension, dyslipidemia) ATC codes from DNPD were also applied, e.g., a patient is categorized with hypertension if 1 of the following ICD10 codes I10*–I15* is registered or the patient has redeemed 2 or more prescriptions in the same ATC group (antihypertensives, C02–C03, C07–C09) 5 years before the index date.
A complete overview of the definition of comorbidity variables, logical consistency classification, and procedures is provided in the Supplementary data.
Statistics
Non-normally distributed data was reported as median with interquartile range (IQR).
Risk of re-amputation is stated as cumulative risk, considering death as a competing event to re-amputation. To estimate the time to first re-amputation, the cumulative incidence was assessed using the Aalen–Johansen method, stratified by index level.
Potential risk factors associated with re-amputation were analyzed using Cox regression. Due to multiple observations for some of the included subjects, the data was clustered at patient ID using clustered robust standard errors. Hazard ratios (HR) were reported for ≤ 30 and > 30 days with 95% confidence intervals (CI). The risk factors investigated were age, sex, index level, diabetes, dyslipidemia, PAD, CVD, prior vascular surgery, and prior minor amputation. Model assumptions were checked with a proportional hazard test using Schoenfeld residuals and were for most variables not statistically significant when time was split into ≤ 30 days and > 30 days. For the variables that were significant (prior minor amputation and sex) after the time-split, the Schoenfeld residuals were plotted graphically and found to be acceptable. Sensitivity analyses for Cox regression were conducted as a competing risk regression model, taking the competing risk of death into account. All analysis were conducted in STATA v17.0 (Stata Statistical Software: Release 17.0, 2021, StataCorp. College Station, TX, USA).
Ethics, data sharing, funding, and disclosures
The Danish Data Protection Agency in the Region of Southern Denmark approved this study (no. 21/27110). Ethical approval was not relevant due to the study design. The Region of Southern Denmark and Odense University Hospital funded this study. Sharing of raw data is not available due to Danish law. No artificial intelligence tools were used to prepare this manuscript. The authors have no competing interests to declare. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2024.39963
Results
Our study included 11,743 MLEAs performed on 10,052 patients (Figure 1, Table 1). Of these, 3,726 were TTAs, 489 KDs, and 7,528 TFAs. 1,691 patients (14%) became bilaterally amputated during the study period. The overall median follow-up time was 17 months (IQR 1–43).
Re-amputation risk
The 1-year cumulative re-amputation risk between 2010 and 2021 ranged from 13–18% with an overall cumulative reamputation risk of 15% (CI 15–16) (Figure 2).
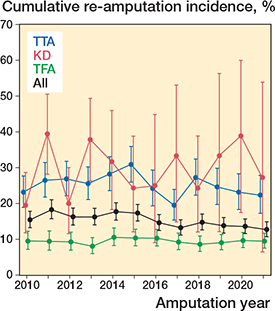
Figure 2. 1-year cumulative re-amputation risk from 2010–2021.
Transtibial amputation (TTA)
3,726 had TTA as index procedure and 1,048 (28%) were reamputated. The cumulative ≤ 30 days re-amputation risk was 17% (CI 16–18) and the cumulative 1-year re-amputation risk was 25% (CI 24–27) (Table 2). 79% ended on the same amputation level, while 21% ended with a higher amputation level, the majority a TFA (Table 3). Of the 1,048 re-amputated, 276 (7.2%) underwent more than 1 re-amputation. The median follow-up time for index TTAs was 29 months (IQR 8–58), and 2,627 (71%) had at least 1-year of follow-up.
| End level | Index level | ||
| TTA | KD | TFA | |
| 3,726 (32) | 489 (4.2) | 7,528 (64) | |
| TTA | 2,944 (79) | - | - |
| KD | 16 (0.4) | 353 (72) | - |
| TFA | 766 (21) | 136 (28) | 7,493 (100) |
| Hip disarticulation | a | a | 35 (0.5) |
| a Exact value < 5. Due to Danish Law regulation, the exact value is censored. The number is added to the row above (TFA) | |||
Knee disarticulation (KD)
489 had KD as index procedure and 147 (30%) were re-amputated. The cumulative ≤ 30 days re-amputation risk was 20% (CI 17–24) and the cumulative 1-year re-amputation risk was 29% (CI 25–33). Of the KDs, 72% ended on the same amputation level, while 28% ended with a higher amputation level. 25 (5.1%) experienced more than 1 re-amputation. The median follow-up time was 15 months (IQR 1–46) and 259 (53%) had at least 1-year follow-up time.
Transfemoral amputation (TFA)
7,528 had TFA as index procedure and 815 (11%) were reamputated. The cumulative ≤ 30 days re-amputation risk was 5.6% (CI 5.1–6.1) and the cumulative 1-year re-amputation risk was 9.5% (CI 8.8–10.2). Re-amputation was in 99% of cases at the same amputation level, but 0.5% were converted to a hip disarticulation. Of the 815 re-amputated, 238 (3.2%) underwent more than 1 re-amputation. The median follow-up time was 13 months (IQR 1–34) and 3,901 (52%) had at least 1-year of follow-up.
Time to re-amputation
Among all re-amputations, 1,820 (91%) were conducted within the first year, with the majority, 1,160 (58%), within the first 30 days (Figure 3).
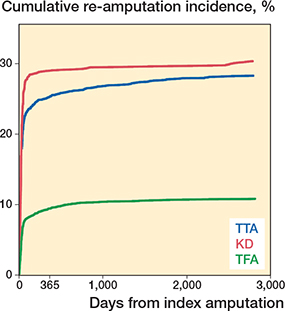
Figure 3. Time to re-amputation after TTA, KD, and TFA visualized with Aalen–Johansen cumulated incidence plot.
Risk factors
Crude HRs via Cox regression for re-amputation ≤ 30 days were dyslipidemia (HR 1.2, CI 1.0–1.3), renal insufficiency (HR 1.2, CI 1.1–1.4), and prior vascular surgery (HR 1.3, CI 1.2–1.5) (Table 4). Conversely, increasing age (HR 0.9, CI 0.9–0.9) was associated with lower HR for re-amputation within 30 days.
| Factor | ≤ 30 days crude HR (CI) | > 30 days crude HR (CI) |
| Age | 0.9 (0.9–0.9) a | 0.9 (0.9–0.9) a |
| Male sex | 1.0 (0.9–1.2) | 1.1 (0.9–1.3) |
| KD (TTA reference) | 1.3 (1.1–1.6) a | 1.1 (0.8–1.5) |
| TFA (TTA reference) | 0.3 (0.3–0.4) a | 0.5 (0.5–0.6) a |
| Diabetes | 0.9 (0.8–1.3) | 0.8 (0.7–0.9) a |
| PAD | 1.1 (0.9–1.3) | 2.0 (1.6–2.6) a |
| CVD | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) |
| Hypertension | 0.9 (0.8–1.1) | 0.8 (0.7–0.9) a |
| Dyslipidemia | 1.2 (1.0–1.3) a | 1.1 (0.9–1.3) |
| Renal insufficiency | 1.2 (1.1–1.4) a | 1.0 (0.9–1.3) |
| Prior minor amputation | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) |
| Vascular surgery | 1.3 (1.2–1.5) a | 1.5 (1.3–1.6) a |
| a P < 0.05. For Abbreviations, see Table 1. | ||
Analysis of crude HRs with death as competing risk was also conducted (Table 5). Renal insufficiency does not significantly increase the risk of re-amputation when death was treated as a competing risk.
| Factor | ≤ 30 days crude HR (CI) | > 30 days crude HR (CI) |
| Age | 0.98 (0.97–0.99) a | 0.96 (0.96–0.97) a |
| Male sex | 1.1 (0.9–1.2) | 1.1 (0.9–1.3) |
| KD (TTA reference) | 1.2 (1.1–1.5) a | 0.9 (0.7–1.3) |
| TFA (TTA reference) | 0.3 (0.3–0.4) a | 0.5 (0.4–0.5) a |
| Diabetes | 0.9 (0.8–1.1) | 0.8 (0.7–0.9) a |
| PAD | 1.2 (0.9–1.4) | 1.9 (1.5–2.4) a |
| CVD | 1.0 (0.9–1.2) | 0.9 (0.9–1.2) |
| Hypertension | 0.9 (0.8–1.1) | 0.7 (0.6–0.9) a |
| Dyslipidemia | 1.2 (1.1–1.4) a | 1.2 (0.9–1.3) |
| Renal insufficiency | 1.1 (0.9–1.3) | 0.9 (0.7–1.1) |
| Prior minor amputation | 1.1 (0.9–1.2) | 1.1 (0.9–1.3) |
| Vascular surgery | 1.4 (1.2–1.6) a | 1.5 (1.3–1.8) a |
| a P < 0.05. For Abbreviations, see Table 1. | ||
Discussion
We investigated the risk of re-amputation and amputation end level after TTA, KA, and TFA procedures, the time to re-amputation, and factors associated with risk for re-amputation. The main finding was a more than twice as high risk of re-amputation after TTA and KD compared with TFA, with no change in 1-year re-amputation risk within the study period.
Re-amputation risk
The overall cumulative re-amputation risks were 29%, 30%, and 11% for TTA, KD, and TFA respectively. These results match the findings from other studies finding a higher re-amputation risk for below-knee amputations compared with above-knee amputations [1-4]. Previously reported re-amputation risk within 30 days ranges between 1.8% and 23% depending on index level, with a higher re-amputation risk for TTA than for TFA [1,3,6,16-19]. The 1-year re-amputation risk is estimated as between 23% and 25% for TTA and 9–14% for TFA, which is similar to our results [4,20]. None of the studies specified the 30 days or 1-year re-amputation risk for KD.
Several attempts to improve the outcome after MLEA have been investigated [21-23]. First, a single-center study observed a decline in re-amputations (22% to 5%) after ceasing to perform KD [23]. Another single-center study investigated whether it was possible to reduce re-amputation rate by scheduling MLEA during the daytime and they found a reduction in 30 days re-amputations from 16% to 11% but without changes in risk of re-amputations after 6 months [21].
Previous efforts to enhance the outcome after MLEA have resulted in no improvements on a nationwide basis. Whether this absence of improvement is due to limited attention to the patient group in general or because the patient group is too frail to optimize is uncertain. To investigate this, a national program for the pre- and postoperative care of MLEA patients would need to be initiated and the effect on mortality and re-amputation risk should be examined continuously using real-time data.
We found that the re-amputation risk for KD was 30% higher compared with TTA, and almost 3 times higher than TFA. This may be explained by low experience with the KD procedure and the anatomic challenge with poor soft tissue coverage. Our results contrast with global guidelines on management of chronic limb-threatening ischemia from 2019 [19], which state that a well-performed KD would present healing rates that are close to TFA.
In Denmark, the surgical strategy has changed in the last decade, and the most common index procedure is TFA, while TTA is declining (personal communication, Brix ATH, 2023). This decline in TTA procedures was thought to be due to better selection of patients for a TTA and we expected a resulting lower risk of re-amputation. However, the 1-year cumulative risk of re-amputation for TTAs did not change in the study period and the selection of patients for TTA may not have been improved. This calls for reflection: what mediates this trend toward a higher proximal index level, and do the patients benefit from the more proximal index amputation level when the re-amputation risk is not changed?
Time to re-amputation
We found that 91% of all re-amputations were conducted within the first year, with 58% re-amputations in the first 30 days after index procedure. Norvell et al. [4] reported a median time to final level of re-amputation of 35 days for TTA and 38 days for TFA. Even though data is not directly comparable as we analyzed time to first re-amputation and not final re-amputation, data from both studies emphasizes that complications leading to re-amputation occur in the early postoperative phase.
Risk factors
Dyslipidemia, renal insufficiency, and prior vascular surgery were associated with increased risk of re-amputation within 30 days. These factors may explain the severity of the patient’s illness, which may impair the healing potential [3-6]. However, comparison of risk factors with other studies is a challenge, due to study heterogeneity in terms of selection of study population and design. Some studies focus on a specific cohort, e.g., with diabetes [24], PAD, or previous revascularization [8,14], while others include both minor and major amputations [4,6,7], or differ in analysis, definition of variables, and design [3,5,17].
Moreover, it is noteworthy that, among the factors examined, only a few demonstrated strong predictive power for re-amputation. This limited evidence may offer minimal guidance to the surgeon when making decisions regarding the optimal amputation level.
Strengths and limitations
The study’s main strength is that we included the population of interest through nationwide registers, thus minimizing the risk of selection bias. The Danish health registers are known for high-quality data and high completeness, which reduce the overall risk of information bias in Danish registry studies [25].
A limitation is that our registers do not contain lifestyle data and results of clinical procedures, e.g., smoking status, alcohol consumption, BMI, blood sample results, related imaging, angiography results, and clinical testing such as perfusion measures. Hence, it is not possible to detect any lifestyle changes during the study period or changes in level of disease over time. Another limitation is that the indication for amputation is not available through registry data. Furthermore, the data is dependent on the surgeons to code the procedure correctly and to add the site (left/right) precisely [26]. The inaccurate reporting of data, especially regarding site, may have caused information bias in this study. The coding of site was missing in 2,013 cases in this study and interpretation of outcomes of these procedures is difficult. The coding of site is not validated. We have no reason to believe that amputations without site registration are different from those with. Site registration is performed by the surgeons independently of patient characteristics and hence may be missing at random. However, if patients are re-amputated several times there may be an increased risk of incomplete site registration due to more procedures. This might result in an underestimation of the reamputation risk and especially an underestimation of the frequency of patients having ≥ 1 re-amputation.
Conclusion
The risk of re-amputation was more than twice as high after TTA (29%) and KD (30%) compared with TFA (11 %). Most re-amputations were conducted within 30 days after index amputation. The 1-year risk for re-amputation after all types of MLEA was stable from 2010–2021 even though an increasing number of patients had a TFA as the first major amputation, calling for reflection on choice of surgical strategies. Dyslipidemia, renal insufficiency, and prior vascular surgery were associated with higher risk of re-amputation.
Perspective
The results are of importance in decision-making and involving the patients on appropriate initial amputation level; knowledge of expected outcomes is essential. The results illuminate the paradox that the rate of TFAs is increasing, but the risk of re-amputation is not decreasing. This controversy needs to be explored further in more detailed prospective studies.
Supplementary data
Surgical and disease codes, definitions, and criteria used in the study are available as supplementary data on the article page, doi: 10.2340/17453674.2024.39963
- Columbo J A, Nolan B W, Stucke R S, Rzucidlo E M, Walker K L, Powell R J, et al. Below-knee amputation failure and poor functional outcomes are higher than predicted in contemporary practice. Vasc Endovascular Surg 2016; 50(8): 554-8. doi: 10.1177/1538574416682159.
- Jensen P S, Petersen J, Kirketerp-Møller K, Poulsen I, Andersen O. Progression of disease preceding lower extremity amputation in Denmark: a longitudinal registry study of diagnoses, use of medication and healthcare services 14 years prior to amputation. BMJ Open 2017; 7(11): e016030. doi: 10.1136/bmjopen-2017-016030.
- Zambetti B R, Stiles Z E, Gupta P K, Stickley S M, Brahmbhatt R, Rohrer M J, et al. Analysis of early lower extremity re-amputation. Ann Vasc Surg 2022; 81: 351-7. doi: 10.1016/j.avsg.2021.10.030.
- Norvell D C, Czerniecki J M. Risks and risk factors for ipsilateral re-amputation in the first year following first major unilateral dysvascular amputation. Eur J Vasc Endovasc Surg 2020; 60(4): 614-21. doi: 10.1016/j.ejvs.2020.06.026.
- Phair J, DeCarlo C, Scher L, Koleilat I, Shariff S, Lipsitz E C, et al. Risk factors for unplanned readmission and stump complications after major lower extremity amputation. J Vasc Surg 2018; 67(3): 848-56. doi: 10.1016/j.jvs.2017.08.061.
- O’Brien P J, Cox M W, Shortell C K, Scarborough J E. Risk factors for early failure of surgical amputations: an analysis of 8,878 isolated lower extremity amputation procedures. J Am Coll Surg 2013; 216(4): 836-42; discussion 42-4. doi: 10.1016/j.jamcollsurg.2012.12.041.
- Font-Jiménez I, Llaurado-Serra M, Roig-Garcia M, De Los MozosPerez B, Acebedo-Urdiales S. Retrospective study of the evolution of the incidence of non-traumatic lower-extremity amputations (2007-2013) and risk factors of reamputation. Prim Care Diabetes 2016; 10(6): 434-41. doi: 10.1016/j.pcd.2016.04.001.
- Torbjörnsson E, Fagerdahl A M, Blomgren L, Boström L, Ottosson C, Malmstedt J. Risk factors for reamputations in patients amputated after revascularization for critical limb-threatening ischemia. J Vasc Surg 2021; 73(1): 258-66.e1. doi: 10.1016/j.jvs.2020.03.055.
- Benchimol E I, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12(10): e1001885. doi: 10.1371/journal.pmed.1001885/.
- Schmidt M, Schmidt S A, Sandegaard J L, Ehrenstein V, Pedersen L, Sørensen H T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449-90. doi: 10.2147/clep.S91125.
- Mainz J, Hess M H, Johnsen S P. The Danish unique personal identifier and the Danish Civil Registration System as a tool for research and quality improvement. Int J Qual Health Care 2019; 31(9): 717-20. doi: 10.1093/intqhc/mzz008.
- Quan H, Li B, Couris C M, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173(6): 676-82. doi: 10.1093/aje/kwq433.
- Pottegård A, Schmidt S A J, Wallach-Kildemoes H, Sørensen H T, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol 2017; 46(3): 798-f. doi: 10.1093/ije/dyw213.
- Londero L S, Hoegh A, Houlind K, Lindholt J. major amputation rates in patients with peripheral arterial disease aged 50 years and over in Denmark during the period 1997–2014 and their relationship with demographics, risk factors, and vascular services. Eur J Vasc Endovasc Surg 2019 ; 58(5): 729-37. doi: 10.1016/j.ejvs.2019.06.007.
- Glintborg D, Petersen T G, Rubin K H, Andersen M S. Diabetes mellitus mediates risk of depression in Danish women with polycystic ovary syndrome: a national cohort study. Biomedicines 2022; 10(10). doi: 10.3390/biomedicines10102396.
- Nelson M T, Greenblatt D Y, Soma G, Rajimanickam V, Greenberg C C, Kent K C. Preoperative factors predict mortality after major lower-extremity amputation. Surgery 2012; 152(4): 685-94; discussion 94-6. doi: 10.1016/j.surg.2012.07.017.
- Belmont P J Jr, Davey S, Orr J D, Ochoa L M, Bader J O, Schoenfeld A J. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg 2011; 213(3): 370-8. doi: 10.1016/j.jamcollsurg.2011.05.019.
- Kristensen M T, Holm G, Gebuhr P. Difficult to predict early failure after major lower-extremity amputations. Dan Med J 2015; 62(12): A5172.
- Conte M S, Bradbury A W, Kolh P, White J V, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limbthreatening ischemia. Eur J Vasc Endovasc Surg 2019; 58(1s): S1-S109. e33. doi: 10.1016/j.ejvs.2019.05.006.
- Dillingham T R, Pezzin L E, Shore A D. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil 2005; 86(3): 480-6. doi: 10.1016/j.apmr.2004.06.072.
- Ignatiussen M E, Pedersen P, Holm G, Thomsen M G, Kristensen M T. Daytime and scheduled surgery for major dysvascular lower extremity amputation. Dan Med J 2023; 70(3): A07220435.
- Kristensen M T, Holm G, Krasheninnikoff M, Jensen P S, Gebuhr P. An enhanced treatment program with markedly reduced mortality after a transtibial or higher non-traumatic lower extremity amputation. Acta Orthop 2016; 87(3): 306-11. doi: 10.3109/17453674.2016.1167524.
- Schmiegelow M T, Sode N, Riis T, Lauritzen J B, Duus B R, Lindberg-Larsen M. Re-amputations and mortality after below-knee, through-knee and above-knee amputations. Dan Med J 2018; 65(12).
- Thorud J C, Plemmons B, Buckley C J, Shibuya N, Jupiter D C. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg 2016; 55(3): 591-9. doi: 10.1053/j.jfas.2016.01.012.
- Thygesen L C, Ersbøll A K. When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol 2014; 29(8): 551-8. doi: 10.1007/s10654-013-9873-0.
- Nymark T, Thomsen K, Röck N D. [Diagnosis and procedure coding in relation to the DRG system]. Ugeskr Laeger 2003; 165(3): 207-9.