Larger 5-year migration but similar polyethylene wear of cementless hemispherical cups with electrochemically applied hydroxyapatite (BoneMaster) coating compared with porous plasma-spray titanium: a randomized 5-year RSA study
Sebastian Breddam MOSEGAARD 1,2, Peter Bo JØRGENSEN 1,3, Stig Storgaard JAKOBSEN 2, Henrik DAUGAARD 4, Kjeld SØBALLE 2,3, and Maiken STILLING 1–3
1 AutoRSA Research Group, Orthopaedic Research Unit, Aarhus University Hospital, Aarhus; 2 Department of Orthopedic Surgery, Aarhus University Hospital, Aarhus; 3 Department of Clinical Medicine, Aarhus University, Aarhus; 4 Department of Orthopedics, Slagelse Hospital, Slagelse, Denmark
Background and purpose — BoneMaster (BM) is a thin electrochemically applied hydroxyapatite (HA) implant coating marketed with expectations of improved osseointegration properties but less polyethylene (PE) wear. We compared the midterm cup migration and PE wear of cementless porous-coated hemispherical cups with and without BM.
Patients and methods — In this patient-blinded, randomized controlled trial, 53 patients with a mean age of 64 years (55–75) received total hip arthroplasty with a porouscoated (P) or porous and BoneMaster (PBM) coated Exceed cup and ArCom E1 infused PE. Patients were followed with RSA, Hip Osteoarthritis Outcome Score (HOOS), and Euro-Qol-5-3L (EQ-5D) at 3 and 6 months, and 1-, 2-, and 5-year follow-up.
Results — At 5-year follow-up, total translation and maximum total point motion was 0.28 mm (95% CI 0.08; 0.47) and 0.52 mm (CI 0.12; 0.93) higher in the PBM group than in the P group. PE wear was comparable between PBM and P cups, and 2D wear rate from 1 year follow-up to last follow-up was 0.03 mm (CI 0.02–0.03). The 5-year anterior translation was 0.05 mm (CI –0.10 to 0.21) in the normal BMD group and 0.40 mm (CI 0.22–0.57) in the osteopenia group.
Interpretation — At 5-year follow-up, Exceed cups in the PBM group migrated more than in the P group but the PE wear rate was low and similar. This study does not indicate any advantage of additional BoneMaster coating compared with porous coating alone on cementless hemispherical cups with regards to migration, polyethylene wear, and clinical outcomes.
Citation: Acta Orthopaedica 2022; 93: 658–664. DOI http://dx.doi.org/10.2340/17453674.2022.3976.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2021-12-03. Accepted: 2022-05-30. Published: 2022-07-14.
Correspondence: sebmos@rm.dk
MS, SSJ, and KS formulated the study hypothesis and design. PBJ, SSJ, and HD included, operated on, and followed the patients. MS, PB, and SBM ensured data collection and analysis. SBM performed the statistical data analysis. MS, SMB, and PB interpreted the data. All authors were involved in the revision of the final manuscript.
Acta thanks Cyrus Brodén and Stephan Maximilian Röhrl for help with peer review of this study.
In total hip replacements in Denmark approximately 86% of the cups are cementless and, of these, 35% are hydroxyapatite (HA) coated (1). Experimental studies have shown that plasmasprayed HA coating provides better osseointegration and early implant fixation (2,3). However, neither midterm (4,5) nor long-term (6,7) clinical studies have confirmed superior fixation of cementless cups with HA coating over porous coating. Furthermore, plasma-sprayed HA coating has been associated with excessive polyethylene (PE) wear due to third-body wear from HA debris found in the joint fluid and PE during late-term revision surgeries (8). Using electrochemical techniques HA can be applied in a very thin coating of 5 µm compared with the 30–250 µm thickness of plasma-sprayed HA coating. Electrochemically applied thin HA coating—BoneMaster—has been shown to resorb quickly in the experimental setting (3,9) and has the potential to alleviate third-body PE wear, but no clinical studies have evaluated this. The positive properties of BM coating on osseointegration have been confirmed experimentally (3,9) and in clinical femoral stem studies (10,11), whereas the benefit is less clear for cup fixation (12).
Radiostereometry (RSA) is recommended in phased introduction of, e.g., new surgical methods and new arthroplasty component designs (13). RSA of cup migration until 2-year follow-up may predict the risk of revision using suggested thresholds of > 0.2 mm proximal cup migration (= at risk) and > 1.0 mm proximal cup migration (= unacceptable) (14). We compared the RSA-measured migration and PE wear of cups porous coated and with BoneMaster (PBM) vs. porous (P) coated at 5-year follow-up. The primary hypothesis was that the PBM group would have lower proximal cup migration than the P group at 5 years follow-up.
Patients and methods
Study design
From January 2013 to March 2015, 82 patients were assessed for eligibility in this randomized controlled patient-blinded study. The patient randomization was done in 10-patient blocks (5 porous with BoneMaster [PBM] and 5 porous [P]). Written consent was obtained from 56 patients meeting the inclusion criteria: severe coxarthrosis, age 55–75 years, and preoperatively non-osteoporotic identified using dual-energy X-ray absorptiometry (DEXA) (Figure 1, Table 1). We formerly presented cup migration until 2-year follow-up of cementless porous-coated hemispherical cups with or without BM and found no advantage of BM at 2-year follow-up (12). The exclusion criteria are listed in the study by Jørgensen et al. (12).
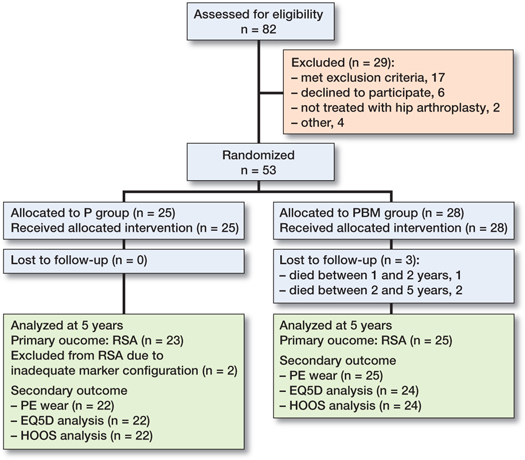
Figure 1. Consort flowchart of patient allocation.
Sample size
Based on a clinically relevant proximal cup migration difference of 0.2 mm (SD 0.2) at 5 years, power of 90%, and alpha of 0.05, the sample size was estimated to 23 patients in each group (4). We then aimed for 25 patients in each group to balance potential dropout/exclusions. The inclusion per block randomization was continued until a minimum of 25 patients were included in each group, leading to a total of 53 patients in the study.
Implants
51/53 patients received a cementless Exceed cup (Exceed ABT RingLoc-x solid shell) with an ArCom E1 infused PE liner and cementless Bi-Metric stem (Zimmer Biomet, Warsaw, IN, USA) with cobalt-chromium-molybdenum modular femoral heads. 2 patients received an ArcomXL highly crosslinked liner. The 25 patients in the P group had the cup and stem coated with plasma-sprayed porous titanium with 45% porosity and a pore size of mean 250 μm (range, 100–1,000 μm), providing scratch fit (15). The remaining 28 patients in the PBM group received similar components additionally coated with electrochemically applied HA (BoneMaster, Zimmer Biomet). The BoneMaster (BM) coating was 5 μm thick, consisting of 70% crystalline HA with a 2.0 Ca/P ratio. The amorphous phase in the coating was mainly amorphous calcium phosphate (ACP) but also β-tricalcium phosphate (TCP).
Surgery
All 53 patients were operated on at Aarhus University Hospital, Denmark. The preoperative planning was done using AGFA OT3000 digital templating software (Agfa-Gevaert NV, Mortsel, Belgium). All patients were operated on using a posterolateral approach and under-reamed by 1 mm of the acetabulum. 6 to 8 1-mm tantalum beads were inserted to the periprosthetic pelvic bone during surgery. Prior to surgery, all patients received one dose of tranexamic acid 10 mg/kg IV and 1.5 grams of cefuroxime intravenously. Postoperatively, all patients received 3 doses of 1.5 grams cefuroxime IV within 24 hours, and 1 dose of tranexamic acid IV. Using a fast-track protocol, patients were immediately mobilized with full weight-bearing, and walking aids as needed.
Radiostereometric analysis
RSA imaging was performed using a standard RSA system as previously described (12).
The RSA analyses were conducted with Model-Based RSA 4.1 (RSAcore, Leiden, Netherlands) using computer-aided design surface implant models (Zimmer Biomet) for evaluation of cup migration in addition to an EGS sphere for analysis of PE wear. The mean condition number was 102 (CI 91–112) and the mean rigid body error was 0.18 (CI 0.16–0.20). 2 patients in the P group were excluded from the RSA analyses due to inadequate marker configuration (1 patient with CN > 200 and 1 patient with only 2 markers). Supine stereoradiographs were taken after weight-bearing on the first postoperative day, at 3 and 6 months, and at 1, 2, and 5 years postoperatively. To evaluate the RSA precision, double examinations were performed at 6-month follow-up in accordance with the ISO 2013 standards for RSA (16). The precision of the RSA measurements is presented with mean difference, standard deviation difference, and coefficient of repeatability in Table 2 (see Supplementary data).
PE wear
PE wear was calculated as the relative migration of the femoral head to the metal shell. PE wear was adjusted for side and presented as y-wear (proximal), 2D wear (the vectorial sum of x- and y-wear), and 3D wear (the vectorial sum of x-, y-, and z-wear) in the coordinate system of the calibration box. Wear is presented in 3 timeframes: bedding-in was defined as wear within the first year, femoral head penetration was defined as the wear from baseline to the 5-year follow-up and wear rate was defined as the wear from 1-year follow-up to the 5-year follow-up (17). The precision of the wear analyses was evaluated using double examinations.
Radiographs and DXA scans
Medio-lateral and anterior-posterior radiographs of the hip were recorded preoperatively and at 5-year follow-up. Cup position was measured by 1 experienced hip surgeon (SSJ) on postoperative radiographs. Radiolucent lines larger than 1 mm were described according to DeLee and Charnley (18).
Preoperatively, DXA scans were performed on both hips and the lumbar spine using a fan-beam GE Lunar iDXA with Encore software version 13 (Minneapolis, MN, USA). Patients with a T-score > –1 were classified as having normal bone mineral density (BMD), and patients with T-score ranging from –2.5 to –1.0 were classified as osteopenic. Patients with a T-score < –2.5 on either the lumbar spine or hip were defined as osteoporotic (exclusion criteria).
Clinical outcomes
Patient data was collected on hip disability and HOOS, EQ5D, and self-reported pain at rest and activity on a visual analogue scale (VAS). HOOS has been validated for use in total hip arthroplasty patients, and consists of 5 subscales (pain, symptoms, activities of daily living, quality of life, and sport and recreational activities) (19). The EQ5D is a questionnaire used to assess quality of life and general health and has been suggested for use in total hip arthroplasty patients (20). Clinical outcomes were collected at baseline, 3 and 6 months, and at 1-, 2-, and 5-year follow-up.
Statistics
RSA measured cup migration was reported as signed migrations along and rotations around the three axes (x, y, z) and additionally total rotations (TR = √(Rx2 + Ry2 + Rz2)), total translation (TT = √(Tx2 + Ty2 + Tz2)) and maximum total point motion (MTPM).
Normality of continuous data was evaluated using quantile–quantile plots, to ensure no statistical assumptions were violated.
Linear mixed models for repeated measurements were used to analyze cup migration from baseline to 5-year follow-up as dependent continuous variable and P/PBM coating as the independent dichotomous variable. The data distribution assumptions were evaluated using model residual quantile–quantile plots and residual vs. fitted plots. A likelihood-ratio test was used to find differences between models. The Wald test was used to detect differences within the model.
PE wear was compared between the P and PBM group using Student’s t-test. Confidence intervals (CI) are 95% confidence intervals. STATA (v. 16.1, StataCorp LLC, College Station, TX, USA) was used for statistical analyses. The level of significance was 0.05.
Ethics, registration, funding, data sharing, potential conflicts of interest
The study was conducted in accordance with the Helsinki II declaration, and all patients gave their informed consent to participate. Approvals were obtained from the local ethics committee (M-20110224), the Data Protection Agency (1-16-02-175-11) and registered at ClinicalTrials.gov (NCT02311179). The RSA analyses were financed by Zimmer Biomet but the company had no influence on the data analyses, manuscript preparation, or publication. Data sharing is possible. The authors declare no conflicts of interest.
Results
RSA-measured cup migration (Figure 2, Table 3, see Supplementary data)
Proximal migration was 0.14 mm (95% CI –0.00 to 0.26) higher in the PBM group than the P group at 6-month followup (p = 0.054). At 5-year follow-up, 6/23 P group and 10/25 PBM group exceeded the proximal migration precision limit of 0.235 mm, and the mean proximal cup migration was 0.10 mm (CI –0.00 to 0.20) for the P group and 0.19 mm (CI 0.10–0.29) for the PBM group.
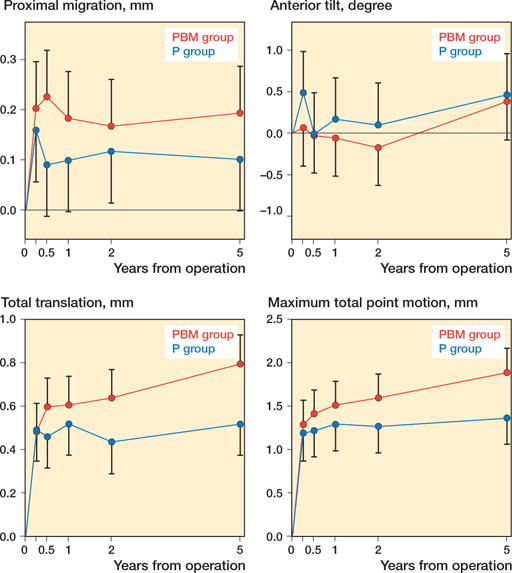
Figure 2. Proximal translation, anterior tilt, total translation, and maximum total point motion of the 2 groups. Graphs are presented with predicted means from linear mixed models and 95% confidence intervals.
Total translations were 0.20 mm (CI 0.01–0.40) and 0.28 mm (CI 0.08–0.47) higher for the PBM group than the P group at 2- and 5-year follow-up, respectively. In the PBM group, the TT increased by 0.12 mm (CI 0.01–0.23) from 3 to 6 months and by 0.16 mm (CI 0.05–0.27) from 2- to 5-year follow-up. In the P group there was no statistically significant difference in TT between any of the consecutive follow-ups.
MTPM was 0.52 mm (CI 0.12–0.93) higher in the PBM group compared with the P group at 5-year follow-up. From 2- to 5-year follow-up, MTPM increased by 0.29 mm (CI 0.04–0.54) in the PBM group, while the P group remained stable.
Medial/lateral translations, anterior/posterior translations, anterior/posterior tilt, and internal/external rotations were not statistically significantly different between the 2 groups at all follow-ups.
DXA-measured bone mineral density (Figure 3)
21 patients (8 P/13 PBM) had osteopenia (T-score < –1), which was not statistically significantly unevenly distributed between groups. There was no statistically significant difference between patients with osteopenia and normal BMD with regards to proximal migration at any follow-up. Only anterior/posterior translations were statistically significantly different between patients with osteopenia and normal BMD. Patients with osteopenia had statistically significantly higher anterior translations of 0.31 mm (CI 0.08–0.55) at 2-year and of 0.34 mm (CI 0.11–0.58) at 5-year follow-up. The 5-year anterior translation was 0.05 mm (CI –0.10 to 0.21) in the normal BMD group and 0.40 mm (CI 0.22–0.57) in the osteopenia group.
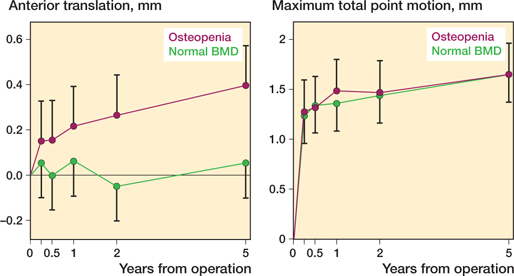
Figure 3. Anterior/posterior translation and maximum total point motion of the patients with osteopenia and the patients with normal BMD. Graphs are presented with predicted means from linear mixed models and 95% confidence intervals.
Polyethylene wear (Table 2 and Table 4)
PE wear was comparable between groups regarding all parameters. The majority of the PE wear occurred during the bedding-in phase. Additionally, the annual wear rate from 1 year postoperatively to 5-year follow-up ranged from 0.00 to 0.01 mm/year, indicating that minimal PE wear occurred after the bedding-in phase.
Cup position and radiolucent lines
The cup inclination at baseline was not statistically significantly different between groups with a mean of 41° (CI 39–43) in the P group and 42° (95% CI 40–44) in the PBM group, and remained similar at 5-year follow-up.
The distribution of heterotopic bone formation using Brooker’s classification was similar between groups at 5-year follow-up. Further, there were no patients presenting with radiolucent lines at the 5-year follow-up.
Clinical outcomes (Figure 4)
At 5-year follow-up, VAS measured pain showed clinically relevant improvements of 21 (CI 10–32) at rest and 31 (CI 18–43) at activity, with no statistically significant difference between groups (21). At 5-year follow-up, the PBM group had improved their EQ-5D by a mean of 0.34 (CI 0.24–0.45), which was statistically significantly higher than the improvement of 0.14 (CI 0.04–0.25) in the P group.
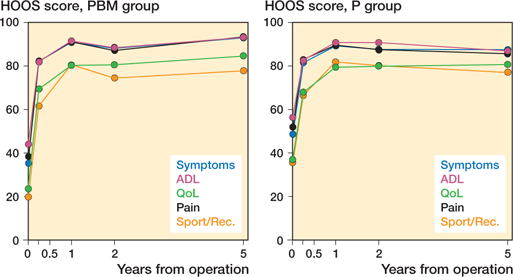
Figure 4. Mean HOOS scores for the PBM group and the P group with 5 years’ follow-up.
At baseline, the P group scored statistically significantly higher on all 5 HOOS subscales. 3 months postoperatively, the HOOS score differences were not present, and the groups remained comparable all the way to the 5-year follow-up. The 5-year mean HOOS improvements ranged from 45 (CI 38–53) for HOOS pain to 53 (CI 45–61) HOOS quality of life.
Discussion
In this 5-year RCT study, we found similar early cup migration but a pattern of continued migration in the PBM group resulting in a higher 5-year TT and MTPM in the PBM group compared with the P group. This is contrary to our expectation of similar or superior fixation of PBM cups compared with P cups, as experimental study results suggested improved fixation using BM coating (3,22). The PE wear rate was barely measurable in both groups, indicating no harmful effect on PE wear of BM coating.
RSA
The 5-year results in the present study do not change the interpretation of the previously published 2-year migration (12). These 2 publications are based on the only available RCT of electrochemically applied HA coating on cementless cups. Flatøy et al. compared migration of BM-coated and plasma-sprayed HA coated femoral stems and found no difference in 5-year migration (11). Other studies have evaluated the effect of plasma-sprayed HA coating on cups with 5 and 8 years’ follow-up, showing either little or no positive effect of HA coating on cup fixation (4,5). This complies with the results from the present study on PBM cups, and is further in accordance with a large meta-analysis and registry study on cementless cups coated with HA (7,23).
Nilsson et al. evaluated the 5-year migration pattern of cementless porous acetabular cups in 11 patients who were age-comparable to our patients after THA and found a mean proximal migration of 0.36 mm (24), which is slightly larger and outside the 95% CI of the 0.10 and 0.19 mm in the P and PBM group of the present study. Pijls et al. published a paper with proximal migration thresholds indicating 0.2 mm at 2-year follow-up to increase the risk of later aseptic loosening (14). 7/23 patients in the P group and 8/25 patients in the PBM group had 2-year proximal migration ≥ 0.2 mm, indicating no effect of BM.
DXA-measured bone mineral density
We found a difference only between patients with normal BMD and patients with osteopenia in anterior/posterior translation, where the osteopenia group had larger migration at 2- and 5-years follow-up. Finnilä et al. found increased 3- and 6-month proximal cup migration in female patients with low systemic BMD, which is in accordance with the difference in anterior translation in the present study (25).
Polyethylene wear
Plasma-sprayed HA coating on cementless cups has been associated with higher UHMWPE wear rates in several studies (8,26). In this study, BM was not associated with higher wear, likely because it is a thin coating and quickly resorbed. Bergvinsson et al. reported RSA measured wear rates (proximal wear) of 0.12 mm/year for UHMWPE and 0.02 mm/year for HXLPE in porous-coated cups considering a 3-month bedding-in period and 10 years’ follow-up (27).
Shareghi et al. evaluated the 5-year penetration rate of uncemented cups with vitamin E-infused highly cross-linked polyethylene liners (E1) compared with highly cross-linked liners without vitamin E (ArComXL) (28). They found annual 3D wear rates of mean 0.04 mm/year (CI 0.03–0.05) for E1 and mean 0.07 mm/year (CI 0.06–0.10) for ArComXL. The annual 3D wear rates for E1 are comparable to the 0.05 mm/year (P group) and 0.04 mm/year (PBM group) in the present study (28).
Clinical outcomes
The PBM group had larger 5-year improvements than the P group on all 5 subscales of HOOS and EQ-5D. However, there was no difference between the 2 groups at 5-year follow-up in any of the 5 HOOS subscales or EQ-5D, and probably the larger improvement in the PBM group is due to the lower baseline score. The lowest 5-year HOOS improvements were found in the pain and activities of daily living subscales, and all were larger than the minimal clinical important improvements (29). Patients reached mean HOOS scores comparable to the patient-acceptable symptom states (PASS) for HOOS and the PASS value for EQ-5D was contained within the confidence interval internal for both the P and PBM group (29).
Strengths and weaknesses
The strength of this study is the blinded randomized design, the high precision of the RSA method used for measurements, and the high patient compliance rate.
Conclusion
Contrary to expectations, we found higher 2- and 5-year cup migration in the group with porous and BoneMaster coating compared with the group with only porous coating. Both the 5-year PE wear rates and the 5-year clinical outcomes were comparable between the two groups. The results of this study do not indicate any advantage of BoneMaster coating on cementless hemispherical cups compared with porous coating alone.
- DHR. The Danish Hip Arthroplasty Registry. National annual report; 2016.
- Søballe K, Hansen E S, B-Rasmussen H, Jørgensen P H, Bünger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10(2): 285-99.
- Daugaard H, Elmengaard B, Bechtold J E, Jensen T, Søballe K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J Biomed Mater Res A 2010; 92(3): 913-21.
- Röhrl S M, Nivbrant B, Ström H, Nilsson K G. Effect of augmented cup fixation on stability, wear, and osteolysis: a 5-year follow-up of total hip arthroplasty with RSA. J Arthroplasty 2004; 19(8): 962-71.
- Valancius K, Søballe K, Nielsen P T, Laursen M B. No superior performance of hydroxyapatite-coated acetabular cups over porous-coated cups. Acta Orthop 2013; 84(6): 544-8.
- Otten V T, Crnalic S, Röhrl S M, Nivbrant B, Nilsson K G. Stability of uncemented cups—long-term effect of screws, pegs and HA coating: a 14-year RSA follow-up of total hip arthroplasty. J Arthroplasty 2016; 31(1): 156-61.
- Lazarinis S, Mäkelä K T, Eskelinen A, Havelin L, Hallan G, Overgaard S, et al. Does hydroxyapatite coating of uncemented cups improve long-term survival? An analysis of 28,605 primary total hip arthroplasty procedures from the Nordic Arthroplasty Register Association (NARA). Osteoarthritis Cartilage 2017; 25(12): 1980-7.
- Stilling M, Rahbek O, Søballe K. Inferior survival of hydroxyapatite versus titanium-coated cups at 15 years. Clin Orthop Relat Res 2009; 467(11): 2872-9.
- Wang H, Eliaz N, Xiang Z, Hsu H P, Spector M, Hobbs L W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006; 27(23): 4192-203.
- Bøe B, Heier T, Nordsletten L. Measurement of early bone loss around an uncemented femoral stem. Acta Orthop 2011; 82(3): 321-4.
- Flatøy B, Röhrl S M, Bøe B, Nordsletten L. No medium-term advantage of electrochemical deposition of hydroxyapatite in cementless femoral stems. 5-year RSA and DXA results from a randomized controlled trial. Acta Orthop 2016; 87(1): 42-7.
- Jørgensen P B, Daugaard H, Jakobsen S S, Lamm M, Søballe K, Stilling M. Higher early proximal migration of hemispherical cups with electrochemically applied hydroxyapatite (BoneMaster) on a porous surface compared with porous surface alone: a randomized RSA study with 53 patients. Acta Orthop 2020; 91(1): 26-32.
- Nelissen R G, Pijls B G, Kärrholm J, Malchau H, Nieuwenhuijse M J, Valstar E R. RSA and registries: the quest for phased introduction of new implants. J Bone Joint Surg 2011; 93(Suppl 3): 62-5.
- Pijls B G, Nieuwenhuijse M J, Fiocco M, Plevier J W, Middeldorp S, Nelissen R G, et al. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survivalstudies. Acta Orthop 2012; 83(6): 583-91.
- Lindgren V, Galea V P, Nebergall A, Greene M E, Rolfson O, Malchau H. Radiographic and clinical outcomes of porous titanium-coated and plasma-sprayed acetabular shells: a five-year prospective multicenter study. J Bone Joint Surg 2018; 100(19): 1673-81.
- Valstar E R, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76(4): 563-72.
- Callary S A, Solomon L B, Holubowycz O T, Campbell D G, Munn Z, Howie D W. Wear of highly cross-linked polyethylene acetabular components. Acta Orthop 2015; 86(2): 159-68.
- DeLee J G, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res 1976(121): 20-32.
- Nilsdotter A K, Lohmander L S, Klässbo M, Roos E M. Hip disability and osteoarthritis outcome score (HOOS): validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003; 4:10.
- Greene M E, Rader K A, Garellick G, Malchau H, Freiberg A A, Rolfson O. The EQ-5D-5L improves on the EQ-5D-3L for health-related quality-of-life assessment in patients undergoing total hip arthroplasty. Clin Orthop Relat Res 2015; 473(11): 3383-90.
- Danoff J R, Goel R, Sutton R, Maltenfort M G, Austin M S. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplasy 2018; 33(7s): S71-S5.e2.
- Schmidmaier G, Wildemann B, Schwabe P, Stange R, Hoffmann J, Südkamp N P, et al. A new electrochemically graded hydroxyapatite coating for osteosynthetic implants promotes implant osteointegration in a rat model. J Biomed Mater Res 2002; 63(2): 168-72.
- Chen Y L, Lin T, Liu A, Shi M M, Hu B, Shi Z L, et al. Does hydroxyapatite coating have no advantage over porous coating in primary total hip arthroplasty? A meta-analysis. J Orthop Relat Res 2015; 10: 21.
- Nilsson K G, Theodoulou A, Mercer G, Quinn S J, Krishnan J. Midterm migration of a cementless, porous acetabular cup: a 5 year radiostereometric analysis. J. Orthop 2017; 14(4): 454-60.
- Finnilä S, Moritz N, Svedströ M E, Alm J J, Aro H T. Increased migration of uncemented acetabular cups in female total hip arthroplasty patients with low systemic bone mineral density: a 2-year RSA and 8-year radiographic follow-up study of 34 patients. Acta Orthop 2016; 87(1): 48-54.
- Gottliebsen M, Rahbek O, Ottosen P F, Søballe K, Stilling M. Superior 11-year survival but higher polyethylene wear of hydroxyapatitecoated Mallory-Head cups. Hip Int 2012; 22(1): 35-40.
- Bergvinsson H, Zampelis V, Sundberg M, Flivik G. Highly cross-linked polyethylene still outperforms conventional polyethylene in THA: 10-year RSA results. Acta Orthop 2021: 1-7.
- Shareghi B, Johanson P E, Kärrholm J. Wear of vitamin e-infused highly cross-linked polyethylene at five years. J Bone Joint Surg 2017; 99(17): 1447-52.
- Paulsen A, Roos E M, Pedersen A B, Overgaard S. Minimal clinically important improvement (MCII) and patient-acceptable symptom state (PASS) in total hip arthroplasty (THA) patients 1 year postoperatively. Acta Orthop 2014; 85(1): 39-48.
Supplementary data
| Factor | Translations, mm | Rotations, ° | |||||||
| Cup migration | x | y | z | TT a | MTPM | x | y | z | TR b |
| Mean diff. | 0.019 | –0.008 | –0.003 | 0.079 | 0.110 | –0.149 | –0.152 | 0.114 | 0.111 |
| SD diff. | 0.209 | 0.120 | 0.278 | 0.180 | 0.427 | 0.744 | 0.820 | 0.399 | 0.764 |
| CR | 0.41 | 0.235 | 0.545 | 0.353 | 0.837 | 1.458 | 1.607 | 0.782 | 1 .497 |
| PE wear | x | y | z | W2D c | W3D d | ||||
| Mean diff. | 0.019 | 0.018 | –0.045 | –0.005 | –0.035 | ||||
| SD diff. | 0.070 | 0.062 | 0.203 | 0.066 | 0.176 | ||||
| CR | 0.137 | 0.122 | 0.398 | 0.129 | 0.345 | ||||
| Mean diff. represents the systematic measurement error. | |||||||||
| SD diff. represents the random variation within the measurement comparing the double examinations. | |||||||||
| CR (1.96 * SD diff.) represents the precision on individual measurements. | |||||||||
| a TT (total translation) was calculated using the Pythagorean theorem (TT = √(Tx2+Ty2+Tz2)). | |||||||||
| b TR (total rotation) was calculated using the Pythagorean theorem (TR = √(Rx2+Ry2+Rz2)). | |||||||||
| c W2D was defined as the vectorial sum of x- and y-wear. | |||||||||
| d W3D was defined as the vectorial sum of x-, y-, and z-wear. | |||||||||
| Axis | P group | PBM group | |||||||
| Translations, mm | |||||||||
| x-axis (+medial/–lateral) | |||||||||
| 3 months | 0.07 (–0.10 to 0.24) | 0.01 (–0.14 to 0.16) | |||||||
| 6 months | 0.12 (–0.05 to 0.28) | –0.01 (–0.16 to 0.15) | |||||||
| 1 year | 0.16 (–0.00 to 0.33) | 0.02 (–0.13 to 0.18) | |||||||
| 2 years | 0.09 (–0.08 to 0.25) | 0.01 (–0.15 to 0.16) | |||||||
| 5 years | 0.16 (–0.01 to 0.33) | 0.06 (–0.09 to 0.22) | |||||||
| y-axis (+proximal/–distal) | |||||||||
| 3 months | 0.16 (0.06 to 0.26) | 0.20 (0.11 to 0.30) | |||||||
| 6 months | 0.09 (–0.01 to 0.19) | 0.23 (0.13 to 0.32) | |||||||
| 1 years | 0.10 (–0.00 to 0.20) | 0.18 (0.09 to 0.28) | |||||||
| 2 years | 0.12 (0.01 to 0.22) | 0.17 (0.07 to 0.26) | |||||||
| 5 years | 0.10 (–0.00 to 0.20) | 0.19 (0.10 to 0.29) | |||||||
| z-axis (+anterior/–posterior) | |||||||||
| 3 months | 0.19 (0.01 to 0.36) | 0.02 (–0.15 to 0.18) | |||||||
| 6 months | 0.10 (–0.07 to 0.28) | 0.03 (–0.13 to 0.19) | |||||||
| 1 year | 0.17 (–0.00 to 0.35) | 0.09 (–0.07 to 0.25) | |||||||
| 2 years | 0.10 (–0.08 to 0.27) | 0.08 (–0.08 to 0.24) | |||||||
| 5 years | 0.18 (0.00 to 0.36) | 0.23 (0.07 to 0.39) | |||||||
| TT a | |||||||||
| 3 months | 0.49 (0.35 to 0.63) | 0.48 (0.35 to 0.61) | |||||||
| 6 months | 0.46 (0.31 to 0.60) | 0.60 (0.47 to 0.73) | |||||||
| 1 year | 0.52 (0.37 to 0.66) | 0.61 (0.47 to 0.74) | |||||||
| 2 years | 0.43 (0.29 to 0.58) | 0.64 (0.51 to 0.77) | |||||||
| 5 years | 0.52 (0.37 to 0.66) | 0.80 (0.66 to 0.93) | |||||||
| MTPM, mm c | |||||||||
| 3 months | 1.19 (0.89 to 1.49) | 1.29 (1.01 to 1.57) | |||||||
| 6 months | 1.22 (0.92 to 1.52) | 1.41 (1.14 to 1.69) | |||||||
| 1 year | 1.29 (0.99 to 1.59) | 1.51 (1.24 to 1.79) | |||||||
| 2 years | 1.27 (0.97 to 1.58) | 1.60 (1.32 to 1.87) | |||||||
| 5 years | 1.37 (1.06 to 1.67) | 1.89 (1.61 to 2.17) | |||||||
| Rotations, ° | |||||||||
| x-axis (+anterior/–posterior tilt) | |||||||||
| 3 months | 0.49 (–0.01 to 0.98) | 0.07 (–0.39 to 0.53) | |||||||
| 6 months | –0.01 (–0.51 to 0.49) | –0.03 (–0.48 to 0.42) | |||||||
| 1 year | 0.17 (–0.33 to 0.67) | –0.06 (–0.51 to 0.40) | |||||||
| 2 years | 0.10 (–0.40 to 0.28) | –0.17 (–0.63 to 0.28) | |||||||
| 5 years | 0.46 (–0.04 to 0.96) | 0.38 (–0.08 to 0.84) | |||||||
| y-axis (+internal/–external rotation) | |||||||||
| 3 months | 0.55 (–0.02 to 1.12) | 0.68 (0.16 to 1.21) | |||||||
| 6 months | 0.09 (–0.47 to 0.66) | 0.58 (0.07 to 1.09) | |||||||
| 1 year | 0.21 (–0.36 to 0.78) | 0.21 (–0.30 to 0.73) | |||||||
| 2 years | –0.07 (–0.65 to 0.50) | 0.24 (–0.28 to 0.76) | |||||||
| 5 years | 0.27 (–0.29 to 0.84) | 0.83 (0.31 to 1.35) | |||||||
| z-axis (+decreased/–increased inclination) | |||||||||
| 3 months | 0.01 (–0.43 to 0.44) | –0.18 (–0.58 to 0.22) | |||||||
| 6 months | 0.20 (–0.24 to 0.64) | –0.16 (–0.56 to 0.23) | |||||||
| 1 year | 0.24 (–0.20 to 0.68) | –0.10 (–0.50 to 0.30) | |||||||
| 2 years | 0.17 (–0.27 to 0.61) | –0.16 (–0.56 to 0.24) | |||||||
| 5 years | 0.16 (–0.27 to 0.60) | –0.37 (–0.77 to 0.03) | |||||||
| TR b | |||||||||
| 3 months | 1.80 (1.38 to 2.21) | 1.64 (1.26 to 2.02) | |||||||
| 6 months | 1.69 (1.28 to 2.11) | 1.72 (1.35 to 2.10) | |||||||
| 1 year | 1.81 (1.40 to 2.23) | 1.85 (1.47 to 2.23) | |||||||
| 2 years | 1.87 (1.45 to 2.29) | 2.01 (1.63 to 2.39) | |||||||
| 5 years | 1.94 (1.52 to 2.35) | 2.36 (1.98 to 2.75) | |||||||
| a Total translation (TT), for calculation, see Table 2 | |||||||||
| b Total rotation (TR), for calculation, see Table 2. | |||||||||
| c Maximum total point motion (MTPM). | |||||||||