Internal fixation or hip replacement for undisplaced femoral neck fractures? Pre-fracture health differences reflect survival and functional outcome
Stina EK 1, Helen AL-ANI 2, Katarina GREVE 2,3, Karin MODIG 1, and Margareta HEDSTRÖM 2,4
1 Unit of Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm; 2 Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institutet, Stockholm; 3 Perioperative Medicine and Intensive Care, Karolinska University Hospital, Stockholm; 4 Trauma and Reparative Medicine Theme (TRM), Karolinska University Hospital, Stockholm, Sweden
Background and purpose — Internal fixation (IF) has been the standard procedure for undisplaced femoral neck fractures (FNFs). However, there is a changing trend towards hip replacement (HR). Yet there is a knowledge gap regarding the benefits of this surgical method. We investigated functional outcomes in patients ≥ 70 years following HR compared to IF for undisplaced FNFs.
Patients and methods — Patients ≥ 70 years with undisplaced FNF registered in the Swedish National Hip Fracture Registry (SHR) who underwent either IF or HR (hemiarthroplasty [HA)] or total hip arthroplasty [THA]) were investigated in terms of 1-year survival and proportion of reoperation. In a subsample with 4-month follow-up data (n = 3,623), pain, changes in living status, and physical function were additionally analyzed.
Results — 7,758 patients were included with a mean age of 85 years. 93% of the patients were operated on with IF, 5% with HA, and 2% with THA. Patients with THA more often lived independently and were able to walk outdoors, both before and after the hip fracture. The IF and HA groups were similar in baseline characteristics, and in functional and survival outcomes. The THA group had a 54% lower adjusted risk of 1-year mortality. The proportion of reoperations within 1 year was 9.5% for IF, 5.3% for HA, and 7% for THA.
Interpretation — The pre-fracture difference in health and function between patients operated on with IF, HA, and THA maked it difficult to compare outcomes of the 2 methods. Decision on surgical method must be taken on an individual level, considering patients’ well-being and allocation of resources.
Citation: Acta Orthopaedica 2022; 93: 643–651. DOI http://dx.doi.org/10.2340/17453674.2022.3974.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2021-05-31. Accepted: 2022-06-02. Published: 2022-07-11.
Correspondence: stina.ek@ki.se
The study was conceived by HAA, MH, KM, KG, and SE. SE performed the analyses. HAA, SE, and MH wrote the draft. All authors contributed to the interpretation of the data and to revision of the manuscript.
The authors would like to thank the patients and staff involved in RIKSHÖFT (SHR), who made this study possible.
Acta thanks Alma B Pedersen for help with peer review of this study.
The recommended routine surgical procedure for undisplaced femoral neck fractures (FNFs) is internal fixation (IF) with 2 or more screws or nails, preserving the femoral head with similar functional outcomes to hip replacements (HR) (1,2). Recently, it has been suggested that HR may be a better choice of surgical method with fewer reoperations (3). The good results of HR treatment in older patients with displaced FNF have inspired the idea that treatment with HR in undisplaced FNF may be similarly beneficial (4,5). Consequently, the best surgical method for undisplaced FNF for older patients is now debated (5). Therefore, we compared outcomes for patients ≥ 70 years with undisplaced FNF operated with IF compared with hemiarthroplasty (HA) and total hip arthroplasty (THA). Outcomes were function and pain after 4 months and mortality within 1 year, as well as frequency and types of reoperations.
Patients and methods
Study design and population
This nationwide cohort study included patients with undisplaced (Garden I+II), non-pathological femoral neck fractures aged 70 years or older. Patient data between 2014 and 2019 was extracted from the Swedish National Registry for Hip Fractures, RIKSHÖFT (SHR) (6). The SHR is a clinical register with an estimated coverage of 80–90% of all hip fractures (7).
Baseline and 4-month follow-up data until December 31, 2019 was extracted from the SHR. The date of death was obtained from the National Cause of Death Register and information on reoperations from the Swedish National Patient Register (NPR). The NPR contains information on all hospital admissions within Sweden, including classifications of surgical procedures assigned by physicians. The NPR has close to full coverage of all inpatient care (8) and the correct classification for injuries has been shown to be up to 95% (9). All citizens in Sweden are assigned a personal identification number. Using this number, data from the different registries was linked by the National Board of Health and Welfare and pseudonymized before being handed over to the investigators.
Demographics and comorbidities: baseline data
Age, sex, fracture type, and surgical method were recorded and registered in the SHR in conjuncture with the initial hospitalization for hip fracture. ASA classification was assessed as part of standard preoperative practice and in this study used to denote comorbidity (10). For the analysis, ASA 1–2 was combined into one group and ASA 3–5 in the other.
Walking ability was recorded in SHR by interview of the patient or next of kin, with set options. In this study it was divided into 3 categories (walked outdoors [with/without aid]/walked indoors [with/without aid]/not able to walk). Information on usage of walking aid was collected by the same procedure and divided into 3 categories (no/one aid [crutch, stick]/two aids [crutch, stick], or rollator/wheelchair or not able to walk). The type of residence at admission was dichotomized as: independent (own home/service housing) or care home and health care (care home/rehabilitation/healthcare facility). Primary surgical procedure was divided into 3 groups for the analysis: IF (1, 2, or 3 screws/pins/nails) or HR, separating HA and THA. Cognitive status at the time of admission was assessed by medical records and observation and recorded as: normal cognitive functioning, suspected dementia/delirium, or a known diagnosis of dementia.
Follow-up data
All patients either received a questionnaire from the register or were called by telephone 4 months after the primary operation. Patients who had died (n = 1,089) and those with missing information on the 4-month follow-up (n = 3,046) were excluded in the second part of the analysis regarding functional outcomes (Figure 1). Type of residence, walking ability, and need for walking aids were divided into groups as described above. Hip pain was categorized into 4 groups: transient/no pain, intermittent/mild, severe/substantial, or not able to answer.
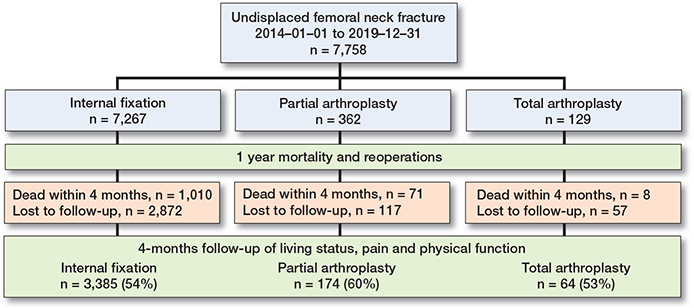
Figure 1. Flowchart of the study populations per surgical method.
Reoperation data
Reoperations within 1 year from the fracture date were collected from the NPR between 2014 and 2020. To ensure that the reoperation was related to the primary fracture, a restriction in reoperation codes was made for the different surgical procedures. Reoperation for IF patients included primary arthroplasty (ICD-10 code NFB), removal of implant, i.e., screws (NFU), deep infection (NFS), Girdlestone resection (NFG), and other minor reoperations (NFW and NFL). Reoperation for both types of HR included secondary arthroplasty (NFC), dislocation (NFH), removal of implant (NFU), deep infection (NFS), Girdlestone resection (NFG), and other minor reoperations (NFW and NFL). Primary and secondary arthroplasty overwrote any coexisting code and luxation, removal of implant, and deep infection, and a Girdlestone resection overwrote any of the other minor reoperations.
Statistics
Descriptive information on the full study population at baseline and the subsample at 4 months after fracture was stratified by type of surgery and presented in percentages or mean values. The association between surgical procedure and time to death was investigated by Cox proportional hazards regression models, for all and for strata of sex and age groups. The hazard ratio with 95% confidence intervals (CI) for HA and THA (using IF as a reference group) was estimated in survival analysis models accounting for the potential confounders age, sex, and ASA. Time to death for the different surgical procedures was also plotted with Kaplan–Meier curves. The change in function and residency before and after the hip fracture was plotted on an individual level with ggplot graphs. Finally, time trends in proportion of surgery procedures between 2014 and 2019 were plotted.
Sensitivity analyses
Descriptive information on individuals who died within 4 months and those missing at the 4-month follow-up was displayed in separate descriptive tables. Kaplan–Meier curves for strata of age groups, sex, and ASA score were created. To estimate the effect of cognitive impairment on the selection into type of surgery and outcome, an additional Kaplan–Meier curve was created among dementia-free patients only. Statistical analyses were performed using STATA version 16 (Stata Corp, College Station, TX, USA) and RStudio (RStudio Team [2020]. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA).
Ethics, funding, and possible conflict of interest
The study was approved by the regional Ethics Committee of Stockholm Dnr 2017/1088-31, 2011/136-31/5 and amendment Dnr 2018/84-32. The study was funded by grants provided by Region Stockholm (ALF project), Stiftelsen Promobilia, as well as the Kamprad Family Foundation for Entrepreneurship, Research and Charity [grant number 20190135]. The funding source did not play an active role in the investigation. The authors declare no conflicts of interest.
Results
7,758 patients (68% women) with undisplaced FNF and a mean age of 86 (SD 6) years were included in the study. 7,267 underwent IF, 362 HA and 129 THA. The patients who underwent THA were on average younger, had lower ASA scores, better walking ability, and were more likely to live independently before the fracture than patients who underwent IF or HA. Patients who underwent IF and HA were similar in terms of baseline characteristics, although patients with HA were on average older and had somewhat lower walking ability (Table 1). Waiting time to surgery was 22 hours for patients operated on with IF, 25 hours for HA and 34 hours for THA. The subsample with 4-month follow-up after the surgery (n = 3,046) was, overall, similar to the whole group at inclusion (Table 1), although with a higher proportion of individuals living independently and better walking abilities across the surgical groups, compared with the full sample. Table 2 (see Supplementary data) shows that individuals who died before the 4-month follow-up were older and in poorer health, as expected. Patients that were lost to follow-up at 4 months were less independent and in worse health, mainly with a larger proportion of dementia. These differences were most visible in the IF and HA groups.
| Factor | Full sample n = 7,758 a | 4 months’ sample n = 3,623 | ||||
| IF | HA | THA | IF | HA | THA | |
| n = 7,267 | n = 362 | n = 129 | n = 3,385 | n = 174 | n = 64 | |
| Age (SD) | 83 (7) | 86 (6) | 79 (5) | 83 (7) | 85 (6) | 79 (5) |
| Women | 4,942 (68) | 243 (67) | 81 (63) | 2,339 (69) | 118 (68) | 45 |
| Coming from | ||||||
| Independent living | 4,934 (68) | 239 (66) | 114 (88) | 2,545 (75) | 128 (74) | 60 |
| Care home or health care | 2,333 (32) | 123 (34) | 15 (12) | 842 (25) | 46 (26) | 4 |
| Walking ability | ||||||
| Outside | 4,907 (68) | 244 (67) | 110 (86) | 2,493 (74) | 134 (77) | 55 |
| Inside | 2,113 (29) | 111 (31) | 16 (12) | 800 (24) | 39 (22) | 7 |
| Not walking | 247 (3) | 7 (2) | 3 (2) | 92 (3) | 1 (1) | 2 |
| Walking aid | ||||||
| None or one stick/cane | 3,634 (50) | 162 (45) | 89 (69) | 1,900 (56) | 86 (49) | 46 |
| Two sticks or rollator | 3,305 (45) | 192 (53) | 36 (28) | 1,372 (41) | 86 (49) | 15 |
| Wheelchair/Not walking | 328 (5) | 8 (2) | 4 (3) | 113 (3) | 2 (2) | 3 |
| ASA score | ||||||
| 1–2 | 2,792 (38) | 123 (34) | 74 (57) | 1,498 (44) | 67 (39) | 39 |
| 3–5 | 4,475 (62) | 239 (66) c | 55 (43) | 1,887 (56) | 107 (61) d | 25 |
| Dementia | ||||||
| Intact | 3,450 (62) | 173 (58) | 81 (92) | 1,89 (70) | 102 (68) | 45 |
| Suspected/delirium | 824 (15) | 49 (17) | 4 (5) | 327 (12) | 22 (15) | 2 |
| Diagnosis of dementia | 1,283 (23) | 73 (25) | 3 (3) | 460 (18) | 26 (17) | 0 |
| Reoperation within 1 year | 690 (10) | 19 (5) | 9 (7) | 311 (9) | 9 (5) | 2 |
| Dead within 30 days | 441 (6) | 34 (9) | 4 (3) | |||
| Decad within 1 year | 1,722 (24) | 109 (30) | 10 (8) | 302 (9) | 18 (10) | 1 |
| a The analytical sample for survival and reoperations | ||||||
| b The analytical sample with SHR follow-up data for 4 months’ function. | ||||||
| c 1,818 missing (23%) | ||||||
| d 810 missing (22%) | ||||||
Functional outcome
At 4-month follow-up, the functional differences between the groups seen at baseline remained similar, although with a general decline in function (Table 3). All patients operated on with THA who lived independently at baseline still did so, while the percentage of patients operated on with IF and lived independently at baseline decreased from 75% to 70% and among HA from 74% to 68%. Despite a decline in walking ability and increase in walking aids, patients with THA maintained their ability to walk. Patients with HA had the biggest drop in walking ability, from 77% walking outside before the fracture to 50% walking outside 4 months after, compared with 74% to 52% and 86% to 84% for IF and THA, respectively. Around 10% could no longer walk at all in both the IF and HA groups, compared with 3% and 1% at baseline, respectively. Figure 2 shows a flow plot of change in living status, walking ability, and walking aid before and after surgery. To consider death as a functional outcome at 4 months, the figure shows both individuals who had 4-month follow-up data and individuals who died before having the possibility to undergo the 4-month follow-up. Patients with IF and HA both had similar baseline function and changes in function, with the difference that HA patients used more walking aids both before and after the fracture. The THA group stands out as being better off on all measures, as well as a lower proportion who died within 4 months.
| Factor | IF n = 3,385 | HA n = 174 | THA n = 64 |
| Living status | |||
| Independent living | 2,361 (70) | 118 (68) | 60 |
| Care home or health care | 2,024 (30) | 56 (32) | 4 |
| Walking ability | |||
| Outside | 1,768 (52) | 87 (50) | 54 |
| Inside | 1,258 (37) | 70 (40) | 9 |
| Not walking | 359 (11) | 17 (10) | 1 |
| Walking aid | |||
| None or one stick/cane | 1,242 (37) | 38 (22) | 39 |
| Two sticks or rollator | 1,717 (51) | 116 (66) | 22 |
| Wheelchair/not walking | 426 (12) | 20 (12) | 3 |
| Pain | |||
| None/transient | 1,893 (56) | 120 (69) | 41 |
| Mild/intermittent | 972 (29) | 31 (18) | 17 |
| Substantial/severe | 283 (8) | 7 (4) | 1 |
| Cannot answer | 237 (7) | 16 (9) | 5 |
| Still on pain medication a | 1,097 (34) | 46 (29) | 18 |
| a 143 missing (4%). | |||
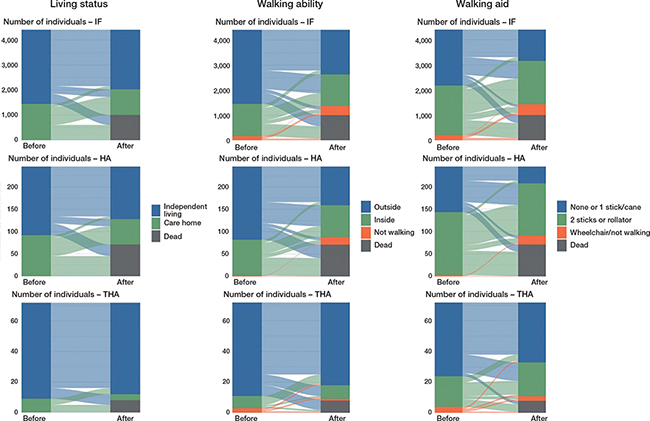
Figure 2. Change in living status, walking ability, and use of walking aid prior to the hip fracture and 4 months after the hip fracture, per surgical method, the 4-month follow-up sample (n = 3,623) plus those who died during the 4 months after the hip fracture (n = 1,089), total n = 4,712.
Pain
Pain 4 months after the fracture showed a different pattern than walking ability, with HA patients experiencing least pain (69% with none/transient pain compared with 56% for IF and 64% for THA) but IF patients having the highest proportion of pain (8% with substantial/severe pain compared with 4% among HA patients and 2% for THA). 34% among IF patients were still taking pain medication due to pain from the hip; the percentages for HA and THA were 29% and 31%, respectively. In general, very few (2–8%) patients answered that they had substantial or severe pain.
Mortality
Compared with IF, HA had a 35% increased risk of dying in the first year after the hip fracture and the risk was even stronger in women and the 70–79 years old, with a 45% and 88% increase, respectively. When controlling for age and sex, the increased risk was only statistically significant for individuals aged 70–79 years. In the final model, also controlling for ASA score, there were no increased risks for any of the subgroups. THA had a lower risk compared with IF in all subgroups except for the 70–79 years old, with a risk reduction between 72% and 67%. The protective effect remained when controlling for age and sex but only remained in the full sample when additionally controlling for ASA score (Table 4). The Kaplan–Meier curves showed that patients who died after THA did so shortly after the fracture while the curves for HA and IF had a similar smoother curve (Figure 3). Kaplan–Meier curves stratified for age, sex, and ASA score showed that older individuals, men, and individuals with ASA score 3–5 had steeper survival curves but that the pattern between the groups followed the one for the whole sample (Figure 4, see Supplementary data). The survival curves for dementia-free individuals showed that, although attenuated, the same pattern remained (Figure 4, see Supplementary data).
| Factor | crude HR (CI) | adjusted HR (CI) a | adjusted HR (CI) b |
| All | |||
| HA | 1.4 (1.1–1.6) | 1.2 (0.9–1.4) | 1.1 (0.9–1.4) |
| THA | 0.3 (0.2–0.6) | 0.4 (0.2–0.7) | 0.5 (0.3–0.9) |
| Women | |||
| HA | 1.5 (1.1–1.9) | 1.2 (0.9–1.6) | 1.3 (0.9–1.6) |
| THA | 0.3 (0.1–0.7) | 0.4 (0.2–0.9) | 0.4 (0.2–1.0) |
| Men | |||
| HA | 1.2 (0.9–1.6) | 1.0 (0.8–1.4) | 0.9 (0.7–1.3) |
| THA | 0.3 (0.1–0.7) | 0.4 (0.2–0.9) | 0.5 (0.2–1.2) |
| 70–79 years | |||
| HA | 1.9 (1.1–3.1) | 1.7 (1.1–2.9) | 1.4 (0.9–2.4) |
| THA | 0.4 (0.2–1.1) | 0.4 (0.1–1.0) | 0.5 (0.2–1.3) |
| ≥ 80 years | |||
| HA | 1.1 (0.9–1.4) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) |
| THA | 0.3 (0.2–0.7) | 0.4 (0.2–0.9) | 0.5 (0.2–1.1) |
| a Adjusted for age and sex (unless stratified for) | |||
| b Adjusted for age, sex, and ASA score. | |||
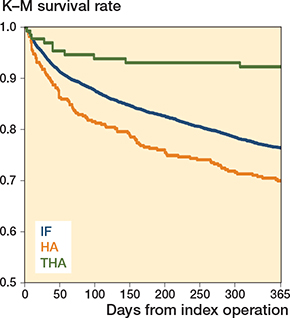
Figure 3. Kaplan–Meier curves showing time of death up to 1 year for the different surgical methods (n = 7,758).
Reoperations (Table 5)
IF patients had the highest proportion of reoperations (9.5%). The proportion was 7% among THA patients and 5% among HA patients. The most common types of reoperation among IF patients were arthroplasty or removal of screws while the most common reoperation for both HA and THA were a secondary arthroplasty and intervention for deep infection.
Time trends
There was an increase over time for undisplaced FNF that were treated with arthroplasty, although still at low levels. This increase seems to be driven by an increase in HA among the oldest old (Figure 5).
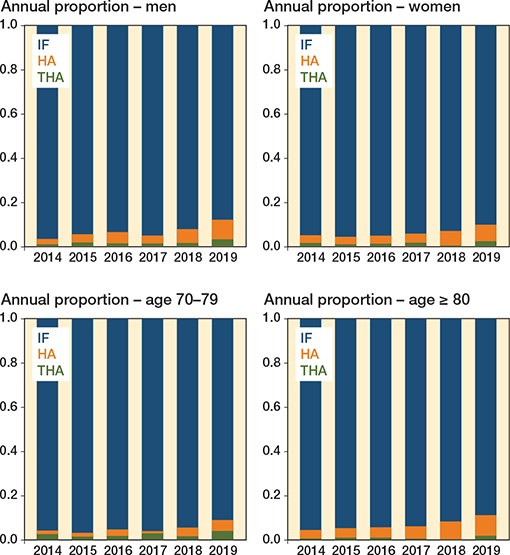
Figure 5. Time trends of proportion of surgical methods for undisplaced FNF between 2014 and 2019, stratified by sex and age groups.
Discussion
The purpose of this study was to evaluate the outcome in patients 70 years and older, following HR compared with IF for undisplaced FNF in terms of pain, walking ability, mortality, and reoperation. Only 5% of patients with undisplaced FNF were operated on with HA and only 2% with THA. Patients who received THA were younger, healthier, had higher physical function, and lived independently to a higher extent than HA and IF patients. This makes comparison between the surgical methods problematic, as THA patients are likely to have better outcomes. As expected, the same pattern was also seen after 4 months. When trying to account for some of these differences by adjusting for age, sex, and ASA score, THA still showed a better effect in 1-year survival compared with IF.
Functional outcome
That baseline function and sociodemographic characteristics differed between the surgical groups is in line with a Danish study, although comparing both displaced and undisplaced FNF (11). This indicates that the worse functional outcome for IF and HA is driven by selection of patients into surgical procedure. In a randomized controlled trial (RCT) of 218 Norwegian patients > 70 years of age with an undisplaced FNF allocated to screw fixation or HA, no clinically significant differences in regaining hip function or in postoperative pain were found. Yet HA patients had slightly better results for mobility measured by Timed Up and Go (3). These results are in contradiction to our observational findings, reinforcing our conclusion that the difference in post-surgery function is driven by pre-surgery status. Improved mobility soon after HR is an expected benefit, as it provides fracture stability soon after surgery. IF is a hip-preserving method, thus one could theorize that the fracture may not have fully healed 4 months after surgery. However, both our study and a recent study by Laubach et al. show that, compared with HA (but not THA), IF patients recover as well or better in terms of mobility even within 4 months (12). 2 recent review studies reported no clinical benefits of THA compared with HA when adjusting for confounders (13,14). This strengthens our idea that the differences we saw between the 2 HR groups were driven by selection of surgical method. Dolatowski et al. reported that pain reached pre-fracture level in the first 24 months after the fracture among both IF and HA patients (3). Therefore, comparison of pain and mobility earlier after surgery might not be ideal. Instead, a longer follow-up period after surgery may be more appropriate. However, in our study, most patients already rated their pain as none to intermittent 4 months after the fracture, indicating that pain might not be the mechanism behind poorer function. Also, patients with HA (with the worst outcomes of the three surgical methods) rated their pain as least severe.
Reoperations
Reoperation within 1 year was, overall, low in our study: 10% after IF, 5% after HA, and 7% after THA. Complications after IF, such as avascular necrosis of the femoral head, produces symptoms that often develop later than 12 months after surgery. Likewise, for arthroplasty, the possibility of detecting femoral loosening and acetabular erosion increases with longer follow-up (15). This suggests that a longer follow-up time than our 4 months might be important when evaluating the risk of reoperations.
Age likely affects the risk of reoperation. Griffin et al. found a higher risk of reoperation following IF with increasing age (16). However, the study by Gjertsen et al. on patients with undisplaced FNFs found no association between the risk of reoperation with age, sex, or ASA class (15). Due to the low number of reoperations in our study, we did not have the possibility to compare subgroups.
Mortality
HA was associated with an increased risk of 1-year mortality and THA with a lower risk of mortality, compared with IF. After controlling for age, sex, and ASA score the lower risk remained for THA, but the difference between IF and HA was no longer statistically significant. There might be several reasons why THA patients have lower mortality. Residual confounding related to health is one, a better possibility to be active and mobile once rehabilitated is another. In line with our study, Sikand et al. found higher mortality at both 1 month and 1 year after HA compared with IF (17). In our study, most deaths were observed in the early months following surgery in the THA group, which may be explained by HR being a more invasive surgery with an increased risk of peroperative blood loss and postoperative complications (2). THA patients waited longer for surgery compared with IF and HA patients, which could be due to THA sometimes requiring more experienced surgeons than IF. This could contribute to the early mortality pattern seen in the HA group, since a long waiting time to surgery is associated with higher short-term mortality, although it does not explain the similar curves seen for IF and HA (18).
Clinical implications and future research
There is still a lack of knowledge regarding the optimal treatment for undisplaced FNFs, as pointed out by Rogmark et al. (19). In our study, we found that IF and HA patients had similar characteristics and outcomes while HA and THA patients were different in terms of baseline age, function, and independence. The reasons for this could be several: a surgeon might reason that a healthy and independent hip fracture patient would benefit from receiving a THA, which would mean more invasive surgery but also give more freedom in terms of mobility and less risk of reoperations. That healthier, mobile patients with displaced FNF should have the option to receive a THA is in line with National Institute for Health and Care Excellence (NICE) Guidelines; however, that is not the recommendation for undisplaced fractures, reflected in the low proportion of 1.7 % undergoing THA in our study (20). A frail patient with poor bone quality might have a lower risk of reoperation with an HA instead of an IF, which is still a less invasive method than THA. There might also be strategic incentives for choosing one surgical method over another, a factor that is not possible to capture with our study design. In the end, these 2 patient profiles are different and if put together in a risk analysis would mask any potential associations between surgical method and adverse outcomes. In a clinical reality scenario with heterogenic patients, it might not be possible to decide on surgical method for a specific type of fracture on a group level, but a person-centered approach is required. According to our findings, the surgery type decision today seems to be person-centered. Other factors also play a role in decision on surgical method, such as the longer operation time and a demand for more experienced surgeons that an HR requires. In our study, THA patients had a longer waiting time to surgery compared with IF and HA patients, and still had a better outcome, strengthening the results by Greve et al. showing that healthier patients are not as affected by long waiting time until surgery (21). A reason for a small increase in HR during the last few years could be due to the recent knowledge regarding a large posterior tilt increasing the risk of fixation failure, which implies a reason to choose HR (22).
There is a lack of RCTs comparing the results of IF and HR in treating undisplaced FNFs (15). An RCT (the HipSTHeR study) is currently being conducted in Sweden, where 1,440 patients with an undisplaced FNF are randomized to IF or hip arthroplasty. That study focuses on mortality and reoperations, and not on functional outcome (23), thus the question of which method is preferable may partly remain unanswered.
Strengths and limitations
Many RCTs exclude frail patients, especially the large group of patients with cognitive impairments. We present results from a national clinical quality register, which adds to the generalizability of our results. However, dementia was more common among individuals who were lost to follow-up, indicating that cognitive impairment was one of the mechanisms behind dropout. This is a well-known issue in most observational studies that needs to be addressed as a limitation. Also, difference in mortality could be explained by the cognitive status of the patients, as dementia is a well-known risk factor for mortality after a hip fracture (24), and there was almost no one with delirium or dementia in the THA groups, who also had better survival. We did not have the possibility to adjust for dementia in our main analysis but conducted sensitivity analyses of dementia-free patients. These showed similar patterns in the survival curves to those in the main analyses, although attenuated, suggesting dementia patients having higher mortality but not necessarily a different pattern depending on surgical method. Another drawback is that patients were not randomized to treatment and thus the groups differed at baseline, with THA-treated patients being healthier. Nevertheless, observational studies like this reflect a clinical reality and mirror the surgeon’s choice of method. Moreover, the small sample of HR-treated patients as well as the short follow-up time may obscure the full impact of the different surgical methods (by missing real differences due to wide confidence intervals). The fact that only 40–47% of the patients were alive and eligible at 4-month follow-up introduces uncertainty regarding the results for functional outcome. However, the proportion of those lost to follow-up was similar between groups and is unlikely to take away the difference between the groups. A strength of the study is that data on mortality and reoperation was complete. Last, we cannot rule out the risk of residual confounding in observational studies. One such factor is information concerning the share of uncemented HR, to which we did not have access. A recent review by Lewis et al. indicates that cemented HRs might have a better outcome (14) and a lower reoperation rate than uncemented HRs (25). However, uncemented HRs are rarely used for hip fractures in Sweden (26).
Conclusion
Patients operated on with THA were healthier, more mobile, and lived independently to a higher degree before the fracture than patients operated on with IF and HA. This difference remained postoperatively. HA patients shared baseline characteristics with IF patients and had slightly worse function and mortality after 4 months and 1 year, respectively. Our findings imply that it is not possible to compare surgical methods for undisplaced FNF in real-world data without considering differences in patient characteristics prior to the surgery. There is a health selection into surgical method, which is also reflected in mortality and function after the surgery. Decision on surgery method must be taken on an individual level, considering patients’ well-being and allocation of resources.
- LeBlanc K E, Muncie H L Jr, LeBlanc L L. Hip fracture: diagnosis, treatment, and secondary prevention. Am Fam Physician 2014; 89(12): 945-51.
- Bhandari M, Swiontkowski M. Management of acute hip fracture. N Engl J Med 2017; 377(21): 2053-62.
- Dolatowski F C, Frihagen F, Bartels S, Opland V, Šaltytė Benth J, Talsnes O, et al. Screw fixation versus hemiarthroplasty for nondisplaced femoral neck fractures in elderly patients: a multicenter randomized controlled trial. J Bone Joint Surg Am 2019; 101(2): 136-44.
- Gjertsen J E, Vinje T, Engesaeter L B, Lie S A, Havelin L I, Furnes O, et al. Internal screw fixation compared with bipolar hemiarthroplasty for treatment of displaced femoral neck fractures in elderly patients. J Bone Joint Surg Am 2010; 92(3): 619-28.
- Oñativia I J, Slullitel P A, Diaz Dilernia F, Gonzales Viezcas J M, Vietto V, Ramkumar P N, et al. Outcomes of nondisplaced intracapsular femoral neck fractures with internal screw fixation in elderly patients: a systematic review. Hip Int 2018; 28(1): 18-28.
- RIKSHÖFT. Swedish National Registry for Hip Fractures 2022. Available from: https://www.xn--rikshft-e1a.se/english.
- Meyer A C, Hedström M, Modig K. The Swedish Hip Fracture Register and National Patient Register were valuable for research on hip fractures: comparison of two registers. J Clin Epidemiol 2020; 125: 91-9.
- Ludvigsson J F, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11(1): 450.
- Bergström M F, Byberg L, Melhus H, Michaelsson K, Gedeborg R. Extent and consequences of misclassified injury diagnoses in a national hospital discharge registry. Inj Prev 2011; 17(2): 108-13.
- Saklad M. Grading of patients for surgical procedures. Anesthesiology (Philadelphia) 1941; 2(3): 281-4.
- Viberg B, Frøslev T, Overgaard S, Pedersen A B. Mortality and revision risk after femoral neck fracture: comparison of internal fixation for undisplaced fracture with arthroplasty for displaced fracture: a population-based study from Danish National Registries. Acta Orthop 2021; 92(2): 163-9.
- Laubach M, Bläsius F M, Volland R, Knobe M, Weber C D, Hildebrand F, et al. Internal fixation versus hip arthroplasty in patients with nondisplaced femoral neck fractures: short-term results from a geriatric trauma registry. Eur J Trauma Emerg Surg 2022 Jun;48(3):1851-9.
- Ekhtiari S, Gormley J, Axelrod D E, Devji T, Bhandari M, Guyatt G H. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fracture: a systematic review and meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2020; 102(18): 1638-45.
- Lewis S R, Macey R, Parker M J, Cook J A, Griffin X L. Arthroplasties for hip fracture in adults. Cochrane Database Syst Rev 2022; 2(2): Cd013410.
- Gjertsen J E, Fevang J M, Matre K, Vinje T, Engesæter L B. Clinical outcome after undisplaced femoral neck fractures. Acta Orthop 2011; 82(3): 268-74.
- Griffin J, Anthony T L, Murphy D K, Brennan K L, Brennan M L. What is the impact of age on reoperation rates for femoral neck fractures treated with internal fixation and hemiarthroplasty? A comparison of hip fracture outcomes in the very elderly population. J Orthop 2016; 13(1): 33-9.
- Sikand M, Wenn R, Moran C G. Mortality following surgery for undisplaced intracapsular hip fractures. Injury 2004; 35(10): 1015-19.
- Pincus D, Ravi B, Wasserstein D, Huang A, Paterson J M, Nathens A B, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA 2017; 318(20): 1994-2003.
- Rogmark C, Flensburg L, Fredin H. Undisplaced femoral neck fractures: no problems? A consecutive study of 224 patients treated with internal fixation. Injury 2009; 40(3): 274-6.
- NICE. Hip Fracture Management. Clinical Guidelines. Available from: https://www.nice.org.uk/guidance/cg124.
- Greve K, Modig K, Talbäck M, Bartha E, Hedström M. No association between waiting time to surgery and mortality for healthier patients with hip fracture: a nationwide Swedish cohort of 59,675 patients. Acta Orthop 2020; 91(4): 396-400.
- Dolatowski F C, Adampour M, Frihagen F, Stavem K, Erik Utvåg S, Hoelsbrekken S E. Preoperative posterior tilt of at least 20° increased the risk of fixation failure in Garden-I and -II femoral neck fractures. Acta Orthop 2016; 87(3): 252-6.
- Wolf O, Sjöholm P, Hailer N P, Möller M, Mukka S. Study protocol: HipSTHeR—a register-based randomised controlled trial—hip screws or (total) hip replacement for undisplaced femoral neck fractures in older patients. BMC Geriatr 2020; 20(1): 19.
- Bai J, Zhang P, Liang X, Wu Z, Wang J, Liang Y. Association between dementia and mortality in the elderly patients undergoing hip fracture surgery: a meta-analysis. J Orthop Surg Res 2018; 13(1): 298.
- Kristensen T B, Dybvik E, Kristoffersen M, Dale H, Engesæter L B, Furnes O, et al. Cemented or uncemented hemiarthroplasty for femoral neck fracture? Data from the Norwegian Hip Fracture Register. Clin Orthop Relat Res 2020; 478(1): 90-100.
- Swedish National Hip Register. Annual report 2019. Available from: https://registercentrum.blob.core.windows.net/slr/r/2019-B1xpW-MUSPO.pdf.
Supplementary data
| Factor | Dead before 4-month FU, n = 1,089 | Alive but missing at 4-month FU, n = 3,046 | ||||
| IF | HA | THA | IF | HA | THA | |
| n = 1,010 | n = 71 | n = 8 | n = 2,872 | n = 117 | n = 57 | |
| Age (SD) | 86 (7) | 88 (6) | 85 (7) | 83 (7) | 84 (6) | 79 (6) |
| Women | 565 (56) | 44 | 3 | 2,038 (71) | 81 (69) | 33 |
| Coming from | ||||||
| Independent living | 400 (40) | 26 | 3 | 1,991 (69) | 85 (73) | 51 |
| Care home or health care | 610 (60) | 45 | 5 | 881 (31) | 32 (27) | 6 |
| Walking ability | ||||||
| Outside | 443 (44) | 30 | 6 | 1,971 (69) | 80 (68) | 49 |
| Inside | 491 (49) | 40 | 1 | 822 (28) | 32 (28) | 8 |
| Not walking | 76 (7) | 1 | 1 | 79 (3) | 5 (4) | 0 |
| Walking aid | ||||||
| None or one stick/cane | 315 (31) | 16 | 2 | 1,419 (49) | 60 (51) | 41 |
| Two sticks or rollator | 595 (59) | 54 | 5 | 1,338 (47) | 52 (45) | 16 |
| Wheelchair/not walking | 100 (10) | 1 | 1 | 115 (4) | 5 (4) | 0 |
| ASA score | ||||||
| 1–2 | 152 (15) | 16 | 1 | 1,142 (40) | 40 (34) | 34 |
| 3–5 | 858 (85) | 55a | 7 | 1,730 (60) | 77 (66)b | 23 |
| Dementia | ||||||
| Intact | 263 (35) | 18 | 3 | 1,358 (62) | 53 (60) | 33 |
| Suspected/delirium | 159 (21) | 12 | 0 | 338 (15) | 15 (17) | 2 |
| Known dementia | 327 (44) | 26 | 1 | 496 (23) | 21 (23) | 2 |
| Reoperation within 1 year | 25 (3) | 4 | 1 | 354 (12) | 6 (5) | 6 |
| Dead within 30 days | 441 (44) | 34 | 4 | |||
| Dead within 1 year | – | – | – | 410 (14) | 20 (17) | 1 |
| a 280 missing (26%) | ||||||
| b 728 missing (24%) | ||||||
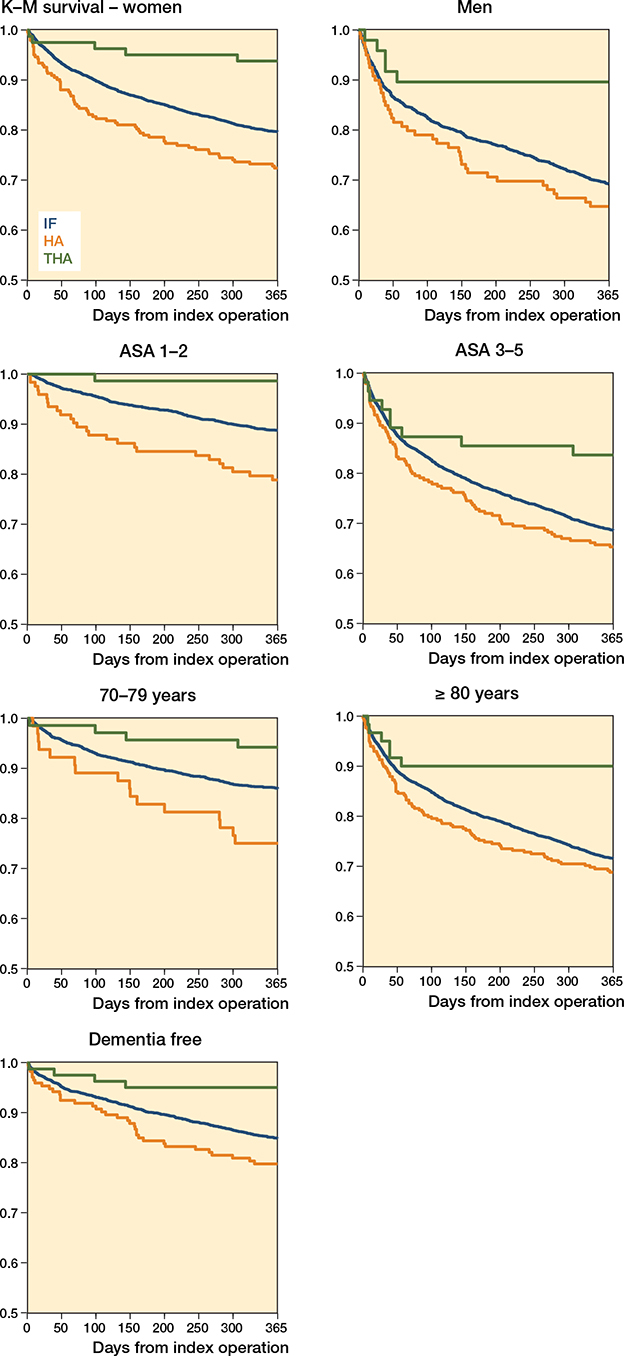
Figure 4. Kaplan–Meier curves showing time of death up to 1 year for the different surgical methods, stratified by sex, ASA score and age groups, as well as for a dementia-free subsample.