Impact of patient and prosthesis characteristics on common reasons for total knee replacement revision: a registry study of 36,626 revision cases from Australia, Sweden, and USA
Peter L LEWIS 1,3, Annette W-DAHL 2,3, Otto ROBERTSSON 2,3, Heather A PRENTICE 4, and Stephen E GRAVES 1
1 Australian Orthopaedic Association National Joint Replacement Registry, South Australian Health and Medical Research Institute, Adelaide, South Australia; 2 Swedish Knee Arthroplasty Register, Lund, Sweden; 3 Department of Orthopedics, Clinical Science Lund, Faculty of Medicine, Lund University, Lund, Sweden; 4 Surgical Outcomes and Analysis, Kaiser Permanente, San Diego, CA, USA
Background and purpose — Total knee replacement (TKR) studies usually analyze all-cause revision when considering relationships with patient and prosthesis factors. We studied how these factors impact different revision diagnoses.
Patients and methods — We used data from 2003 to 2019 of TKR for osteoarthritis from the arthroplasty registries of Sweden, Australia, and Kaiser Permanente, USA to study patient and prosthesis characteristics for specific revision diagnoses. There were 1,072,924 primary TKR included and 36,626 were revised. Factors studied included age, sex, prosthesis constraint, fixation method, bearing mobility, polyethylene type, and patellar component use. Revision diagnoses were arthrofibrosis, fracture, infection, instability, loosening, pain, patellar reasons, and wear. Odds ratios (ORs) for revision were estimated and summary effects were calculated using a meta-analytic approach.
Results — We found between-registry consistency in 15 factor/reason analyses. Risk factors for revision for arthrofibrosis were age < 65 years (OR 2.0; 95% CI 1.4–2.7) and mobile bearing designs (MB) (OR 1.7; CI 1.1–2.5), for fracture were female sex (OR 3.2; CI 2.2–4.8), age ≥ 65 years (OR 2.8; CI 1.9–4) and posterior stabilized prostheses (PS) (OR 2.1; CI 1.3–3.5), for infection were male sex (OR 1.9; CI 1.7–2.0) and PS (OR 1.5; CI 1.2–1.8), for instability were age < 65 years (OR 1.5; CI 1.3–1.8) and MB (OR 1.5; CI 1.1–2.2), for loosening were PS (OR 1.5; CI 1.4–1.6), MB (OR 2.2; CI 1.6–3.0) and use of ultra-high molecular weight polyethylene (OR 2.3; CI 1.8–2.9), for patellar reasons were not resurfacing the patella (OR 13.6; CI 2.1–87.2) and MB (OR 2.0; CI 1.2–3.3) and for wear was cementless fixation (OR 4.9; CI 4.3–5.5).
Interpretation — Patients could be counselled regarding specific age and sex risks. Use of minimally stabilized, fixed bearing, cemented prostheses, and patellar components is encouraged to minimize revision risk.
Citation: Acta Orthopaedica 2022; 93: 623–633. DOI http://dx.doi.org/10.2340/17453674.2022.3512.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-14. Accepted: 2022-06-13. Published: 2022-07-05.
Correspondence: plewis@aoanjrr.org.au
PLL: Literature search, study design, methodology determination, data collection, data analysis, data synthesis, manuscript writing. AWD: Study design, interpretation of data, manuscript preparation. OR: Data analysis. HAP: Data analysis, manuscript preparation. SEG: Interpretation of data, manuscript preparation.
The authors would like to thank the surgeons and patients of the SAR, AOANJRR, and the KPJRR for contributing their valuable data as without these, studies like theirs would not be possible. They also thank Dr Sophie Rainbird, Prof David Campbell, and Professor Richard De Steiger for their help and advice during manuscript preparation.
Acta thanks Oystein Johannes Gothesen and Mika Niemelšinen for help with peer review of this study.
More than 100,000 annual total knee replacement (TKR) procedures are recorded by the combined arthroplasty registries of Sweden, Australia, and Kaiser Permanente from the USA (1). Although survivorship of TKR is over 95% at 10 years, the frequency of the procedure creates a considerable number requiring revision (2,3).
Data from large arthroplasty registries is useful for studying TKR revision, and this gives “real-world” assessments as it describes community experience rather than that of a tertiary referral service (4). Combining data from multiple registries can not only increase the number available to study, but also reduce practice variation limitations and increase generalizability (1). Although individual patient data is ideal, there can be difficulties obtaining this due to patient privacy regulations and data security and ownership concerns, so, alternatively, summary data can be obtained and combined using meta-analytic techniques (1,5).
Studies of patient and prosthesis characteristics affecting TKR revision usually analyze all-cause revision (1,6). While there have been summaries of common revision diagnoses, including arthrofibrosis (7,8), fracture (9,10), infection (11,12), instability (13,14), loosening (15,16), pain (17,18), patellar pain (19,20), and wear (21,22), how patient and prosthesis factors relate to these different reasons for revision remains unclear.
In a previous study using a multi-registry approach we found characteristics associated with overall revision rates of primary TKR (1). Now we analyze patient and prosthesis factors in relation to each common reason for revision.
Patients and methods
We obtained aggregate annual data for the period January 1, 2003 to December 31, 2019 for all first revision TKR procedures recorded in the Swedish Knee Arthroplasty Register (SKAR), the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), and the Kaiser Permanente Joint Replacement Registry (KPJRR). We included revision TKR only where the primary TKR procedure was recorded in the study period (between 2003 and 2019) and the initial diagnosis was osteoarthritis (OA). Revisions of partial knee arthroplasties, revisions of primary TKR for pathologies other than OA, with primary procedures prior to 2003 or subsequent revisions of a previous revision, were excluded. The completeness of these registries exceeds 95% and loss to follow-up was less than 8% over the study period. Validation and quality control methods of these registries have been published (2,23,24).
During this period, there were 1,072,924 primary TKRs for OA recorded (188,290 from the SKAR, 663,982 from the AOANJRR, and 220,652 from the KPJRR). Of these, 36,626 were revised (5,613 from the SKAR, 24,931 from the AOANJRR, and 6,082 from the KPJRR) and this constitutes the study group. Revision knee replacements included revision procedures of a previous total knee replacement where 1 or more components were added, removed, or exchanged.
The reason for revision was determined from the revision diagnosis selected by the surgeon at the time of the revision procedure from a predetermined list, or specifically added. Multiple reasons could be listed. In Sweden, all operative reports were methodically read and from these the primary reason for revision was interpreted by registry staff. In the AOANJRR and KPJRR, when multiple reasons for revision were recorded, a diagnosis hierarchy was used to determine the most important reason for revision. In this study only 1 reason for revision was permitted for each revision procedure. Revision diagnoses were classified by a previously created harmonized table of equivalent diagnosis groups (25).
Patient factors recorded (for both primary and revision procedures) were age, sex, ASA class, and BMI. As the SKAR and AOANJRR began collecting ASA and BMI data at later time points, these categories permitted limited analyses. We analyzed 5 prosthesis factors: (i) Prosthesis constraint was divided into minimally stabilized (MS) (those that have a flat or dished tibial articulation, regardless of congruency), posterior stabilized (PS) (implants that provide posterior stability using a peg and box design), fully stabilized (FS) (implants with a large peg and box design designed to give some collateral as well as posterior stability), and hinged (implants with a hinge mechanism to link the femoral and tibial components); (ii) Fixation was cemented (both femoral and tibial components cemented), cementless (both components inserted without cement), and hybrid (tibial or femoral component only cemented); (iii) Bearing mobility was either mobile (inserts designed to move relative to the tibial base-plate) or fixed (inserts designed not to move relative to the tibial base-plate); (iv) Patellar resurfacing components were either used or not used; (v) Polyethylene type was ultra-high molecular weight polyethylene (UHMWPE), highly cross-linked (XLPE, classified as ultrahigh molecular weight polyethylene that has been irradiated by high dose [> 50 kGy] gamma or electron beam radiation) and highly cross-linked polyethylene with antioxidant (combining the anti-oxidants vitamin E and Covernox [DePuy Synthes. Warsaw, IN, USA]) (XLPE + AntiOx ).
For the patient and prosthesis factor comparisons the 2 most common categories were selected for analysis. Age was divided into < 65 years and compared with ≥ 65 years of age. For the analyses of prosthesis constraint, MS were compared with PS, cement fixation was compared with cementless fixation, and for polyethylene type XLPE and XLPE + AntiOx were combined for comparison with UHMWPE.
Statistics
Proportions of the individual registry’s reasons for revision and revision procedures were determined and compared. Mean time to revision for each reason was calculated.
As time-to-event data was not available for this study, categorical data was used to calculate odds ratios (OR) with 95% confidence intervals (CI) for patient and prosthesis factors for each reason for revision using the on-line GIGAcalculator (26). The number revised was considered with respect to the total number of primary procedures having that factor. Where the odds ratios for each registry were concordant and all either above or below 1, and the confidence interval did not contain 1 (i.e., a consistent and statistically significant association was found), these were chosen for meta-analysis. The Mantel– Haenszel random-effects method was used due to the event rate being low and the presence of inter-registry heterogeneity. RevMan version 5.4 (27) was used for the calculations. Heterogeneity was determined by I2.
Ethics, data sharing, funding, and potential conflicts of interest
Ethics approval covering the SKAR data use was approved by the Ethics Board of Lund University (LU20-02). The AOANJRR is a declared a Commonwealth of Australia Quality Assurance Activity under section 124X of the Health Insurance Act, 1973. All AOANJRR studies are conducted in accordance with ethical principles of research (Helsinki Declaration II). Approval for inclusion of data from the Kaiser Permanente Joint Replacement Registry Institutional Review Board approval (#5488) was granted on August 18, 2021. A data sharing agreement for the purpose of this study was finalized on December 10, 2020 by the directors of the SKAR, AOANJRR, and KPJRR. There was no funding. There are no conflicts of interest.
Results
The mean patient ages of primary TKR were similar to those revised, while there were marginally greater proportions of males undergoing revision compared with primary TKR. ASA status and BMI for revised patients both showed a small shift in proportion to higher categories. Prosthetic factor comparisons between primary TKR and those revised showed small increases in proportions of PS components revised, mobile-bearing (MB) prostheses, and those using UHMWPE, but no consistent differences regarding fixation type or patellar component use (Table 1, see Supplementary data).
Reasons for revision
Infection, loosening, patellar reasons, and instability were the most common reasons for revision, except for the KPJRR where revisions for patellar reasons were infrequent (Figure 1). The mean times to revision were shortest for infection and arthrofibrosis and longest for wear, loosening, and fracture (Figure 2).
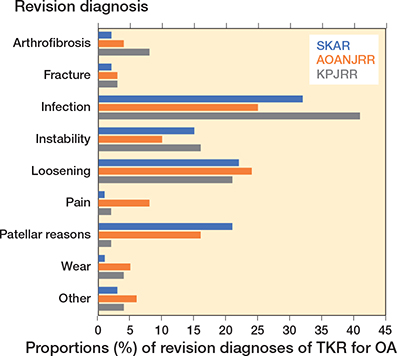
Figure 1. Proportions of revision diagnoses of TKR for OA.
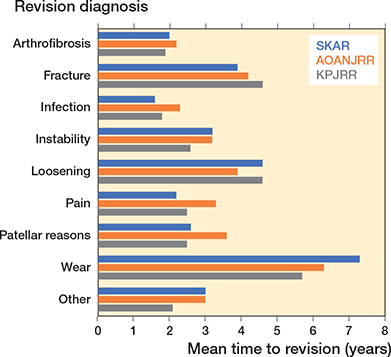
Figure 2. Mean time to revision (years) by revision reason.
Patient factors for each of the more common reasons for revision were analyzed (Table 2). The mean age at time of revision ranged from 64 to 77 years, being lowest for revisions for arthrofibrosis and highest for revisions for fracture. The proportion of females revised ranged from 39% to 88%, being lowest for revisions for infection and highest for revisions for fracture.
Prosthesis factors for the individual reasons for revision are presented in Table 3. These are the characteristics of the components used in the primary procedure that was revised. For each registry, the number and proportions for these factors for all primary procedures from which these revisions were derived are also listed for comparison. The proportions of revised TKR to primary TKR in each registry were compared for the 8 revision diagnoses, giving 24 comparisons. The proportion of revised compared with primary TKR using UHMWPE was higher in 21 of 24 comparisons, for both cementless and PS prostheses in 15 comparisons, while use of a fixed bearing was only higher in 2 comparisons.
Association of prosthesis factors with reasons for revision
Odds ratio determinations for individual reasons for revision showed that there was inconsistency between registries for many of the factors studied (Table 4, see Supplementary data). There was insufficient data for analysis of ASA or BMI. Where consistency was found, a summary effect for revision risk was determined by meta-analysis (Table 5).
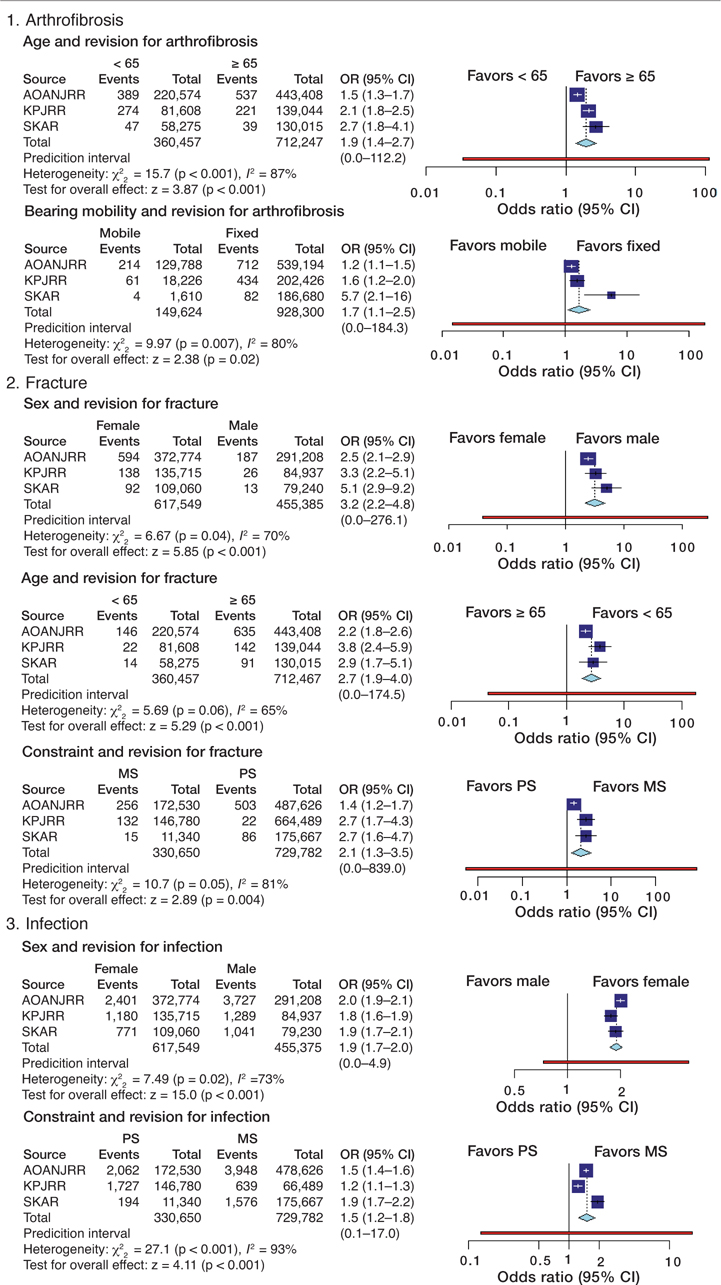
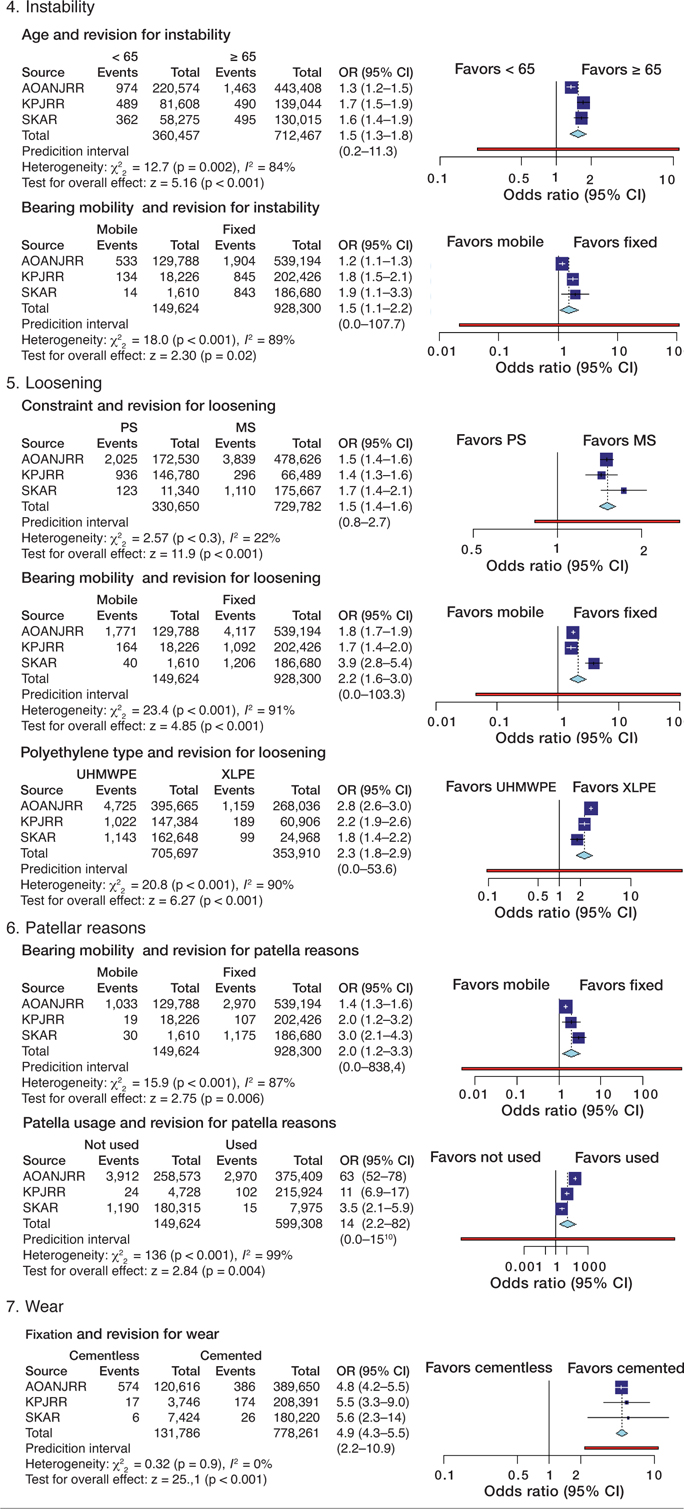
Table 5. Meta-analysis of odds ratios for the selected patient and prosthesis factors for each reason for revision, using the Mantel–Haenszel random-effects method
Analysis of arthrofibrosis showed that young age (OR 2.0; CI 1.4–2.7) and MB designs (OR 1.7; CI 1.1–2.5) were risk factors for revision. Higher odds for revision for fracture were seen for females (OR 3.2; CI 2.2–4.8), age 65 years and over (OR 2.8; CI 1.9–4), and with PS prostheses (OR 2.1; CI 1.3–3.5). Factors affecting revision for infection were male sex (OR 1.9; CI 1.7–2.1), and PS components (OR 1.5; CI 1.2–1.8). Younger age (OR 1.5; CI 1.3–1.8) and MB designs (OR 1.5; CI 1.1–2.2) had higher odds for revision for instability, while PS (OR 1.5; CI 1.4–1.6), as well as MB designs (OR 2.2; CI 1.6–3) and UHMWPE inserts (OR 2.3; CI 1.8–2.9) had higher odds for revision for loosening. There was no consistency found when factors were assessed with regard to revision for pain. MB components (OR 2.0; CI 1.2–3.3) and not using a patellar component (OR 13.6; CI 2.1–87.2) increased odds for revision for patellar reasons. Cementless fixation (OR 4.9; CI 4.3–5.5) increased the odds for revision for wear. The meta-analyses showed substantial or considerable statistical heterogeneity for all but 2 measures (fixation and revision for wear and prosthesis constraint and revision for loosening). It was only for the analysis of fixation for revision for wear that the prediction interval did not contain 1.
Discussion
We found infection, loosening, instability, and patellar reasons were the most common diagnoses for revision of TKR for OA in all 3 registries, with the only difference seen in the KPJRR where 98% of TKR had a primary patellar resurfacing and patellar causes for revision were rare. These findings, along with the timing of these revisions, are consistent with previous studies (25,28,29). When patient and prosthesis factors for revision reasons were studied, 15 of 56 possible factor/reason combinations showed between-registry consistency, varying from 3 concordant factors for each of revision for fracture and loosening, and no factor consistency for revision for pain.
Where discrepancy in odds ratio was found, this could mean that there is no link between the factor and revision reason (such as patellar component use and revision for instability), the numbers revised were too small to detect a difference (such as revisions for wear in the SKAR), or there may have been practice variations and individual prosthesis performance differences within the factor studied. There may also be between-surgeon differences in selecting revision diagnoses (for instance, due to multiple failure mechanisms being present, loosening recorded when a low-grade infection is detected later, or distinguishing between pain and patellar pain).
Younger age and MB designs were risk factors for revision for arthrofibrosis. Arthrofibrosis, or postoperative stiffness, is poorly understood but thought to involve an exaggerated soft tissue inflammation (8). While there is no consensus regarding the influence of age on postoperative stiffness shown in a systematic review (7), arthrofibrosis was the most frequent cause for revision in a recent MB study (30).
Higher likelihood for revision for fracture was seen with female sex and age ≥ 65 years, consistent with previous findings (31), and the association with PS components has been related to excessive or eccentric “box” cuts and increased internal prosthetic constraint compared with cruciate-retaining designs (10,32).
The odds ratio for revision for infection was higher for males, as has previously been described (11,33). The increase in revision for infection with PS components has been reported (12,34), attributed to polyethylene debris and the associated joint response seen with these designs (34).
Revision for instability was associated with younger age, consistent with other results (13,14), while instability with MB prostheses has been described (35,36). Due to inter-registry differences in the odds ratios (which may relate to prosthesis use patterns) prosthesis constraint was not a factor chosen for further analysis for instability.
The fixation method had inconsistent odds ratios for revision for loosening, but there were associations identified with factors relating to the bearing. UHMWPE showed higher odds for revision for loosening than XLPE, while there was a greater risk with PS and MB designs. Previous studies have been variable, with some showing no difference between “conventional” polyethylene and XLPE (37), while others report findings similar to ours that may relate to biological reactions to wear products (16). Osteolysis may also be a common mechanism to explain the association of loosening with PS and MB components (21,38), which others claim relates simply to component design (39).
Odds ratio summaries showed no factor consistency in the analysis of revision for pain. Post-TKR pain is considered multifactorial, and often related to systemic conditions such as depression and catastrophizing (17), and it is not surprising that prosthesis factors showed no association in this study.
The strongest association between prosthesis factors and revision was shown in the analysis of patellar component use and revision for patellar reasons, where not using a patellar component showed much higher odds for revision for these diagnoses. Although some controversy may remain around function and pain relief regarding patellar component use (40), there seems less doubt that using a patellar component leads to a lower rate of revision for this reason (41). The finding that MB prostheses also showed higher odds for revision for patellar reasons is contrary to a study from the New Zealand Joint Registry that found fixed-bearing PS prostheses had a higher rate of secondary patellar resurfacing than mobile-bearing designs (42). This difference may relate to the specific prostheses used in each registry, or possibly to the common “gap balancing” technique for implantation, where femoral component rotation is determined after ligament releases (43).
Prostheses with cementless fixation had higher odds for revision for wear compared with cemented components. While there seems no direct link of the implant–bone interface to the bearing, cementless fixation may have been selected for more active patients (44). Surgeons may also have difficulty deciding between wear and loosening as the primary mechanism leading to revision (25). The lack of further associations with wear may be due to the relative rarity of revisions for this diagnosis, particularly in Sweden.
This study has a number of limitations. Despite the study design that included large data sets from each registry, some revision reasons still have only small numbers for analysis and more robust conclusions would require the inclusion of data from more registries. Only 7 patient and prosthesis factors were included, and there may be other influences on revision. We used a categorical distinction of revised/not revised as the outcome measure, and perhaps more detail could have been obtained had time-to-event data been available. In addition, odds ratios for each reason for revision were considered separately, comparing the number revised for that diagnosis with the number not revised, and this method does not take into account TKR revised for other competing reasons, or those who died. Additionally, the number revised for each reason most likely understates the true number of revisions as, in order to assess the proportion of primary TKRs revised, toward the censoring endpoint some TKRs were included that did not have time to be revised. This aspect would have a greater effect on those reasons with longer times to revision. Each factor was analyzed independently and there may be interactions between factors. We used categories for each prosthesis factor analyzed and there may be prosthesis performance differences within these categories. We used the Mantel–Haenszel method for calculating a summary effect size, and this is known to become less accurate with small event rates (45).
In summary, age < 65 years was associated with lower odds of revision for fracture but higher odds of revision for arthrofibrosis and instability. Females had higher odds of revision for fracture, but lower odds of revision for infection. PS prostheses had a higher likelihood of revision for infection, fracture, and loosening, while MB prostheses had higher odds of revision for arthrofibrosis, instability, and for patellar reasons, cementless fixation showed higher odds of revision for wear, and not using a patellar component increased the likelihood of revision for patellar reasons.
Patients could be counselled regarding specific risks for their age and sex, while the use of minimally stabilized, fixed-bearing, cemented prostheses and patellar components is encouraged to minimize revision risk. However, the final choice of implant characteristics may need to be modified according to specific patient circumstances.
- Lewis P L, W-Dahl A, Robertsson O, Lorimer M, Prentice H A, Graves S E, et al. The effect of patient and prosthesis factors on revision rates after total knee replacement using a multi-registry meta-analytic approach. Acta Orthop 2022; 93: 284-93. doi: 10.2340/17453674.2022.1997.
- AOANJRR. AOANJRR Hip, knee and shoulder arthroplasty: 2020 annual report. Available from: https://aoanjrr.sahmri.com/annualreports-2020. (Accessed February 18, 2022).
- SKAR. Swedish Knee Arthroplasty Register Annual Report 2020. Available from: https://www.myknee.se/pdf/SVK_2020_Eng_1.0.pdf (Accessed February 18, 2022).
- Ner E, Nakamura N, Lattermann C, McNicholas M J. Knee registries: state of the art. J ISAKOS 2022; in press. doi: 10.1136/jisakos-2021-000625.
- Paxton E W, Mohaddes M, Laaksonen I, Lorimer M, Graves S E, Malchau H, et al. Meta-analysis of individual registry results enhances international registry collaboration. Acta Orthop 2018; 89: 369-73. doi: 10.1080/17453674.2018.1454383.
- Vertullo C J, Graves S E, Peng Y, Lewis P L. An optimum prosthesis combination of low-risk total knee arthroplasty options in all five primary categories of design results in a 60% reduction in revision risk: a registry analysis of 482,373 prostheses. Knee Surg Sports Traumatol Arthrosc 2019; 27: 1418-26. doi: 10.1007/s00167-018-5115-z.
- Tibbo M E, Limberg A K, Salib C G, Ahmed A T, van Wijnen A J, Berry D J, et al. Acquired idiopathic stiffness after total knee arthroplasty: a systematic review and meta-analysis. J Bone Joint Surg Am 2019; 101: 1320-30. doi: 10.2106/jbjs.18.01217.
- Thompson R, Novikov D, Cizmic Z, Feng J E, Fideler K, Sayeed Z, et al. Arthrofibrosis after total knee arthroplasty: pathophysiology, diagnosis, and management. Orthop Clin North Am 2019; 50: 269-79. doi: 10.1016/j.ocl.2019.02.005.
- Yoo J D, Kim N K. Periprosthetic fractures following total knee arthroplasty. Knee Surg Relat Res 2015; 27: 1-9. doi: 10.5792/ksrr.2015.27.1.1.
- Lombardo D J, Siljander M P, Sobh A, Moore D D, Karadsheh M S. Periprosthetic fractures about total knee arthroplasty. Musculoskelet Surg 2020; 104: 135-43. doi: 10.1007/s12306-019-00628-9.
- Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J 2017; 14: 529-36. doi: 10.1111/iwj.12640.
- Lenguerrand E, Whitehouse M R, Beswick A D, Kunutsor S K, Foguet P, Porter M, et al. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis 2019; 19: 589-600. doi: 10.1016/s1473-3099(18)30755-2.
- Lewis P L, Campbell D G, Lorimer M F, Requicha F, W-Dahl A, Robertsson O. Primary total knee arthroplasty revised for instability: a detailed registry analysis. J Arthroplasty 2022; 37: 286-97. doi: 10.1016/j.arth.2021.11.002.
- Wilson C J, Theodoulou A, Damarell R A, Krishnan J. Knee instability as the primary cause of failure following total knee arthroplasty (TKA): a systematic review on the patient, surgical and implant characteristics of revised TKA patients. Knee 2017; 24: 1271-81. doi: 10.1016/j.knee.2017.08.060.
- Cherian J J, Jauregui J J, Banerjee S, Pierce T, Mont M A. What host factors affect aseptic loosening after THA and TKA? Clin Orthop Relat Res 2015; 473: 2700-9. doi: 10.1007/s11999-015-4220-2.
- Gkiatas I, Karasavvidis T, Sharma A K, Xiang W, Malahias M A, Chalmers B P, et al. Highly cross-linked polyethylene in primary total knee arthroplasty is associated with a lower rate of revision for aseptic loosening: a meta-analysis of 962,467 cases. Arch Orthop Trauma Surg 2022; 142: 1177-84. doi: 10.1007/s00402-021-03887-z.
- Lewis G N, Rice D A, McNair P J, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth 2015; 114: 551-61. doi: 10.1093/bja/aeu441.
- Park C N, White P B, Meftah M, Ranawat A S, Ranawat C S. Diagnostic algorithm for residual pain after total knee arthroplasty. Orthopedics 2016; 39: e246-52. doi: 10.3928/01477447-20160119-06.
- Assiotis A, To K, Morgan-Jones R, Pengas I P, Khan W. Patellar complications following total knee arthroplasty: a review of the current literature. Eur J Orthop Surg Traumatol 2019; 29: 1605-15. doi: 10.1007/s00590-019-02499-z.
- Putman S, Boureau F, Girard J, Migaud H, Pasquier G. Patellar complications after total knee arthroplasty. Orthop Traumatol Surg Res 2019; 105: S43-S51. doi: 10.1016/j.otsr.2018.04.028.
- Fraser J F, Werner S, Jacofsky D J. Wear and loosening in total knee arthroplasty: a quick review. J Knee Surg 2015; 28: 139-44. doi: 10.1055/s-0034-1398375.
- Wilhelm S K, Henrichsen J L, Siljander M, Moore D, Karadsheh M. Polyethylene in total knee arthroplasty: Where are we now? J Orthop Surg (Hong Kong) 2018; 26: 2309499018808356. doi: 10.1177/2309499018808356.
- Paxton E W, Inacio M C, Khatod M, Yue E J, Namba R S. Kaiser Permanente National Total Joint Replacement Registry: aligning operations with information technology. Clin Orthop Relat Res 2010; 468: 2646-63. doi: 10.1007/s11999-010-1463-9.
- Robertsson O, Ranstam J, Sundberg M, W-Dahl A, Lidgren L. The Swedish Knee Arthroplasty Register: a review. Bone Joint Res 2014; 3: 217-22. doi: 10.1302/2046-3758.37.2000289.
- Lewis P L, Robertsson O, Graves S E, Paxton E W, Prentice H A, W-Dahl A. Variation and trends in reasons for knee replacement revision: a multi-registry study of revision burden. Acta Orthop 2021; 92: 182-8. doi: 10.1080/17453674.2020.1853340.
- Georgiev G Z. Odds ratio calculator. Available from https://www.gigacalculator.com/calculators/odds-ratio-calculator.php (Accessed February 18, 2022).
- Review Manager Web (RevMan Web). [Computer program]. Version 5.4.1. The Cochrane Collaboration, September 2020. Available from http://revman.cochrane.org/ (Accessed February 9, 2022).
- Sharkey P F, Lichstein P M, Shen C, Tokarski A T, Parvizi J. Why are total knee arthroplasties failing today: has anything changed after 10 years? J Arthroplasty 2014; 29: 1774-8. doi: 10.1016/j.arth.2013.07.024.
- Lum Z C, Shieh A K, Dorr L D. Why total knees fail: a modern perspective review. World J Orthop 2018; 9: 60-4. doi: 10.5312/wjo.v9.i4.60.
- Breugem S J M, Linnartz J, Sierevelt I, Bruijn J D, Driessen M J M. Evaluation of 1031 primary titanium nitride coated mobile bearing total knee arthroplasties in an orthopedic clinic. World J Orthop 2017; 8: 922-8. doi: 10.5312/wjo.v8.i12.922.
- Quinzi D A, Childs S, Lipof J S, Soin S P, Ricciardi B F. The treatment of periprosthetic distal femoral fractures after total knee replacement: a critical analysis review. JBJS Rev 2020; 8: e2000003. doi: 10.2106/jbjs.Rvw.20.00003.
- Kuzyk P R T, Watts E, Backstein D. Revision total knee arthroplasty for the management of periprosthetic fractures. J Am Acad Orthop Surg 2017; 25: 624-33. doi: 10.5435/jaaos-d-15-00680.
- Resende V A C, Neto A C, Nunes C, Andrade R, Espregueira-Mendes J, Lopes S. Higher age, female gender, osteoarthritis and blood transfusion protect against periprosthetic joint infection in total hip or knee arthroplasties: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2021; 29: 8-43. doi: 10.1007/s00167-018-5231-9.
- Vertullo C J, de Steiger R N, Lewis P L, Lorimer M, Peng Y, Graves S E. The effect of prosthetic design and polyethylene type on the risk of revision for infection in total knee replacement: an analysis of 336,997 prostheses from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am 2018; 100: 2033-40. doi: 10.2106/jbjs.17.01639.
- Diamond O J, Doran E, Beverland D E. Spinout/dislocation in mobilebearing total knee arthroplasty: s report of 26 cases. J Arthroplasty 2018; 33: 537-43. doi: 10.1016/j.arth.2017.09.016.
- Quinlan N D, Wu Y, Chiaramonti A M, Guess S, Barfield W R, Yao H, et al. Functional flexion instability after rotating-platform total knee arthroplasty. J Bone Joint Surg Am 2020; 102: 1694-1702. doi: 10.2106/jbjs.19.01403.
- Bistolfi A, Giustra F, Bosco F, Faccenda C, Viotto M, Sabatini L, et al. Comparable results between crosslinked polyethylene and conventional ultrahigh molecular weight polyethylene implanted in total knee arthroplasty: systematic review and meta-analysis of randomised clinical trials. Knee Surg Sports Traumatol Arthrosc 2022; in press. doi: 10.1007/s00167-022-06879-7.
- Goodman S B, Gallo J. Periprosthetic osteolysis: mechanisms, prevention and treatment. J Clin Med 2019; 8. doi: 10.3390/jcm8122091.
- Gøthesen O, Espehaug B, Havelin L, Petursson G, Lygre S, Ellison P, et al. Survival rates and causes of revision in cemented primary total knee replacement: a report from the Norwegian Arthroplasty Register 1994-2009. Bone Joint J 2013; 95-b: 636-42. doi: 10.1302/0301620x.95b5.30271.
- McConaghy K, Derr T, Molloy R M, Klika A K, Kurtz S, Piuzzi N S. Patellar management during total knee arthroplasty: a review. EFORT Open Rev 2021; 6: 861-71. doi: 10.1302/2058-5241.6.200156.
- Parsons T, Al-Jabri T, Clement N D, Maffulli N, Kader D F. Patella resurfacing during total knee arthroplasty is cost-effective and has lower re-operation rates compared to non-resurfacing. J Orthop Surg Res 2021; 16: 185. doi: 10.1186/s13018-021-02295-8.
- Wyatt M C, Frampton C, Horne J G, Devane P. Mobile- versus fixedbearing modern total knee replacements—which is the more patellafriendly design?: The 11-year New Zealand Joint Registry study. Bone Joint Res 2013; 2: 129-31. doi: 10.1302/2046-3758.27.2000159.
- Moon Y W, Kim H J, Ahn HS , Park C D, Lee D H. Comparison of soft tissue balancing, femoral component rotation, and joint line change between the gap balancing and measured resection techniques in primary total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016; 95: e5006. doi: 10.1097/md.0000000000005006.
- Massin P, Achour S. Wear products of total hip arthroplasty: the case of polyethylene. Morphologie 2017; 101: 1-8. doi: 10.1016/j.morpho.2016.06.001.
- Deeks J, Higgins J, Altman D. Analysing data and undertaking metaanalyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V, editors. Cochrane handbook for systematic reviews of interventions version 62. Updated February 2021. ed: Cochrane; 2021.
Supplementary data
| Patient factors | Prosthesis factors | ||||||
| Sex F vs. M | Age< 65 vs. ≥ 65 | Constraint PS vs. MS | Cement no vs. yes | Bearing mobility Mobile vs. fixed | Poly type UHMWPE:XLPE | Patellar component used no vs. yes | |
| Arthrofibrosis | |||||||
| SKAR | 0.7 (0.5—1.1) | 2.7 (1.8—4.1) a | 2.3 (1.2—4.4) a | 1.5 (0.6—3.7) | 5.7 (2.1—16) a | 0.6 (0.4—1.0) | 0.3 (0.2—0.7) b |
| AOANJRR | 0.7 (0.6—0.8) b | 1.5 (1.3—1.7) a | 1.2 (1.1—1.4) a | 1.5 (1.3—1.8) a | 1.3 (1.1—1.5) a | 1.5 (1.3—1.7) a | 1.6 (1.0—2.3) |
| KPJRR | 0.9 (0.8—1.1) | 2.1 1.8—2.5) a | 0.8 (0.6—0.9) b | 1.3 (0.7—2.4) | 1.6 (1.2—2.0) a | 1.2 (1.0—1.5) | 0.9 (0.4—1.6) |
| Fracture | |||||||
| SKAR | 5.1 (2.9—9.2) a | 0.3 (0.2—0.6) b | 2.7 (1.6—4.7) a | 0.5 (0.1—1.9) | 1.1 (0.2—8.0) | 1.6 (0.8—3.2) | 0.4 (0.2—0.8) b |
| AOANJRR | 2.5 (2.1—2.9) a | 0.5 (0.4—0.6) b | 1.4 (1.2—1.7) a | 1.5 (1.2—1.7) a | 1.2 (1.0—1.4) | 2.0 (1.7—2.4) a | 1.2 (1.0—1.4) |
| KPJRR | 3.3 (2.2—5.0) a | 0.3 (0.2—0.4) b | 2.7 (1.7—4.3) a | 1.1 (0.4—3.5) | 1.2 (0.7—2.0) | 2.4 (1.5—3.8) a | 0.6 (0.1—2.3) |
| Infection | |||||||
| SKAR | 0.5 (0.5—0.6) b | 0.8 (0.7—0.9) b | 1.9 (1.7—2.2) a | 1.1 (0.9—1.4) | 2.1 (1.5—3.0) a | 0.8 (0.7—0.9) b | 0.7 (0.6—0.9) b |
| AOANJRR | 0.5 (0.5—0.5) b | 0.9 (0.9—1.0) | 1.5 (1.4—1.6) a | 0.9 (0.8—0.9) b | 1.0 (1.0—1.1) | 1.0 (0.9—1.0) | 1.0 (1.0—1.1) |
| KPJRR | 0.6 (0.5—0.6) b | 0.9 (0.8—0.9) b | 1.2 (1.1—1.3) a | 0.9 (0.7—1.3) | 1.2 (1.1—1.4) a | 1.2 (1.1—1.3) a | 0.9 (0.7—1.3) |
| Instability | |||||||
| SKAR | 1.5 (1.3—1.7) a | 1.6 (1.4—1.9) a | 1.5 (1.2—1.9) a | 2.2 (1.7—2.8) a | 1.9 (1.1—3.3) a | 1.1 (0.9—1.3) | 0.9 (0.7—1.3) |
| AOANJRR | 1.2 (1.1—1.3) a | 1.3 (1.2—1.5) a | 1.2 (1.1—1.3) a | 1.5 (1.4—1.7) a | 1.2 (1.1—1.3) a | 1.7 (1.5—1.8) a | 1.1 (1.1—1.2) a |
| KPJRR | 1.1 (1.0—1.2) | 1.7 (1.5—1.9) a | 1.0 (0.9—1.2 | 1.2 (0.8—1.9) | 1.8 (1.5—2.1) a | 1.0 (0.9—1.2) | 0.9 (0.6—1.4) |
| Loosening | |||||||
| SKAR | 1.2 (1.1—1.3) a | 1.2 (1.0—1.3) | 1.7 (1.4—2.1) a | 0.4 (0.2—0.6) b | 3.9 (2.9—5.4) a | 1.8 (1.4—2.2) a | 0.4 (0.3—0.4) b |
| AOANJRR | 1.0 (1.0—1.1) | 1.2 (1.2—1.3) a | 1.5 (1.4—1.6) a | 1.7 (1.6—1.8) a | 1.8 (1.7—1.9) a | 2.8 (2.6—3.0) a | 1.3 (1.2—1.3) a |
| KPJRR | 1.1 (0.9—1.2) | 1.3 (1.1—1.4) a | 1.4 (1.3—1.6) a | 2.2 (1.7—3.0) a | 1.7 (1.4—2.0) a | 2.2 (1.9—2.6) a | 0.6 (0.4—1.0) |
| Pain | |||||||
| SKAR | 1.5 (0.9—2.4) | 3.0 (1.9—4.7) a | 0.2 (0.0—1.6) | 0.7 (0.2—2.9) | n.a. | 11 (1.5—79) a | 0.1 (0.0—0.1) b |
| AOANJRR | 1.0 (1.0—1.1) | 1.0 (0.9—1.1) | 1.2 (1.1—1.3) a | 1.5 (1.3—1.6) a | 1.4 (1.3—1.6) a | 2.2 (2.0—2.5) a | 3.3 (3.0—36) a |
| KPJRR | 1.1 (0.8—1.6) | 1.6 (1.1—2.2) a | 0.9 (0.7—1.3) | 5.2 (2.9—9.3) a | 2.1 (1.3—3.3) a | 1.5 (1.0—2.3) | 3.0 (1.5—5.9) a |
| Patellar reasons | |||||||
| SKAR | 1.2 (1.1—1.4) a | 1.1 (1.0—1.3) | 1.1 (0.8—1.3) | 1.3 (1.0—1.7) | 3.0 (2.1—4.3) a | 1.3 (1.1—1.5) a | 3.5 (2.1—5.9) a |
| AOANJRR | 1.1 (1.0—1.1) | 1.0 (0.9—1.0) | 1.0 (1.0—1.1) | 2.0 (1.8—2.1) a | 1.5 (1.4—1.6) a | 2.6 (2.4—2.8) a | 63 (52—78) a |
| KPJRR | 1.0 (0.7—1.5) | 1.2 (0.8—1.7) | 0.4 ((0.3—0.5) b | 3.0 (1.3—6.7) a | 2.0 (1.2—3.2) a | 1.3 (0.8—1.9) | 11 (6.9—17) a |
| Wear | |||||||
| SKAR | 0.6 (0.3—1.3) | 2.0 (1.0—3.9) | n.a. | 5.6 (2.3—14) a | n.a. | 0.7 (0.3—1.6) | 0.1 (0.1—0.3) b |
| AOANJRR | 1.0 (0.9—1.1) | 1.0 (0.9—1.1) | 0.6 (0.5—0.7) b | 4.8 (4.2—5.5) a | 3.0 (2.7—3.4) a | 5.3 (4.4—6.3) a | 1.6 (1.4—1.8) a |
| KPJRR | 0.9 (0.7—1.1) | 1.1 0.8—1.4) | 0.7 (0.6—1.0) | 5.5 (3.3—9.0) a | 1.6 (1.1—2.5) a | 1.0 (0.7—1.4) | 0.7 (0.2—2.2) |
| a Significant > 1 b Significant < 1 n.a. = not applicable (numbers too small to calculate). |
|||||||