Temporal trends in revision rate due to knee periprosthetic joint infection: a study of 115,120 cases from the Danish Knee Arthroplasty Register
Marie ANNEBERG 1, Anders TROELSEN 2, Per GUNDTOFT 3, Henrik T SØRENSEN 1, and Alma B PEDERSEN 1,4
1 Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus University; 2 Department of Orthopedic Surgery, Hvidovre Hospital; 3 Department of Orthopedic Surgery, Aarhus University Hospital; 4 Department of Clinical Medicine, Aarhus University Hospital, Denmark
Background and purpose — We aimed to examine the temporal trends in periprosthetic joint infection (PJI) revision incidence after knee arthroplasty (KA) from 1997 through 2019.
Patients and methods — 115,120 primary KA cases from the Danish Knee Arthroplasty Register were followed until the first PJI revision. We computed cumulative incidences and adjusted hazard ratios (aHRs) of PJI revision by calendar periods and several patient- and surgical-related risk factors. Results were analyzed from 0–3 months and from 3–12 months after KA.
Results — The overall 1-year PJI revision incidence was 0.7%, increasing from 0.5% to 0.7% (1997 through 2019). The incidence of PJI revision within 3 months increased from 0.1% to 0.5% (1997 through 2019). The adjusted hazard ratio (aHR) within 3 months of primary KA was 5.1 comparing 2017–2019 with 2001–2004. The PJI revision incidence from 3–12 months of KA decreased from 0.4% to 0.2%, with an aHR of 0.5 for 2017–2019 vs. 2001–2004. Male sex, age 75–84 (vs. 65–74), and extreme obesity (vs. normal weight) were positively associated with the risk of PJI revision within 3 months, whereas only male sex was associated from 3–12 months. Partial knee arthroplasty (PKA) vs. total KA was associated with a lower risk of PJI revision both within 3 months and 3–12 months of KA.
Conclusion — We observed an increase in PJI revision within 3 months of KA, and a decrease in PJI revision incidence from 3–12 months from 1997 through 2019. The reasons for this observed time-trend are thought to be multifactorial. PKA was associated with a lower risk of PJI revision.
Citation: Acta Orthopaedica 2023; 94: 616–624. DOI https://doi.org/10.2340/17453674.2023.33294.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-05-02. Accepted: 2023-11-16. Published: 2023-12-27.
Correspondence: mab@clin.au.dk
MA analyzed the data and drafted the work. MA and ABP revised the work. All authors participated in the design, interpretation, and final approval of the study.
Handling co-editors: Marianne Westberga and Philippe Wagner
Acta thanks Jan-Erik Gjertsen nd Anna Stefánsdóttir for help with peer review of this manuscript.
Periprosthetic joint infection (PJI) after knee arthroplasty (KA) is a serious complication associated with increased mortality and morbidity, including persistent pain, disability, and impaired quality of life [1,2]. In addition, the economic burden of PJI treatment is substantial [3], making it a major patient and healthcare challenge.
PJI can occur early after KA and is thought to be acquired during the prosthesis implantation, or later as hematogenous seeding [4]. There is an inconsistency in results from studies analyzing the time trend in the incidence of PJI after KA.
Several risk factors [5] associated with PJI have been described, including age, sex, type of prosthesis, BMI, and comorbidity. Existing evidence [5,6] is limited by substantial inter-study heterogeneity, inadequate adjustment for confounders, and older publication date, leaving uncertain the importance of these risk factors.
There is a need for large cohort studies with adequate power to provide evidence concerning the nature and magnitude of associations between time, potential risk factors, and PJI, and to separate the analyses of the time-specific effects of factors associated with an early onset of PJI that are likely to be acquired during the primary KA versus factors associated with a later onset of PJI that are more likely to result from hematogenous spread.
We therefore aimed to examine the time trends in PJI revision incidence after primary KA within the first year after KA surgery and associated risk factors, and their change in prevalence over time, between 1997 and 2019 in Denmark.
Patients and methods
Design
We conducted a nationwide population-based cohort study using data from the Danish Civil Registration System (DCRS), the Danish Knee Arthroplasty Register (DKR), and the Danish National Patient Registry (DNPR) [7,8]. Denmark (5.3 million residents in 1997; 5.8 million in 2019) has tax-supported universal free healthcare with a long tradition of using databases, linkable through an individual’s 10-digit Civil Personal Registration (CPR) number, assigned at birth or on immigration.
The DKR is a nationwide mandatory clinical quality database on primary KA and revisions performed in public and private hospitals in Denmark since 1997. Data is registered by the surgeon immediately after surgery. In 2019, 40 orthopedic departments (23 public and 17 private) reported to the DKR. The PJI variable in the DKR was until ultimo 2012 only “revision due to deep infection,” and thereafter it was split into “deep infection, verified by microbiology” and “deep infection suspected.” The DKR aims to reach a registration completeness of Danish KAs of more than 90% [8]. The completeness of both primary KAs and revisions is evaluated in the annual report against procedure codes from the DNPR [9].
The DNPR holds data on all inpatient admissions to Danish hospitals since 1977, and since 1995 for all outpatient clinics and emergency room visits. Discharge diagnosis codes of each hospital contact are registered as the main reason, and, when relevant, secondary diagnoses related to the contact, e.g., underlying comorbidities [7].
Cohort
From the DKR, we included patients undergoing primary KA due to osteoarthritis (OA) between January 1, 1997, and December 31, 2019. We excluded patients treated with KA for reasons other than OA and if they were revised or died on the day of primary KA. The day of primary KA surgery was considered the index date. If a patient had KA surgery on both knees, these were included as 2 separate cases. The analyses of PJI revisions from 3–12 months were based on the patients still alive and revision free at 90 days.
Outcome
Our outcome was the chronologically first same-side revision within the first 3 months or from 3–12 months after the index date, registered in the DKR due to PJI; “suspected” or “verified by microbiology.” We included both causes, as PJI remains a clinical diagnosis [10]. Aseptic causes of revision in the DKR were aseptic loosening, instability, pain, other, and unknown. Infections treated non-surgically are not registered in the DKR and were not considered. If revision surgery required several stages, every stage was registered separately in the DKR, and the chronologically first revision was considered the outcome date.
Covariates
From the DCRS we assessed median age (years with interquartile range [IQR]), age groups (< 54, 55–64, 65–74, 75–84, > 85 years), and sex.
From the DKR we assessed the year of primary KA, the type of KA: total KA ( TKA, including revision implants used for primary KA; revision implants are hinges, rotating hinges, and implants that are marketed especially for revision cases or difficult primaries, as well as modular TKA using longer stem extensions than typical for a standard implant), partial KA (PKA, including medial and lateral unicompartmental KA and patellofemoral KA), and unknown/other including e.g., hemicap, UniSpacer, or inlays), weight (kg with IQR), and BMI as classified by the World Health Organization (WHO) (normal weight < 25 [under- and normal weight], overweight 25–29.9, obese 30–34.9 [class I], and extremely obese > 35, [class II and III]). The height variable was added to the DKR in 2011 and hence the BMI is only reported thereafter.
From the DNPR we assessed comorbidity history 10 years before the index date measured by the Charlson Comorbidity Index (CCI), including diagnosis codes for both primary and secondary, as well as in-hospital and outpatient clinic visits. We defined 3 levels of comorbidity: low (CCI points = 0), medium (CCI points 1–2), and high (CCI points ≥ 3).
Statistics
Distributions of baseline characteristics at the index date were tabulated as numbers and percentages of cases overall and by calendar periods of primary KA surgery (1997–2000, 2001–2004, 2005–2008, 2009–2012, 2013–2016, and 2017–2019). Each patient was followed from the index date to the date of the first PJI revision, aseptic revision, death, emigration, or until 12 months after index surgery, whichever came first. All cases had a minimum of 1 year of follow-up, and PJI revision incidences were computed for 1 year, within 3 months, and 3–12 months after the index date, by calendar periods.
The net risk of PJI revision was calculated with the 1-minus-survival Kaplan–Meier (KM) method, censoring at aseptic revision and death. This provides a “surgeon’s perspective,” useful for administering resources for PJI revisions. To provide a clinical or “patient’s perspective,” we calculated the risk of PJI revision using the Aalen Johansen (AJ) method, considering aseptic revisions and death as competing risks. This is relatable for the patient, as it provides the risk of being revised due to PJI, taking aseptic revisions and mortality into account [11].
To analyze the time trend, we used Cox regression to estimate cause-specific hazard rate (HR) ratios for PJI revision with a 95% confidence interval (CI) comparing the HR of PJI revision in different calendar periods with the second period, 2001–2004. Assumptions of proportional hazards were tested through log–log plots and were not met if we included the first calendar period, 1997–2000, as the HR of PJI revision in this first period differed from the subsequent periods, perhaps due to incomplete registrations in the first years of the DKR. Therefore, we used the second calendar period, 2001–2004, as a reference. The time trend analysis was adjusted for confounders of age group, sex, type of prosthesis, CCI group, and weight (as a continuous variable) based on previous evidence on risk factors.
To perform an etiological analysis of each risk factor and the association with PJI revision, we estimated the HR by age group, sex, type of prosthesis, BMI group, and CCI group. To avoid the Table 2 fallacy, we analyzed each risk factor separately by repeating a Cox regression for each risk factor. Separate DAG models were drawn to decide on confounders (Figure 1, see Appendix).
The content of this paper follows the STROBE guidelines [12]. Analyses were performed using SAS V.9.4 (SAS Institute, Cary, NC, USA) and R V.3.6.1 (R Foundation for Clinical Computing, Vienna, Austria).
Ethics, registration, data sharing plan, funding, and disclosures
The study was approved by the Danish Data Protection Agency (Record number AU-2016–051–000001, sequential number 880), and was funded by the Health Research Fund of Central Region Denmark and the Research Fund of the Institute of Clinical Medicine, Aarhus University, Denmark. The funders played no role in the investigation. Data was obtained specifically for this project, based on permissions required by the relevant Danish data authorities, which do not allow sharing of data with third parties. The authors declared no conflicts of interest. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2023.33294
Results
Cohort
We included 115,120 cases, who had undergone primary KA due to OA between 1997 and 2019. 26 cases were excluded due to revision or death on the day of primary KA (Figure 2). 114,029 cases were still alive and revision-free after 90 days and were included in the analysis of PJI revision from 3–12 months following KA. Patient characteristics according to calendar periods are presented in Table 1. The median age was stable throughout the study period. The largest age group was 65–74 years of age, accounting for 40% over the study period. The proportion of male patients increased from 32% in 1997–2000 to 42% in 2018–2019. In 1997–2000 83% had a TKA decreasing to 73% in 2018–2019, as the proportion of cases having a PKA increased from 2% in 1997–2000 to 19% in 2018–2019.
| Factor | 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | 2017–2019 | 1997–2019 |
| Total, n | 5,633 | 11,478 | 20,362 | 27,510 | 26,881 | 23,256 | 115,120 |
| Age, median (IQR) | 72 (64–77) | 70 (62–77) | 69 (62–75) | 68 (62–75) | 69 (62–75) | 70 (62–75) | 69 (62–75) |
| Age group | |||||||
| < 55 | 273 (4.9) | 592 (5.2) | 1,279 (6.4) | 2,082 (7.7) | 1,906 (7.2) | 1,472 (6.4) | 7,604 (6.7) |
| 55–64 | 1,187 (21) | 2,870 (25) | 5,567 (28) | 6,942 (26) | 6,220 (24) | 5,488 (24) | 28,274 (25) |
| 65–74 | 2,077 (37) | 4,038 (36) | 7,541 (38) | 10,959 (40) | 11,225 (42) | 9,386 (41) | 45,226 (40) |
| 75–84 | 1,841 (33) | 3,429 (30) | 4,942 (25) | 6,214 (23) | 6,379 (24) | 5,951 (26) | 28,756 (25) |
| ≥ 85 | 206 (3.7) | 383 (3.4) | 738 (3.7) | 911 (3.4) | 775 (2.9) | 617 (2.7) | 3,630 (3.2) |
| Sex | |||||||
| Male | 1,756 (31) | 3,939 (34) | 7,458 (37) | 10,726 (39) | 10,739 (40) | 9,603 (41) | 44,221 (38) |
| Female | 3,877 (69) | 7,539 (66) | 12,904 (63) | 16,784 (61) | 16,142 (60) | 13,653 (59) | 70,899 (62) |
| Type of prosthesisa | |||||||
| TKA | 4,695 (83) | 9,837 (86) | 1,6870 (83) | 23,755 (86) | 22,766 (85) | 17,876 (77) | 95,799 (83) |
| PKA | 114 (2.0) | 571 (5.0) | 1,548 (7.6) | 2,473 (9.0) | 3,483 (13) | 4,507 (19) | 12,696 (11) |
| Other/unknown | 824 (15) | 1,070 (9.3) | 1,944 (9.5) | 1,282 (4.7) | 632 (2.4) | 873 (3.8) | 6,625 (5.8) |
| CCI group | |||||||
| Low (0) | 5,439 (97) | 10,992 (96) | 19,460 (96) | 26,402 (96) | 25,802 (96) | 22,560 (97) | 110,655 (96) |
| Medium (1–2) | 173 (3.1) | 421 (3.7) | 779 (3.8) | 938 (3.4) | 915 (3.4) | 573 (2.5) | 3799 (3.3) |
| High (≥ 3) | 21 (0.4) | 65 (0.6) | 123 (0.6) | 170 (0.6) | 164 (0.6) | 123 (0.5) | 666 (0.6) |
| Weight (kg), median (IQR) b | 80 (70–90) | 80 (70–90) | 81 (72–93) | 82 (73–95) | 84 (74–96) | 85 (75–97) | 83 (72–95) |
| BMI | |||||||
| Normal weight (< 25) | – | – | – | 22,41 (22) | 5,202 (20) | 4,317 (19) | 11,765 (20) |
| Overweight (25–30) | – | – | – | 4,167 (41) | 10,548 (40) | 8,978 (39) | 23,701 (40) |
| Obese (30–35) | – | – | – | 2,562 (25) | 6,757 (26) | 6,219 (27) | 15,541 (26) |
| Extremely obese (> 35) | – | – | – | 1,324 (13) | 3,907 (15) | 3,599 (16) | 8831 (15) |
| Percentage with BMI information c | – | – | – | 57 | 98 | 99 | 52 |
| BMI, body mass index; CCI, Charlson Comorbidity Index; IQR, interquartile range. | |||||||
| a Type of prosthesis: PKA = partial knee arthroplasty (i.e., unicompartmental knee replacement, medial, lateral, or patellofemoral); TKA = total knee arthroplasty with standard or revision implants used for primary KA. Revision implants are hinges, rotating hinges, implants that are marketed especially for revision cases or difficult primaries as well as modular TKA using longer stem extensions than typical for a standard implant.) “Other/unknown” includes other partial knee replacement (n = 51) (e.g., hemicap, UniSpacer or inlays), and unknown (n = 6,574). | |||||||
| b 98% of patients had a registered weight. | |||||||
| c The height variable was added to the DKR in 2011. | |||||||
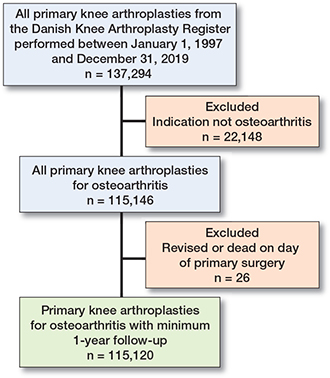
Figure 2. Flowchart for inclusion in and exclusion from the study population.
Of the cases operated on in 1997–200, 97% had a low CCI, 3% a medium CCI, and 0.4% a high CCI. This pattern was the same throughout the study period.
The median weight was 80 kg (IQR 70–90) in 1997–2000 and 85 kg (75–97) in 2017–2019. From 2011 through 2019 an increasing proportion of cases were obese (25% to 27%) or extremely obese (13% to 16%).
Time trend in the incidence of PJI revision
The overall 1-year incidence of PJI revision was 0.7 (CI 0.6–0.7) (Figure 3, Table 2, see Appendix).
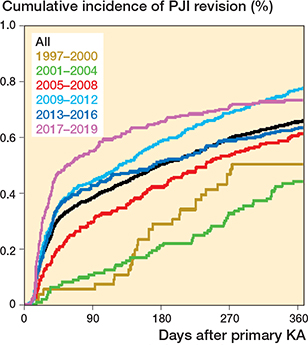
Figure 3. Cumulative incidence of PJI revision (1-minus-survival Kaplan–Meier plot) for all patients and by year of primary surgery.
The KM 1-year incidence of PJI revision increased from 0.5 (0.3–0.7) in 1997–2000 to 0.7 (0.6–0.8) in 2017–2019, driven by an increase from 0.1 (0.0–0.2) to 0.5 (0.5–0.6) in PJI revision within 3 months. We observed a decrease in the incidence of PJI revisions from 3–12 months throughout the study period from 0.4 (0.3–0.7) to 0.2 (0.1–0.2) (Figure 3, Table 2, see Appendix). We found nearly identical results, using the AJ model, when considering aseptic revisions and death as competing risks (Table 2, see Appendix).
The aHR for 1-year PJI revision was 1.6 (1.2–2.2) in 2018–2019 vs. 2001–2004 (Table 3). The aHR for sustaining a PJI revision within 3 months was 5.1 (2.8–9.5) in 2018–2019 vs. 2001–2004; and for PJI revision within 3–12 months of KA this was 0.5 (0.3–0.8) in 2018–2019 vs. 2001–2004 (Table 3).
| At risk at index date | PJI | Cumulative incidence (CI) | aHR (CI) | aHR (CI) | |
| PJI revision within 3 months | |||||
| Year of surgery |  |
||||
| 1997–2000 | 5,597 | – a | 0.1 (0.0–0.2) | – | |
| 2001–2004 | 11,402 | 12 | 0.1 (0–0.2) | Ref | |
| 2005–2008 | 20,171 | 60 | 0.3 (0.2–0.4) | 2.8 (1.4–5.3) | |
| 2009–2012 | 27,221 | 122 | 0.4 (0.4–0.5) | 4.3 (2.3–8.0) | |
| 2013–2016 | 26,613 | 114 | 0.4 (0.3–0.5) | 3.9 (2.1–7.3) | |
| 2017–2019 | 23,028 | 127 | 0.5 (0.5–0.6) | 5.1 (2.8–9.5) | |
| Age group | |||||
| < 54 | 7,552 | 29 | 0.4 (0.2–0.5) | 1.0 (0.7–1.5) | |
| 55–64 | 28,094 | 89 | 0.3 (0.2–0.4) | 0.9 (0.7–1.1) | |
| 65–74 | 44,854 | 168 | 0.4 (0.3–0.4) | Ref | |
| 75–84 | 28,360 | 135 | 0.5 (0.4–0.6) | 1.4 (1.1–1.7) | |
| ≥ 85 | 3,549 | 14 | 0.4 (0.2–0.6) | 1.2 (0.7–2) | |
| Sex | |||||
| Female | 70,341 | 205 | 0.5 (0.5–0.6) | Ref | |
| Male | 43,691 | 233 | 0.3 (0.3–0.3) | 1.8 (1.5–2.1) | |
| Prosthesis | |||||
| TKA | 94,885 | 390 | 0.4 (0.4–0.4) | Ref | |
| PKA | 12,567 | 36 | 0.3 (0.2–0.4) | 0.6 (0.4–0.9) | |
| Unknown/other | 6,580 | 12 | 0.2 (0.1–0.3) | 0.6 (0.3–1.0) | |
| BMI group | |||||
| Normal weight | 11,308 | 56 | 0.5 (0.4–0.6) | Ref | |
| Overweight | 23,498 | 112 | 0.5 (0.4–0.6) | 0.8 (0.6–1.1) | |
| Obese | 15,537 | 59 | 0.4 (0.3–0.5) | 0.9 (0.7–1.2) | |
| Extremely obese | 8,738 | 60 | 0.7 (0.5–0.9) | 1.7 (1.1–2.4) | |
| CCI group | |||||
| Low | 109,623 | 412 | 0.4 (0.3–0.4) | Ref | |
| Medium | 3,751 | 22 | 0.6 (0.8–0.8) | 1.5 (1–2.4) | |
| High | 658 | – a | 0.6 (0–1.2) | 1.5 (0.5–3.9) | |
| PJI revision 3–12 months | |||||
| Year of surgery |  |
||||
| 1997–2000 | 5,471 | 25 | 0.4 (0.3–0.6) | – | |
| 2001–2004 | 11,172 | 38 | 0.3 (0.2–0.4) | Ref | |
| 2005–2008 | 19,740 | 63 | 0.3 (0.2–0.4) | 0.8 (0.6–1.3) | |
| 2009–2012 | 26,628 | 89 | 0.3 (0.3–0.4) | 0.9 (0.6–1.4) | |
| 2013–2016 | 26,163 | 55 | 0.2 (0.2–0.3) | 0.6 (0.4–0.9) | |
| 2017–2019 | 22,687 | 42 | 0.2 (0.1–0.2) | 0.5 (0.3–0.8) | |
| Age group | |||||
| < 54 | 7,369 | 18 | 0.2 (0.1–0.4) | 0.9 (0.6–1.5) | |
| 55–64 | 27,623 | 82 | 0.3 (0.2–0.4) | 1.1 (0.8–1.4) | |
| 65–74 | 44,101 | 119 | 0.3 (0.2–0.3) | Ref | |
| 75–84 | 27,768 | 78 | 0.3 (0.2–0.3) | 1.1 (0.8–1.4) | |
| ≥ 85 | 3,410 | 11 | 0.3 (0.1–0.5) | 1.2 (0.7–2.3) | |
| Sex | |||||
| Female | 69,167 | 141 | 0.2 (0.2–0.2) | Ref | |
| Male | 42,694 | 171 | 0.4 (0.3–0.5) | 2 (1.6–2.5) | |
| Prosthesis | |||||
| TKA | 93,201 | 280 | 0.3 (0.3–0.3) | Ref | |
| PKA | 12,259 | 13 | 0.1 (0–0.2) | 0.4 (0.2–0.6) | |
| Unknown/other | 6,401 | 19 | 0.3 (0.2–0.4) | 0.9 (0.6–1.4) | |
| BMI group | |||||
| Normal weight | 11,102 | 20 | 0.2 (0.1–0.3) | Ref | |
| Overweight | 23,095 | 52 | 0.2 (0.2–0.3) | 1.3 (0.7–2.3) | |
| Obese | 15,284 | 37 | 0.2 (0.2–0.3) | 1.2 (0.7–2.1) | |
| Extremely obese | 8,594 | 24 | 0.3 (0.2–0.4) | 1.7 (0.9–3.2) | |
| CCI group | |||||
| Low | 107,551 | 298 | 0.3 (0.2–0.3) | Ref | |
| Medium | 3,680 | 10 | 0.3 (0.1–0.4) | 1 (0.5–1.8) | |
| High | 630 | – a | 0.6 (0–1.2) | 2.2 (0.8–6) | |
| BMI: body mass index; CCI: Charlson Comorbidity Index; CI: confidence interval; KA: knee arthroplasty; PJI: periprosthetic joint infection; PKA: partial knee arthroplasty; TKA: total knee arthroplasty. | |||||
| Adjustments: Year of surgery: age group, sex, type of prosthesis, CCI group, and weight (as a continuous variable); Age group: sex and year of surgery; Sex: age group, year of surgery; Type of prosthesis: age group, sex, CCI group, year of surgery; BMI group: age group, sex, year of surgery; CCI group: age group, sex, year of surgery. | |||||
| a Numbers too few to be reported. | |||||
Risk factors for PJI revision
Factors associated with an increased risk of PJI revision within 3 months were age 75–84 years (vs age 65–74) aHR: 1.4 (1.1–1.7), male sex (vs female) aHR: 1.8 (1.5–2.1); and extreme obesity (vs normal weight) aHR: 1.7 (1.1–2.4). Only male sex (vs female) remained a risk factor for PJI revision from 3–12 months of KA, aHR: 2 (1.6–2.5). There was a trend towards high CCI (vs low CCI) being associated with a higher risk of PJI revision, both within 3 months and 3–12 months of KA. PKA (vs TKA) was associated with a lower risk of PJI revision, both within 3 months and 3–12 months of KA (aHR within 3 months: 0.6, CI 0.4–0.8; 3–12 months: 0.4 CI, 0.2–0.6) (Table 3). The cumulative PJI revision incidence was lower for PKA than for TKA within the first year after surgery (aHR 0.6, CI 0.4–0.9) (Figure 4).
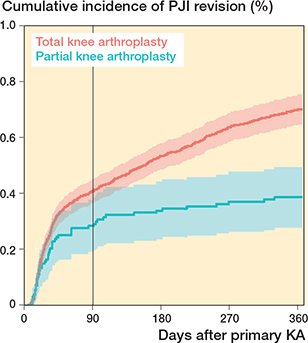
Figure 4. Cumulative incidences of PJI revision with 95% confidence interval stratified by type of prosthesis.
Discussion
We aimed to examine the temporal trends in periprosthetic joint infection (PJI) revision incidence after knee arthroplasty (KA) surgery from 1997 through 2019 in Denmark. We found an increased incidence of PJI revision within 3 months, and a decrease of PJI revisions from 3–12 months of KA, during the last 22 years. However, in general the incidence of PJI revision after KA was low.
High age, male sex, extreme obesity, and high CCI were associated with an increased risk of PJI revision, whereas PKA vs TKA was associated with a lower risk.
Comparisons with other studies
The low overall 1-year PJI revision incidence of 0.7% found in our study is consistent with the previous results [13-16], despite variations in the cohorts (e.g., only TKA patients [15], all indications and not only OA [13], and non-contemporaneous study periods.
A Swedish study [17] on PJI after TKA found a 2-year incidence rate of 1.45%, twice the 1-year incidence found in the present study. Given that the majority of PJIs manifest within the initial year following primary KA surgery, a 1.45% incidence appears high when compared with a 1-year incidence rate of 0.7%. However, it is important to note that in the Swedish study, data from the Swedish Arthroplasty Register was amalgamated with data from the Swedish Prescribed Drug Register to identify cases of PJI treated with antibiotics. This highlights a well-recognized limitation concerning the underreporting of PJI revisions in arthroplasty registers, which could potentially influence the outcomes of our own study. Although some studies report a decrease (Canada, 2002–2016, follow-up 1–15 years) [13] or no change in PJI incidence (United States, 2005–2015, follow-up 1–5 years) [15], others report an increase in PJI incidence (Australia, Sweden, United States, 2003–2017, follow-up 1 year) [14]. Only 1 study analyzed PJI revision within 3 months of KA and found a 2.5-fold increase (England and Wales, 2005–2013) [18].
The inconsistency in time trends reported from previous studies [13-15,18] could be due to methodological differences and differences in completeness and validity of the PJI revision definition used. Apart from one study, they all lacked adjustment for confounders when analyzing the time trend. This 1 study adjusted for several confounders including CCI in the Cox regression model and reported no change in 1-year risk of PJI revision from 2005 through 2015 but did not look at shorter follow-up than 1 year. A separate analysis of risk of PJI revision within 3 months of KA over calendar time was reported only in a large study from England and Wales, and consistent with our results, they found an increased rate of PJI revision within 3 months (2013 vs. 2005, crude rate ratio 2.5, CI 1.2–5.3). However, no adjusted rate ratio estimates were presented.
Interpretation of time trend results
The reasons for the observed historical increase in the risk of PJI revision within 3 months after KA are thought to be multifactorial and likely include a combined effect of a change in (1) the patient characteristics, (2) the diagnosis and treatment patterns, and (3) the causing pathogens. The establishment of expert consensus guidelines for a PJI diagnosis in 2013, and in revised form in 2018 [10,19], may have improved diagnostic confidence and awareness of PJI. Hypothetically this could have led to a more aggressive surgical approach for patients suspected of or diagnosed with PJI, shifting time of PJI revision towards an earlier time frame. This is supported by Figure 3. A Swedish study comparing treatment of PJI in 2 time periods also reported a reduced tendency to treat PJI without surgery [17]. Other shifts in treatment praxis during the study period in Denmark have included a reduction in length of stay [20] and an observed change in the use of antibiotics [21]. No studies have explored these changes in treatment among Danish KA patients. An Australian study [22] investigating PJI following total hip arthroplasty reported elevated antibiotic utilization both preceding and succeeding the initial hip surgery among patients who underwent subsequent PJI revision, as opposed to patients who did not undergo revision or who underwent revision for aseptic causes. This observation suggests a potential association between perioperative antibiotic usage and the risk of PJI revision, although it is important to acknowledge the possibility that bias by indication influenced these findings.
Throughout the study period the patient demographic has changed, with some factors contributing to an increase, others to a decrease in the risk of PJI revision over time.
Changes towards a higher proportion of male and more obese patients have contributed to an increase in the risk of PJI revision during the study period, whereas an increasing percentage of PKA associated with a lower risk of PJI revision has reduced this effect. The comorbidity burden was stable throughout the period. To evaluate the overall effect of the changes in patient demographic on the risk of PJI revision within 3 months, we computed both crude and adjusted HR (Table 4, see Appendix). Similar results led to the conclusion that changes in patient demographics were not the main reason for the increase in the risk of PJI revision within 3 months.
The leading pathogens causing PJI revision within 3 months after KA are Staphylococcus aureus and coagulase-negative staphylococci. Their reduced susceptibility to antimicrobials has been observed, especially for coagulase-negative staphylococci. This could decrease the effectiveness of perioperative antibiotics and potentially contribute to the increase in PJI revision within 3 months [23].
Interpretation of risk factors
In line with our findings, the largest study on risk factors of PJI after KA from the UK [5] identified male sex, comorbidity, and obesity as associated with a higher relative risk of PJI revision, and PKA vs. TKA as associated with a lower relative risk of PJI revision, the latter similar to findings from Sweden [16].
Compared with females, the higher relative risk of PJI revision in males may be due to greater comorbidities and poorer health behaviors [24], which have been shown to increase the risk of PJI revision [5]. Obesity is associated with prolonged operative time, the presence of other medical comorbidities, and an increased risk of wound complications, all factors associated with an increased risk of PJI revision after KA [5].
The negative association between PKA and the risk of PJI revision may be attributed to the smaller implants and incisions used in PKA, and maybe healthier patients compared with TKA. To be considered for a PKA in Denmark, patients must meet specific radiographic criteria for unicompartmental disease. In a UK study [25] it was shown that candidacy for PKA was met by 48% of knees in a series of 200 knees planned for TKA. In our study, 19% received a PKA from 2017 through 2019. Considering the potential differences in PKA and TKA patient phenotypes, further studies are needed to investigate whether confounding by indication has biased the risk results.
Strengths
This study is strengthened by a nationwide population-based study design with large sample size and complete follow-up, reducing potential selection bias.
Limitations
This register study has some limitations [26]. Our outcome “reported PJI revision” includes only deep PJIs that require surgery. Superficial PJIs treated without surgery are not included in our study. There is international consensus that PJI almost always necessitates surgical intervention [27]. Compared with the DNPR, the completeness of the DKR was high throughout the study period (for primary KA: 77% (1997), 92% (2009), and 96% (2019); for revisions: 57% (1997), 89% (2009), and 92% (2019) [8]. The higher completeness in the most recent years would have affected the observed trend if registered TKA cases differed from non-registered cases. Unfortunately, data on completeness of cause-specific revisions is not available.
The study results rely solely on arthroplasty register data to identify PJI revision. However, previous research has revealed that arthroplasty registers underreport PJI revision rates in several settings: by 13% in Finland [28] (knee); by 33% and 44% in Sweden [17,29] (hip and knee respectively); by 37% in New Zealand [30] (hip and knee), and by 40% in Denmark [31] (hip). There are no grounds for considering that the completeness of PJI revision detection within the DKR is higher than in other registers. This should be considered when evaluating our absolute risk estimates.
There is also a risk of misclassification as no microbiological sample results are available at the time of reporting, leading to underestimation of PJI incidences.
Despite adjustment for CCI, there remains the risk of residual confounding because CCI lacks information on the severity of in-hospital comorbidities, and comorbidity treated by general practitioners.
We also cannot eliminate the possibility of unknown confounding and of unmeasured confounding such as smoking or socioeconomic factors, which have been shown to impact the risk of revision after total hip arthroplasty [32].
BMI was available only after 2011 and therefore we used weight as a continuous variable in the adjusted Cox regression models, as this was available for 98% of patients in the cohort. Using the weight variable as a surrogate for BMI could lead to the risk of residual confounding regarding the association between BMI and CCI. The Cox regression analysis stratified by BMI group is limited to patients treated from 2011 and onwards. This introduces the risk of type 2 error in this sub-analysis.
Conclusion
We observed an increase in PJI revision within 3 months and a decrease in PJI revisions from 3–12 months following KA from 1997 through 2019 in patients with OA treated with primary KA.
In perspective, this knowledge can guide patient selection to identify high-risk cases to improve pre- and postoperative rehabilitation and also guide further research into the causes associated with PJI after KA, preferably including access to microbiological data, aiming to limit any increase in this serious complication.
- Lum Z C, Natsuhara K M, Shelton T J, Giordani M, Pereira G C, et al. Mortality during total knee periprosthetic joint infection. J Arthroplasty 2018; 33. doi: 10.1016/j.arth.2018.08.021.
- Boddapati V, Fu M C, Mayman DJ, Su EP, Sculco P K, McLawhorn A S. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty 2018; 33: 521-6. doi: 10.1016/j.arth.2017.09.021.
- Haenle M, Skripitz C, Mittelmeier W, Skripitz R. Economic impact of infected total knee arthroplasty. Scientific World J 2012; 2012. doi: 10.1100/2012/196515.
- Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS 2017; 125: 353-64. doi: 10.1111/apm.12687.
- Lenguerrand E, Whitehouse M R, Beswick A D, Kunutsor SK, Foguet P, Porter M, et al. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis 2019; 19: 589-600. doi: 10.1016/s1473-3099(18)30755-2.
- Kunutsor S K, Whitehouse M R, Blom A W, Beswick A D. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLOS ONE 2016; 11: e0150866. doi: 10.1371/journal.pone.0150866.
- Schmidt M, Schmidt S A J, Adelborg K, Sundboll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019; 11: 563-91. doi: 10.2147/CLEP.S179083.
- Pedersen A, Mehnert, Odgaard A, Schrøder H M. Existing data sources for clinical epidemiology: the Danish Knee Arthroplasty Register. Clin Epidemiol 2012; 10.2147/clep.s30050: 125. doi: 10.2147/clep.s30050.
- Danish Knee Arthroplasty Register. Annual report; 2019. https://www.sundhed.dk/sundhedsfaglig/kvalitet/kliniske-kvalitetsdatabaser/planlagt-kirugi/knaealloplastikregister/, 2019. p 149.
- Parvizi J, Tan T L, Goswami K, Higuera C, Della Valle C, Chen A F, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018; 33: 1309-14. e1302. doi: 10.1016/j.arth.2018.02.078.
- Gillam M H, Ryan P, Graves S E, Miller L N, De Steiger R N, Salter A. Competing risks survival analysis applied to data from the Australian Orthopaedic Association National Joint Replacement Registry. Acta Orthop 2010; 81: 548-55. doi: 10.3109/17453674.2010.524594.
- von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. PLOS Med 2007; 4: e296. doi: 10.1371/journal.pmed.0040296.
- McMaster Arthroplasty Collaborative (MAC). Incidence and predictors of prosthetic joint infection following primary total knee arthroplasty: a 15-year population-based cohort study. J Arthroplasty 2022; 37: 367-72 e361. doi: 10.1016/j.arth.2021.10.006.
- Lewis P L, Robertsson O, Graves S E, Paxton E W, Prentice H A, W-Dahl A. Variation and trends in reasons for knee replacement revision: a multi-registry study of revision burden. Acta Orthop 2021; 92(2): 182-8. doi: 10.1080/17453674.2020.1853340.
- Kurtz S M, Lau E C, Son M-S, Chang E T, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty 2018; 33: 3238-45. doi: 10.1016/j.arth.2018.05.042.
- Swedish Arthroplasty Register. Annual report; 2022.
- Thompson O, W-Dahl A, Lindgren V, Gordon M, Robertsson O, Stefánsdóttir A. Similar periprosthetic joint infection rates after and before a national infection control program: a study of 45,438 primary total knee arthroplasties. Acta Orthop 2022; 93: 3-10. doi: 10.1080/17453674.2021.1977532.
- Lenguerrand E, Whitehouse M R, Beswick A D, Toms A D, Porter M L, Blom A W. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland. BMJ Open 2017; 7: e014056. doi: 10.1136/bmjopen-2016-014056.
- Parvizi J, Gehrke T, Chen A F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 2013; 95-B: 1450-2. doi: 10.1302/0301-620x.95b11.33135.
- Petersen P B, Kehlet H, Jorgensen C C, Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group. Improvement in fast-track hip and knee arthroplasty: a prospective multicentre study of 36,935 procedures from 2010 to 2017. Sci Rep 2020; 10: 21233. doi: 10.1038/s41598-020-77127-6.
- Veimer Jensen M L, Aabenhus R M, Holzknecht B J, Bjerrum L, Jensen J N, Siersma V, et al. Antibiotic prescribing in Danish general practice in the elderly population from 2010 to 2017. Scand J Prim Health Care 2021; 39: 498-505. doi: 10.1080/02813432.2021.2004754.
- De Steiger R N, Pratt N L, Gulyani A, Duszynski K M, Inacio M C, Graves S E, et al. Antibiotic utilisation in primary and revision total hip replacement patients: a registry linkage cohort study of 106 253 patients using the Australian Orthopaedic Association National Joint Replacement Registry. Pharmacoepidemiol Drug Saf 2023; 32(2): 238-47. doi: 10.1002/pds.5522.
- Rosteius T, Jansen O, Fehmer T, Baecker H, Citak M, Schildhauer T A, et al. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J Med Microbiol 2018; 67: 1608-13. doi: 10.1099/jmm.0.000835.
- Pinkhasov R M, Wong J, Kashanian J, Lee M, Samadi D B, Pinkhasov M M, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract 2010; 64: 475-87. doi: 10.1111/j.1742-1241.2009.02290.x.
- Willis-Owen A C, Brust K, Alsop H, Miraldo M, Cobb P J. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee 2009; 16: 473-8. doi: 10.1016/j.knee.2009.04.006.
- Varnum C, Pedersen A B, Gundtoft P H, Overgaard S. The what, when and how of orthopaedic registers: an introduction into registerbased research. EFORT Open Rev 2019; 4: 337-43. doi: 10.1302/2058-5241.4.180097.
- Osmon D R, Berbari E F, Berendt A R, Lew D, Zimmerli W, Steckelberg J M, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56: e1-e25. doi: 10.1093/cid/cis803.
- Jämsen E, Huotari K, Huhtala H, Nevalainen J, Konttinen Y T. Low rate of infected knee replacements in a nationwide series: is it an underestimate? Acta Orthop 2009; 80: 205-12. doi: 10.3109/17453670902947432.
- Lindgren J V, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord 2014; 15: 384. doi: 10.1186/1471-2474-15-384.
- Zhu M, Ravi S, Frampton C, Luey C, Young S. New Zealand Joint Registry data underestimates the rate of prosthetic joint infection. Acta Orthop 2016; 87: 346-50. doi: 10.3109/17453674.2016.1171639.
- Gundtoft P H, Overgaard S, Schonheyder H C, Moller J K, Kjaersgaard-Andersen P, Pedersen A B. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop 2015; 86: 326-34. doi: 10.3109/17453674.2015.1011983.
- Edwards N M, Varnum C, Overgaard S, Pedersen A B. Impact of socioeconomic status on the 90- and 365-day rate of revision and mortality after primary total hip arthroplasty: a cohort study based on 103,901 patients with osteoarthritis from national databases in Denmark. Acta Orthop 2021; 92: 581-8. doi: 10.1080/17453674.2021.1935487.
Appendix
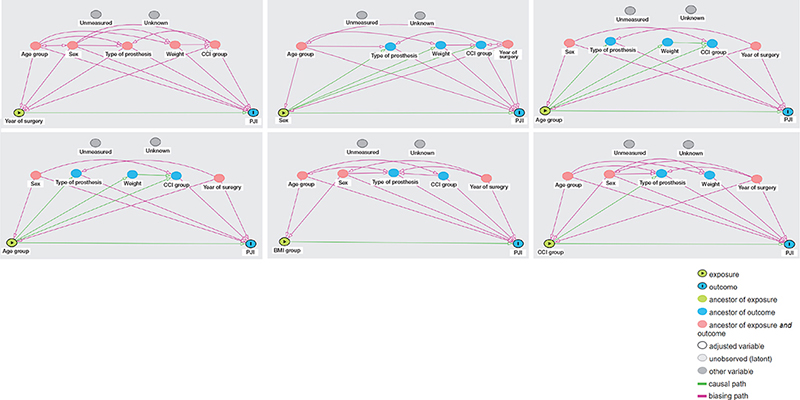
Figure 1. DAGs for adjustment on confounding in the Cox regression model.
DAGs used to decide on adjustment on confounding in the Cox regression analysis. The following variables were found to be path-specific confounders for the association between the following exposures and the risk of PJI:
Year of surgery: age group, sex, type of prosthesis, CCI group, and weight.
Age group: sex and year of surgery.
Sex: age group, year of surgery.
Type of prosthesis: age group, gender, CCI group, year of surgery.
BMI Group: age group, sex, year of surgery.
CCI group: age group, sex, year of surgery.
BMI: body mass index; CCI: Charlson Comorbidity Index; PJI: periprosthetic joint infection; PKA: partial knee arthroplasty.