Increased risk of aseptic loosening for posterior stabilized compared with posterior cruciate-retaining uncemented total knee replacements: a cohort study of 13,667 knees from the Dutch Arthroplasty Registry
Raymond PUIJK 1, Inger N SIEREVELT 1,2, Bart G C W PIJLS 3,4, Anneke SPEKENBRINK-SPOOREN 3, and Peter A NOLTE 1,5
1 Department of Orthopaedic Surgery, Spaarne Gasthuis, Hoofddorp; 2 Department of Orthopaedic Surgery, Xpert Clinics Orthopedic Amsterdam/Specialized Center of Orthopedic Research and Education, Amsterdam; 3 Landelijke Registratie Orthopedische Interventies (LROI; Dutch Arthroplasty Register), Bruistensingel 230, 5232 AD, ’s Hertogenbosch; 4 Department of Orthopaedics, Leiden University Medical Center (LUMC), Leiden; 5 Department of Oral Cell Biology, Academic Centre for Dentistry (ACTA), University of Amsterdam and Vrije Universiteit Amsterdam, Amsterdam, the Netherlands
Background and purpose — While registry studies have suggested a higher risk of revision for posterior-stabilized (PS) compared with posterior cruciate-retaining (CR) total knee replacements (TKR) using cement, it is unknown whether this is also the case for uncemented TKR. We aimed to compare the revision rates of PS and CR designs in patients receiving primary uncemented TKR.
Patients and methods — Data from the Dutch arthroplasty register (LROI) was analyzed, comprising 12,226 uncemented primary CR TKRs and 750 uncemented PS TKRs registered between 2007 and 2022. Competing risk and multivariable Cox regression analyses were used to compare revision rates, risks of revision, and reasons for revision between groups. Sensitivity analyses were performed to analyze the risk, concerning the 5 most commonly used implants and performing hospitals for each group.
Results — Uncemented PS TKRs had higher 10-year revision rates for any reason and aseptic loosening (6.5%, 95% confidence interval [CI] 4.6–9.2 and 3.9%, CI 2.6–6.7) compared with uncemented CR TKRs (4.2%, CI 3.8–4.7 and 1.4%, CI 1.2–1.7). PS TKRs were 1.4 and 2.5 times more likely to be revised for any reason and aseptic loosening, respectively. These results remained consistent after adjustment for age, sex, BMI, previous surgeries, bearing mobility, and surface modification, with sensitivity analyses.
Conclusion — We found that uncemented PS implants have a higher rate of revision than uncemented CR implants, mainly due to a higher risk of aseptic loosening.
Citation: Acta Orthopaedica 2023; 94: 600–606. DOI https://doi.org/10.2340/17453674.2023.33283.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-04-02. Accepted: 2023-11-20. Published: 2023-12-13.
Correspondence: Rpuijk@spaarnegasthuis.nl
RP and BGP conceived the study. AS created the database. RP and INS performed the analyses. INS, BGP, AS, and PAN helped with the interpretation of the results. RP wrote the manuscript.
Handling co-editors: Li Felländer-Tsai and Philippe Wagner
Acta thanks Karl Eriksson and Per-Henrik Randsborg for help with peer review of this manuscript.
In general, the 2 most commonly used total knee replacement (TKR) designs are posterior cruciate-retaining (CR) and posterior stabilized (PS) systems [1]. The use of a PS system is mainly indicated in cases of posterior cruciate ligament (PCL) insufficiency but is currently in most cases dependent on the surgeon’s preference and training. High-evidence studies have shown no clinically significant differences regarding the patient-reported outcome, pain, and function between the systems [2,3]. Yet it is hypothesized that PS systems may increase stress transmission to their interfaces with the polyethylene (PE) and bone, leading to a greater risk of wear, osteolysis, and aseptic loosening [4]. This theory was confirmed by large observational studies, finding a higher revision rate of PS compared with CR implants, but including only cemented implants [5,6]. Recent registry reports from Australia and the Netherlands have also reported a higher risk of revision for cemented implants with a PS compared with CR designs [7,8]. However, no analysis was performed restricted to uncemented implants. The National Joint Registry from the United Kingdom identified a higher revision rate for uncemented TKR with a PS design compared with a CR design, but the analysis lacked correction for confounders and information on reasons for revisions [9].
We aimed to investigate the likelihood of revision for uncemented PS implants compared with CR implants while adjusting for potential confounders and to establish the primary causes of revision. Our hypothesis was that uncemented PS implants have a higher risk of revision than uncemented CR implants, mainly due to aseptic loosening.
Patients and methods
Study design
Our study is an observational study using data from the Dutch Arthroplasty Register (Landelijke Registratie Orthopedische Interventies [LROI]). Data is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [10].
Setting
Since the inception of the LROI in 2007, data on all patients and procedures is anonymized and routinely collected unless patients opt out from the data collection. All Dutch hospitals participated in the data registration from 2012, with a current total data completeness of 97% for both primary and revision TKRs [7]. Reasons for revisions are documented through an online form immediately postoperatively. To validate outcomes, revision rates for various reasons are anonymously compared among healthcare providers, aiming to identify outliers based on performance or registration practices. Encrypted social security numbers are linked to the Dutch national insurance database twice a year, to connect primary and revision TKRs and identify deaths [7].
Participants
All patients who received an uncemented primary TKR for end-stage osteoarthritis between 2007 and 2022 were eligible for the study. Cases were excluded from the study if the fixation was not uncemented, the TKR was neither CR nor PS, or in any case where this was unknown.
Variables
The primary outcome measure was the revision rate for any reason as an endpoint. A revision was defined as the removal, exchange, or addition of 1 or more components. Reasons for revision were registered in the LROI at the time of revision surgery, without incorporation of the results of intraoperative cultures, as these most often were not yet available at the moment of registration. Revisions that include only patellar resurfacing, or debridement, antibiotics, and implant retention (DAIR), with or without insert exchange, were excluded from the endpoint. The secondary outcome measure was the revision rate for aseptic loosening, with removal or exchange of at least a femoral or tibial component (major revision), without the presence of signs of infection. Either endpoint was assessed at 5- and 10-year follow-up.
For each patient, demographic and surgical details, including age, sex, body mass index (BMI), Charnley classification (A, B1, B2, or C), American Society of Anesthesiologists grade (ASA I, II, or III–IV), smoking status, previous surgeries to the index knee (e.g., meniscectomy, osteotomy, ACL reconstruction, osteosynthesis, synovectomy, arthroscopy, and patellar realignment), surgical approach, anonymized implant design, bearing mobility (fixed bearing [FB] or mobile bearing [MB]), polyethylene (PE) material (ultrahigh molecular weight PE [UHMWPE] or highly crosslinked PE [HXLPE]), component surface modification (porous metal—hydroxyapatite (HA), porous metal—uncoated, grit-blasted uncoated, or grit-blasted-titanium-nitride, trabecular metal), and anonymized performing hospital were collected. All data has been collected since 2007, except BMI, smoking status, and Charnley classification, which have only been registered since 2014.
Potential confounders were identified, based on the criteria of Rothman et al. [11] and depicted in a directed acyclic graph (DAG) [12]. Demographic factors such as age, BMI, ASA classification, smoking, and Charnley score have been found to be associated with revision [14]. However, these factors do not affect the choice between CR or PS implants (except possibly for age and BMI). Previous knee surgery was considered a potential confounder because a previous high tibial osteotomy or removed or damaged PCL during an earlier procedure could interfere with the choice between a CR or PS implant. Bearing mobility (e.g., FB or MB) [13] and surface modification [14] are considered to be associated with revision rates but are also part of implant design and hence associated with PS or CR. However, as many manufacturers have multiple bearing-constraint options for the same implant, this is considered minimally influential. Taken together, potential confounders included in the model were considered and visualized in a DAG (Figure 1).
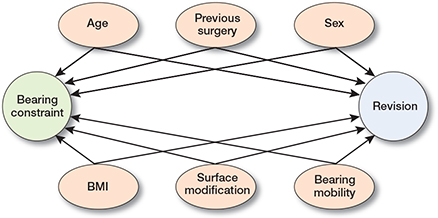
Figure 1. Directed acyclic graph representing the direct causal and associations between variables, the exposure (bearing constraint), and outcome (implant revision risk). Arrows represent the direction of causality or association between variables.
Statistics
Baseline characteristics are presented as means and standard deviation (SD), median and interquartile range (IRQ), or frequency and percentage. Cumulative crude revision incidences were assessed using Kaplan–Meier (KM) and competing-risk time-to-event survival analyses for revision for any reason and aseptic loosening at 5 and 10 years. Survival time was defined as the time from primary arthroplasty to first revision, patient death, or the end of the study period (January 1, 2022). For KM analyses, deaths were censored observations, assuming that the risk of revision is independent of the risk of death. For competing risk analyses, deaths were considered a competing event [16]. Tables and graphs show revision risks for any reason and aseptic loosening and their associated 95% confidence intervals (CI). Furthermore, an overview of other risks of various revision reasons has been calculated and presented. A log-minus-log transformation (with continuity correction in case of zero events) was employed to calculate the CI for the revision risks for different reasons, as the event rates were small. The KM and competing-risk cumulative revision incidences were compared to determine the possible influence of death as a competing risk. Since both analyses gave similar results, this justified using Cox regression models for the univariate and multivariate analyses to estimate hazard ratios (HR) associated with implant revision rates and their corresponding CI. We therefore only used the competing risk model to calculate the revision risks. Schoenfeld residuals were checked to ascertain model fit [17], which showed no violation of the proportional hazard assumption for both models (revisions for any reason and aseptic loosening). KM revision risks are presented in Table 5 (see Appendix). In the Cox regression models, the CR group was used as the reference group. An HR above 1.0 indicates that the PS group had a higher likelihood of revision compared with the CR group, while an HR below 1.0 suggests a lower likelihood of revision for the PS group. The model was adjusted for possible confounders that were included in the DAG (Figure 1). The models’ multicollinearity was assessed using the variance inflation factor (VIF), indicating no significant issues when the VIF is lower than 5.0 [18].
| Factor | Cruciate retaining | Posterior stabilized |
| Patients | 10,679 | 747 |
| Knees | 12,226 | 750 |
| Implant designs | 24 | 16 |
| Follow-up in years, median (IQR) | 7 (3–10) | 5 (2–10) |
| Age at surgery | ||
| < 50 years | 243 (2) | 22 (3) |
| 50–59 years | 1,800 (15) | 121 (16) |
| 60–69 years | 4,191 (34) | 266 (36) |
| 70–79 years | 4,423 (36) | 254 (34) |
| ≥ 80 years | 1,562 (13) | 85 (11) |
| Sex | ||
| Female | 7,894 (65) | 486 (65) |
| Male | 4,314 (35) | 261 (35) |
| BMI, mean (SD) | 29 (5) | 30 (5) |
| Preoperative smoking | 582 (5) | 35 (5) |
| Charnley score a | ||
| A | 2,775 (23) | 228 (30) |
| B1 | 2,072 (17) | 151 (20) |
| B2 | 1,415 (12) | 73 (10) |
| C | 258 (2) | 14 (2) |
| ASA classification | ||
| I | 2,047 (17) | 108 (14) |
| II | 8,295 (68) | 485 (65) |
| III/IV | 1,642 (13) | 107 (14) |
| Previous knee surgery b | 3,099 (25) | 116 (16) |
| Bearing mobility | ||
| Fixed | 2,218 (18) | 644 (86) |
| Mobile | 9,960 (82) | 94 (13) |
| PE material | ||
| Ultrahigh molecular weight PE | 10,345 (85) | 585 (78) |
| Highly crosslinked PE | 1,474 (12) | 146 (20) |
| Highly crosslinked PE + antioxidant | 260 (2) | 14 (2) |
| Femur component surface modifications | ||
| Porous—HA | 1,905 (15) | 99 (13) |
| Porous—uncoated | 8,935 (73) | 23 (3) |
| Porous—TiN | 697 (6) | 2 (0) |
| Grit-blasted—uncoated | 447 (4) | 555 (74) |
| Grit-blasted—TiN | 199 (2) | 30 (4) |
| Trabecular metal | 0 (0) | 0 (0) |
| Tibial component surface modifications | ||
| Porous—HA | 1,879 (15) | 100 (13) |
| Porous—uncoated | 9,502 (78) | 20 (3) |
| Porous—TiN | 81 (1) | 5 (1) |
| Grit-blasted—uncoated | 514 (4) | 470 (63) |
| Grit-blasted—TiN | 186 (2) | 1 (0) |
| Trabecular metal | 13 (0) | 20 (3) |
| Numbers do not add up to total due to missing data. ASA: American Society of Anesthesiologists; BMI: body mass index; HA: hydroxyapatite; IQR: interquartile range; n: number; PE: polyethylene; SD: standard deviation; TiN: titanium nitride. a Charnley score divides patients into 4 categories: A = only 1 affected knee joint, B1 = both knee joints affected, B2 = a knee prosthesis in the contralateral knee joint, and C = multiple joints affected. b Previous surgeries on the same knee were defined as any surgical procedure (e.g., meniscectomy, osteotomy, ACL reconstruction, osteosyntheses, synovectomy, arthroscopy, and patellar realignment). |
||
| Insert | Total, knees n | Revisions for aseptic loosening | Revisions for any reason | At risk a n | ||
| n | %RR (CI) | n | %RR (CI) | |||
| 5-year | ||||||
| CR | 12,226 | 119 | 1.1 (0.9–1.3) | 366 | 3.4 (3.1–3.7) | 7,535 |
| PS | 750 | 13 | 2.1 (1.2–3.5) | 26 | 4.1 (2.8–6.0) | 370 |
| 10-year | ||||||
| CR | 12,226 | 139 | 1.4 (1.1–1.6) | 422 | 4.2 (3.8–4.7) | 3,238 |
| PS | 750 | 19 | 3.9 (2.4–6.0) | 34 | 6.5 (4.6–9.2) | 170 |
| a Total number of knees remaining in the study at the specified follow-up. n: number; CI: confidence interval. |
||||||
To mitigate potential bias from underutilized prostheses or low-volume hospitals, 2 sensitivity analyses were conducted. Multivariable Cox regression analyses were performed on 2 restricted databases, each including only the 5 most commonly used implants and the 5 highest volume hospitals within each group over the follow-up period.
Missing data was addressed by omitting cases with a majority of unknown variables. For variables with feasible imputation, a multiple imputation method was used to generate plausible imputed values based on observed data. This approach aimed to reduce the impact of missing data while considering the probability of incorrect results [19]. R software version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria) using R packages “mstate” and “survival” were used to perform the analyses [20,21].
Ethics, funding, and disclosures
The study was approved by the institutional Scientific Advisory Board (WAR) of the LROI (LROI2022-100) after a comprehensive evaluation regarding the feasibility, relevance, compliance with ethical standards, privacy protection of patients and caregivers, and sound methodology of any study before data was obtained. The protocol of the study (LROI2022-100) can be provided by the authors upon request. Regarding potential conflicts of interest, 2 authors, BP (medical director) and AS (research and quality control), are employees of the LROI. The authors received no financial support for conducting the research and declare no conflict of interest. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2023.33283
Results
In total, 13,667 uncemented primary TKRs initially met the inclusion criteria. Exclusions were made for 32 cases with different bearing designs and 659 cases where it was not possible to determine if the TKR was of CR or PS type. Consequently, the study focused on 12,976 uncemented primary TKRs, of which 12,226 were CR implants and 750 PS implants (Figure 2).
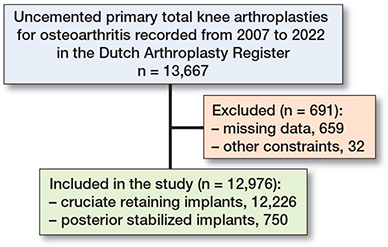
Figure 2. Flowchart of in- and exclusion.
Baseline
The median follow-up was 7 years (IQR 3–10) for the CR group and 5 years (IQR 2–10) for the PS group. Patient demographics were comparable between the groups (Table 1). The data was derived from 88 different hospitals, of which 22 (25%) never utilized a CR TKR system, and 20 (23%) never utilized a PS TKR system. In comparison with the PS group, the CR group had a greater variety of implants available with 24 designs compared with the PS group with 16 designs. The CR group comprised a larger proportion of cases with previous surgery on the same knee (25% vs. 16%), a substantially greater number of mobile bearing cases (82% vs. 13%), and a higher percentage of components with a porous metaluncoated surface modification (Table 1).
There was some missing data for relevant variables such as insert mobility (48 CR [1%]; 12 PS [2%]), surface modification (982 CR [8%]; 205 PS [27%]), previous surgery on the same knee (678 CR [6%]; 69 PS [9%]), BMI (CR 5,756 implants [47%]; PS 290 implants [39%]), smoking (CR 5,970 [49%]; PS 291 [39%]), and Charnley score (CR 5,706 implants [47%]; PS 284 implants [38%]).
Revision for any reason
The 10-year revision rate for any reason was higher for the PS group (6.5%, CI 4.6–9.2) compared with the CR group (4.2%, CI 3.8–4.7) (Table 2 and Figure 3). Revision risks for infection and aseptic loosening were also higher for the PS implants compared with the CR implants (Table 3). Multivariable Cox regression analyses revealed that PS implants had a higher risk of revision for any reason than CR implants (crude HR 1.4, CI 1.0–2.0; Table 4, model 1). Also, after multivariable adjustment, the risk of revision for any reason was higher for PS implants than for CR implants (adjusted HR 1.4, CI 0.6–3.2; Table 4, model 8).
| Factor | Cruciate retaining n = 12,226 | Posterior stabilized n = 750 | ||
| n | % (CI) | n | % (CI) | |
| Infection | 23 | 0.2 (0.1–0.3) | 6 | 0.9 (0.4–2.0) |
| Instability | 183 | 1.9 (1.7–2.3) | 7 | 1.4 (0.6–3.0) |
| Polyethylene wear | 18 | 0.3 (0.2–0.5) | 1 | 0.3 (0.0–2.4) |
| Aseptic loosening | 139 | 1.4 (1.1–1.6) | 19 | 3.9 (2.4–6.0) |
| Arthrofibrosis | 33 | 0.3 (0.2–0.5) | 0 | 0.0 (0.0–2.4) b |
| Patellofemoral pain | 50 | 0.6 (0.5–0.8) | 4 | 1.1 (0.4–3.3) |
| Number of revisions does not add up to the total number of revisions in Table 2 due to the presence of multiple reasons for a single revision. b Continuity correction (1 event) |
||||
| Use of PS | Full dataset | Restricted to 5 implants a | Restricted to 5 hospitals a |
| Revision for any reason | |||
| Crude b | 1.4 (1.0–2.0) | 1.6 (1.1–2.4) | 2.0 (1.2- 3.2) |
| Adjusted for | |||
| Model 1: Age (5 categories c) | 1.4 (1.0–2.0) | 1.6 (1.1–2.4) | 1.8 (1.1–2.9) |
| Model 2: Sex | 1.4 (1.0–2.0) | 1.6 (1.1–2.4) | 2.0 (1.2–3.2) |
| Model 3: BMI | 1.3 (0.8–2.2) | n/a d | n/a d |
| Model 4: Previous knee surgery | 1.5 (1.1–2.2) | 1.7 (1.1–2.6) | 2.2 (1.3–3.7) |
| Model 5: Bearing mobility | 2.1 (1.4–3.1) | 2.2 (1.4–3.5) | 2.6 (1.3–5.0) |
| Model 6: Surface modification femur | 1.3 (0.9–1.8) | 1.4 (1.0–2.2) | 2.1 (1.3–3.4) |
| Model 7: Surface modification tibia | 1.5 (1.3–1.7) | 1.2 (0.8–2.0) | 2.5 (1.4–4.2) |
| Model 8: All above | 1.4 (0.6–3.2) | 1.7 (0.8–3.4) | 2.1 (1.0–4.5) |
| Revision for aseptic loosening | |||
| Crude b | 2.5 (1.5–4.0) | 2.3 (1.3–4.2) | 3.6 (1.8–7.2) |
| Adjusted for | |||
| Model 1: Age (5 categories c) | 2.4 (1.5–3.8) | 2.3 (1.3–4.3) | 3.3 (1.7–6.6) |
| Model 2: Sex | 2.5 (1.5–4.0) | 2.3 (1.3–4.2) | 3.6 (1.8–7.2) |
| Model 3: BMI | 3.0 (1.3–6.7) | n/a d | n/a d |
| Model 4: Previous knee surgery | 2.6 (1.6–4.3) | 2.4 (1.3–4.5) | 4.4 (2.2–8.8) |
| Model 5: Bearing mobility | 4.1 (2.3–7.4) | 3.9 (1.8–8.3) | 7.9 (3.5–17.6) |
| Model 6: Surface modification femur | 2.2 (1.3–3.5) | 2.3 (1.2–4.4) | 4.5 (2.3–9.0) |
| Model 7: Surface modification tibia | 1.9 (1.1–3.2) | 1.8 (0.9–3.5) | 5.6 (2.8–11.1) |
| Model 8: All above | 2.6 (0.6–11.0) | 4.0 (1.4–11.4) | 9.9 (4.0–24.7) |
| PS: posterior stabilized; CR: cruciate retaining; BMI: body mass index. a The restricted datasets contain only data on (1) the 5 most commonly used implants (CR 11,214 [92%]; PS 562 [75%]), (2) the 5 most performing hospitals per group (CR 8,596 [70%]; PS 267 [36%]). b Crude = HR from univariable model. c The 5 age categories include < 50; 50–59; 60–69; 70–79; and ≥ 80 years. d BMI has been registered by the LROI since 2014, resulting in a small sample size that does not allow any meaningful analyses. |
|||
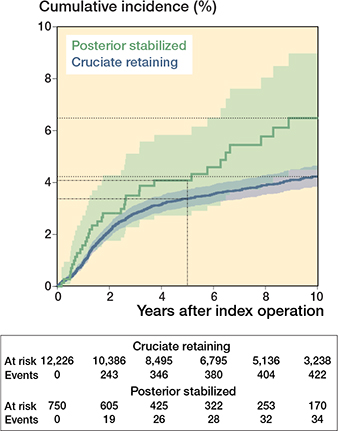
Figure 3. Cumulative incidence with 95% confidence intervals of revisions for any reason of cruciate-retaining and posterior-stabilized implants, calculated by competing risk analysis.
Revision for aseptic loosening
Competing risk analysis, with aseptic loosening as the endpoint, over 10 years, revealed a higher rate of revision for PS implants (3.9%, CI 2.4–6.0) than for CR implants (1.4%, CI 1.1–1.6) (Table 2 and Figure 4). Using multivariable Cox regression analyses, the crude risk of revision for aseptic loosening was estimated to be 2.5 times higher for PS implants than for CR implants (crude HR 2.5, CI 1.5–4.0; see Table 4, model 1). After multivariable adjustment, an approximately 3-fold increased risk (adjusted HR 2.6, CI 0.6–11; Table 4, model 8) of revision due to aseptic loosening was found for PS implants compared with CR implants.
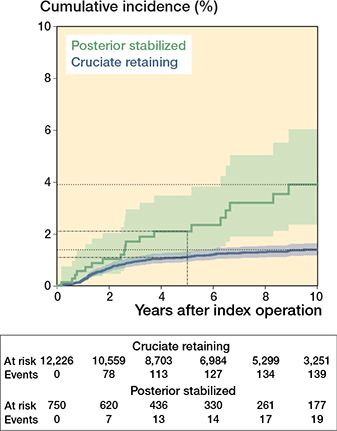
Figure 4. Cumulative incidence with 95% confidence intervals of revisions for aseptic loosening of at least 1 component of cruciate-retaining and posterior-stabilized implants, calculated by competing risk analysis.
The results of sensitivity analyses were consistent with the analyses of the full dataset when restricted to the 5 most commonly used implants or the 5 highest-volume hospitals (Table 4).
Discussion
We aimed, in this registry study, to compare the revision rates of PS and CR designs in patients receiving primary uncemented TKR. The main finding was that uncemented PS implants had a 1.4 times higher risk of revision for any reason and a 2.5 times higher risk for revision for aseptic loosening compared with uncemented CR implants used in the Netherlands from 2007 to 2022 during follow-up of 10 years.
This study represents the first large observational analysis that compares the risks of revision between PS and CR constraints, focused only on uncemented TKR systems. However, our findings are in line with previous observational studies that included only cemented CR and PS TKR systems. The recent Dutch registry study by Spekenbrink-Spooren et al. (2018) found PS cemented TKR systems to be 1.3 (CI 1.2–1.4) times more likely to receive a major revision than the CR cemented TKR systems after a median follow-up of 8 years. Moreover, the most common reason for the revision of both systems was aseptic loosening (PS (41%), CR (27%); P < 0.001) [22]. Similar conclusions were drawn by the Australian registry study of Vertullo et al. (2017), in their effort to circumvent confounding by indication, who compared patient groups treated by high-volume surgeons who preferred CR systems with those who preferred PS systems [6]. The authors found that the PS implants were more at risk of revisions for any reason (HR 1.6, CI 1.3–1.8]), aseptic loosening (HR 1.9, CI 1.6–2.4), and infection (HR 1.5, CI 1.3–1.8), compared with CR implants. Unfortunately, an analysis of uncemented implants was not performed due to an insufficient number of PS implants [6]. In the single high-volume institute study of Abdel et al. (2011), the all-cause revision rate of CR implants was found to be significantly lower than that of PS implants (CR 4.3%, CI 4.9–3.7 vs. PS 7.8%, CI 6.6–9.0, P < 0.001) [5]. This difference remained statistically significant after they attempted to remove a potential selection bias, by stratifying for knees with and without preoperative deformity, which was at the time the main medical indication for using a PS design [5].
Contrary to our findings, the most recent meta-analysis based on 14 randomized controlled trials (RCTs), involving 1,453 patients with follow-up ranging from 0.5 to 6 years, reported equivalent revision rates and functional outcomes between PS and CR designs [23]. However, it is important to note that the present and preceding meta-analyses are based solely on RCTs involving cemented implants and may lack sufficient statistical power and follow-up duration to adequately compare revision rates. In an RCT on uncemented TKR with trabecular metal, Wojtowicz et al. compared PS with CR in terms of fixation, measured with radiostereometric analyses (RSA), and found no clinically relevant differences [24]. Their results predicted that, for trabecular metal, there would be no differences in revision rates of aseptic loosening for PS compared with CR tibial baseplates [24]. Notably, 3 observational studies, which together assessed more than 450 uncemented PS TKRs with trabecular metal, reported no revisions for aseptic loosening after a follow-up period of 5 years [25-27], reinforcing the robustness of the RSA results of the study of Wojtowicz et al. [24]. These finding may seem to contradict our observed higher revision rates for any reason and for aseptic loosening of PS systems. However, only 33 trabecular metal tibial baseplates were included in our study, so the type of uncemented fixation may be an effect modifier. One notable distinction between the 2 groups in our study is that the CR group predominantly consisted of implants with a porous-uncoated surface, whereas the PS group had grit-blasted-uncoated surfaces. This disparity may be associated with the presence of different implant designs in each group, with potentially more modern designs having porous metal surfaces.
The reason why PS implants could be more at risk of revision has not yet been determined, but it is theorized that several factors may contribute to an increased risk. One possible factor could be the release of microparticles by increased post-wear, which can cause inflammation, ultimately leading to implant loosening [28]. Another potential factor is the post and cam mechanism used in PS implants, which can increase load transfer to the tibial tray and result in micromotion at the bone–implant interface. Also, suboptimal implant positioning might increase stress on the bone–implant interface and contribute to the development of micromotion and aseptic loosening [28]. While further research is needed to fully understand the reasons for the increased risk of revision in PS uncemented implants, addressing these potential factors through improved implant designs (e.g., use of trabecular metal implant surface) and surgical techniques could help reduce the risk of aseptic loosening of PS TKR systems.
Limitations
First, the study employs an observational design and there is a notable discrepancy in sample sizes between the PS group (n = 750) and the larger CR group (n = 12,226). This size difference may raise concerns about potential confounding by indication. However, we anticipate that this risk is low, as 2 previous registry and observational studies, in which a possible indication bias was deliberately investigated, reported results consistent with our study. One study included only high-volume surgeons who exclusively employed either a CR or PS design [6], while the other corrected for preoperative deformities [5]. Second, the LROI registers only the indication for revision, without incorporating feedback on the intraoperative cultures. Therefore, it is likely that low-grade infections were misclassified as revisions for aseptic loosening, but it is expected that this misclassification was equal between both groups. Also, the proportion of unsuspected low-grade infections in cases with a preoperative diagnosis of aseptic loosening appears to be low (4–13%) in total hip or knee arthroplasty[29,30]. Third, the inclusion of underutilized prostheses with suboptimal outcomes and the involvement of hospitals with low annual volumes or limited experience in utilizing uncemented TKR, irrespective of design, may contribute to a risk of bias in the study’s findings [15]. However, the sensitivity analyses conducted on the restricted databases yielded results consistent with those obtained from the full database analyses, indicating a minimal risk of introducing bias. Also, unmeasured residual confounding effects may have influenced our findings due to missing variables. For instance, BMI data was available only from 2014 onwards, limiting the sample size for specific analyses. However, after correcting for BMI in the full dataset, our results remained consistent, suggesting that any BMI imbalances between CR and PS groups did not affect the study outcomes. Fourth, it is important to note that the generalizability of the study findings is limited to the specific implants used in this study.
Conclusion
We showed that, during 10 years of follow-up, uncemented PS implants were 1.4 times more at risk of a revision for any reason, and 2.5 times more for aseptic loosening compared with uncemented CR implants. These results remained consistent after adjustment for confounders and sensitivity analyses.
- Wittig U, Moshammer M, Vielgut I, Hauer G, Reinbacher P, Leithner A, et al. Higher use of fixed-bearing over mobile-bearing and posterior-stabilized over medial pivot designs in total knee arthroplasty (TKA): a systematic comparative analysis using worldwide arthroplasty registries. Arch Orthop Trauma Surg 2023; 143: 1021-9. doi: 10.1007/s00402-022-04410-8.
- Verra W C, van den Boom L G H, Jacobs W, Clement D J, Wymenga A A B, Nelissen R G H H, et al. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev 2013. doi: 10.1002/14651858.CD004803.PUB3.
- Jacobs W C H, Clement D J, Wymenga A B. Retention versus removal of the posterior cruciate ligament in total knee replacement: a systematic literature review within the Cochrane framework. Acta Orthop 2005; 76: 757-68. doi: 10.1080/17453670510045345.
- Swedish Arthroplasty Registry. Annual report; 2022.
- Abdel M P, Morrey M E, Jensen M R, Morrey B F. Increased long-term survival of posterior cruciate-retaining versus posterior cruciate-stabilizing total knee replacements. J Bone Joint Surg Am 2011; 93: 2072-8. doi: 10.2106/JBJS.J.01143.
- Vertullo C J, Lewis P L, Lorimer M, Graves S E. The effect on long-term survivorship of surgeon preference for posterior-stabilized or minimally stabilized total knee replacement: an analysis of 63,416 prostheses from the Australian Orthopaedic Association national joint replacement registry. J Bone Joint Surg Am 2017; 99: 1129-39. doi: 10.2106/JBJS.16.01083.
- Dutch Arthroplasty Registry (LROI). Annual report; 2022. https://www.lroi-report.nl.
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, knee & shoulder arthroplasty: annual report 2022. Adelaide: AOANJRR.
- National Joint Registry (NJR). 19th Annual Report 2022 of England, Wales, Northern Ireland, the Isle of Man and the States of Guernsey. https://reports.njrcentre.org.uk/.
- von Elm E, Altman D G, Egger M, Pocock S J, Gøtzsche P C, Vandenbroucke J P, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453-7. doi: 10.1016/s0140-6736(07)61602-x.
- Rothman K J, Greenland S, Lash T L. Modern epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. Available from: www.ebook3000.com
- Textor J, van der Zander B, Gilthorpe M S, Liśkiewicz M, Ellison G T. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 2016; 45: 1887-94. doi: 10.1093/IJE/DYW341.
- Hao D, Wang J. Fixed-bearing vs mobile-bearing prostheses for total knee arthroplasty after approximately 10 years of follow-up: a meta-analysis. J Orthop Surg Res 2021; 16, 437. doi: 10.1186/s13018-021-02560-w.
- Puijk R, Rassir R, Sierevelt I N, Spekenbrink-Spooren A, Nelissen R G H H, Nolte P A, et al. Association between surface modifications for biologic fixation and aseptic loosening of uncemented total knee replacements. J Arthroplasty 2023; 38(12), 2605-2611.e1. doi:10.1016/j.arth.2023.05.094
- van Schie P, van Steenbergen L N, van Bodegom-Vos L, Nelissen R G H H, Marang-van de Mheen P J. Between-hospital variation in revision rates after total hip and knee arthroplasty in the Netherlands: directing quality-improvement initiatives. J Bone Joint Surg Am 2020; 102: 315-24. doi: 10.2106/JBJS.19.00312.
- Maradit Kremers H, Devick K L, Larson D R, Lewallen D G, Berry D J, Crowson C S, et al. Competing risk analysis: what does it mean and when do we need it in orthopedics research? J Arthroplasty 2021; 36: 3362-6. doi: 10.1016/j.arth.2021.04.015.
- Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen A B, et al. Statistical analysis of arthroplasty data: Part 2 guidelines. Acta Orthop 2011; 82: 258-67. doi: 10.3109/17453674.2011.588863.
- Kutner M, Nachtsheim C, Neter J, Li W. Applied linear statistical models. 5th ed. New York: McGraw-Hill Irwin; 2004.
- Graham J W, Olchowski A E, Gilreath T D. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 2007; 8: 206-13. doi: 10.1007/s11121-007-0070-9.
- de Wreede L C, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed 2010; 99: 261-74. doi: 10.1016/J.CMPB.2010.01.001.
- Therneau T M, Grambsch P M. A package for survival analysis in R, R package version 3.5-5. Available from: http://cran.r-project.org/package=survival.
- Spekenbrink-Spooren A, Van Steenbergen L N, Denissen G A W, Swierstra B A, Poolman R W, Nelissen R G H H, et al. Higher mid-term revision rates of posterior stabilized compared with cruciate retaining total knee arthroplasties: 133,841 cemented arthroplasties for osteoarthritis in the Netherlands in 2007–2016. Acta Orthop 2018; 89: 640-5. doi: 10.1080/17453674.2018.1518570.
- Jiang C, Liu Z, Wang Y, Bian Y, Feng B, Weng X. Posterior cruciate ligament retention versus posterior stabilization for total knee arthroplasty: a meta-analysis. PLoS One 2016; 11: e0147865. doi: 10.1371/JOURNAL.PONE.0147865.
- Wojtowicz R, Henricson A, Nilsson K G, Crnalic S. Uncemented monoblock trabecular metal posterior stabilized high-flex total knee arthroplasty: similar pattern of migration to the cruciate-retaining—a prospective radiostereometric analysis (RSA) and clinical evaluation of 40 patients (49 knees). Acta Orthop 2019; 90: 460-6. doi: https://dx.doi.org/10.1080/17453674.2019.1626097.
- Tarazi J M, Salem H S, Ehiorobo J O, Sodhi N, Mont M A, Harwin S F, et al. Cementless tritanium baseplate total knee arthroplasty: survivorship and outcomes at 5-year minimum follow-up. J Knee Surgery 2020; 33: 862-5. doi: 10.1055/S-0040-1712983/ID/JR19DEC0026SSA-14.
- Pulido L, Abdel M P, Lewallen D G, Stuart M J, Sanchez-Sotelo J, Hanssen A D, et al. Trabecular metal tibial components were durable and reliable in primary total knee arthroplasty: a randomized clinical trial. Clin Orthop Relat Res 2015; 473: 34-42. doi: 10.1007/S11999-014-3585-Y/METRICS.
- Kamath A F, Lee G C, Sheth N P, Nelson C L, Garino J P, Israelite C L, et al. Prospective results of uncemented tantalum monoblock tibia in total knee arthroplasty: minimum 5-year follow-up in patients younger than 55 years. J Arthroplasty 2011; 26: 1390-5. doi: 10.1016/J.ARTH.2011.06.030.
- Bourne RB, Baré J V. Failure in cam-post in total knee arthroplasty. In: Total knee arthroscopy. Available from: https://link.springer.com/chapter/10.1007/3-540-27658-0_14.
- Jacobs A M E, Bernard M, Meis J F, Van Hellemondt G, Goosen J H M. The unsuspected prosthetic joint infection: incidence and consequences of positive intraoperative cultures in presumed aseptic knee and hip revisions. B Joint J 2017; 99B: 1482-9. doi: 10.1302/0301-620X.99B11.BJJ-2016-0655.R2.
- Moojen D J F, Van Hellemondt G, Vogely H C, Burger B J, Walenkamp G H I M, Tulp N J A, et al. Incidence of low-grade infection in aseptic loosening of total hip arthroplasty. Acta Orthop 2010; 81: 667-73. doi: 10.3109/17453674.2010.525201.
Appendix
| Insert | Total, knees n | Revisions for aseptic loosening | Revisions for any reason | At risk a n | ||
| n | %RR (CI) | n | %RR (CI) | |||
| 5-year | ||||||
| CR | 12,226 | 119 | 1.1 (0.9–1.3) | 366 | 3.4 (3.1–3.8) | 7,535 |
| PS | 750 | 13 | 2.1 (0.9–3.3) | 26 | 4.1 (2.5–5.7) | 370 |
| 10-year | ||||||
| CR | 12,226 | 139 | 1.4 (1.2–1.7) | 422 | 4.4 (4.0–4.8) | 3,238 |
| PS | 750 | 19 | 4.2 (2.2–6.2) | 34 | 6.8 (4.4–9.2) | 170 |
| For Abbreviations and footnote, see Table 2 | ||||||