Patient-reported outcome of 95% of young patients improves after primary total hip arthroplasty: identification of 3 recovery trajectories in 3,207 patients younger than 55 years from the Dutch Arthroplasty Register
Martijn F L KUIJPERS 1, Liza N VAN STEENBERGEN 2, B Willem SCHREURS 1,2, and Gerjon HANNINK 3
1 Department of Orthopaedics, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen; 2 Dutch Arthroplasty Register (Landelijke Registratie Orthopedische Implantaten), ‘s Hertogenbosch; 3 Department of Operating Rooms, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, The Netherlands
Background and purpose — Little is known about the outcome after receiving total hip arthroplasty (THA), specifically in young patients. We identified different recovery trajectories in young patients using data from the Dutch Arthroplasty Register (LROI). We also explored whether risk factors commonly associated with functional outcome were associated with recovery trajectory.
Patients and methods — We used HOOS-PS score data up to 1 year postoperatively from the LROI from all patients younger than 55 years who received a primary THA between 2014 and 2019. To investigate whether different recovery trajectories could be distinguished, we performed latent class growth analysis (LCGA). Subsequently, we used multinomial logistic regression analyses to explore factors associated with class membership.
Results — 3,207 patients were included. LCGA identified 3 groups of patients: optimal responders (75%), good responders (21%), and poor responders (4.7%). Female sex (RR 1.1; 95% CI 1.1–1.1), ASA II (RR 1.1; CI 1.0–1.1), ASA III–IV (RR 1.1; CI 1.0–1.2), smoking (RR 1.1; CI 1.0–1.1), cemented fixation (RR 1.2; CI 1.1–1.2), and a 22–28 mm head diameter (RR 1.1; CI 1.0–1.2) were associated with “good responder” class membership. ASA II (RR 1.1; 1.0–1.2), ASA III–IV (RR 1.2; 1.1–1.3), smoking (RR 1.2; CI 1.1–1.2), and hybrid fixation (RR 1.2; CI 1.0–1.2) were associated with “poor responder” class membership.
Interpretation — 3 recovery trajectories could be identified. Female sex, higher ASA classifications, smoking, cemented or hybrid fixation, and small head diameter were associated with a suboptimal result after primary THA in young patients. These findings can aid in the process to determine which patients are at risk of a suboptimal outcome.
Citation: Acta Orthopaedica 2022; 93: 560–567. DOI http://dx.doi.org/10.2340/17453674.2022.3140.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2021-05-04. Accepted: 2022-05-02. Published: 2022-06-20.
Correspondence: gerjon.hannink@radboudumc.nl
MK, GH, LN, BS: concept and design. MK, GH, BS: data analysis and interpretation. MK, GH, BS: manuscript preparation. MK, GH, LN, BS: manuscript editing. MK, GH, LN, BS: manuscript review. MK, GH, LN, BS: final approval of the version submitted.
Acta thanks Rob Nelissen and Ola Rolfson for help with peer review of this study.
Around 10–20% of total hip arthroplasty (THA) patients do still report pain and functional disability after THA (1-3). This indicates the existence of different recovery trajectories between patients after THA.
Several reviews report on possible risk factors for a less favorable outcome of THA, such as increased BMI or worse preoperative functioning (4,5). However, results of studies included in these reviews are conflicting. To further evaluate the outcome of THA, it is of importance to have a better understanding of differences in response patterns between patients. A suitable statistical method to gain insight into different patterns in the response after THA is latent class growth modelling (LCGM) (6,7). Only a few studies within the field of orthopedics have used this method to assess possible differences in postoperative response patterns (8,9). However, these studies did not focus on young patients.
THA is used increasingly in young patients (< 55 years), and this number will grow in the coming years (10). The outcome of THA in these young patients is, in terms of prosthesis survival, inferior when compared with older age groups (11,12). However, for these young patients, functional outcome is of even more importance, as these patients want to return to work, sports, and physical and social activities in their daily life (13).
Therefore, we identified recovery trajectories in young patients according to their reported pain, functionality, and physical ability after THA using data from the Dutch Arthroplasty Register (LROI). Additionally, we explored whether risk factors commonly associated with functional outcome were associated with recovery trajectory.
Patients and methods
The LROI (Dutch Arthroplasty Register) is a nationwide population-based register collecting data on joint arthroplasties. Initiated by the Netherlands Orthopaedic Association, data collection started in 2007. The database has coverage of all Dutch hospitals, a completeness of over 95% of primary THA and 88% for revision arthroplasty (14), and 99% for primary THA and 97% for revision THA in recent years (15). Largescale collection of hip-specific and general health-related patient-reported outcome measures (PROMs) was initiated in 2014. As recommended by the Netherlands Orthopaedic Association, PROMs are only collected for patients with primary osteoarthritis as diagnosis. Collecting PROMs of patients with other diagnoses is optional. Hip-specific PROMs in the LROI consist of the EuroQoL 5-Dimensions (EQ-5D-3L) questionnaire with EQ-5D index score and thermometer to measure health perception and health-related quality of life, the Hip disability and Osteoarthritis Outcome Score (HOOS-PS) to assess physical functioning and disability, a numeric rating scale (NRS) measuring pain during activity and at rest and an anchor question (only measured postoperatively). Additionally, the Oxford Hip Score (OHS) is collected in some hospitals, but this score is optional (16).
Data collection
For this study, we obtained collected PROMs data from the LROI from all patients younger than 55 years who received a primary THA between January 1, 2014 and December 31, 2018 (n = 16,429). The outcome of interest of this study was reported hip-specific problems measured with the Hip disability and Osteoarthritis Outcome Score–Physical function Short form (HOOS-PS), as this PROM evaluates physical functioning and disability, which is particularly important for young patients (13). The HOOS-PS is a validated, hipspecific, 5-item measure of physical functioning derived from the items of activity during daily life, sports, and recreational activities. The HOOS-PS aims to measure physical functioning with fewer items and similar validity compared with the full-length measurement instrument (HOOS), resulting in a decrease in burden of the responder and in administrative load (17). The HOOS-PS ranges from 0 to 100. Lower scores indicate a higher level of physical function (18,19).
As PROMs are only routinely collected from patients with primary osteoarthritis, only patients with primary osteoarthritis as diagnosis were selected (n = 11,300). Patients were asked to complete the HOOS-PS preoperatively (a maximum of 182 days preoperatively), at 3 months postoperatively (between 63 and 110 days), and at 12 months postoperatively (between 323 and 407 days), where the ranges are defined by the LROI. Patients who completed the HOOS-PS on at least 2 out of 3 time-points were included. Lastly, patients who underwent a revision procedure within 1 year postoperatively (n = 45), patients with a prosthesis head diameter ≥ 38 mm (n = 9), and patients with metal-on-metal as bearing type (n = 1) were excluded from the analysis (Figure 1), which resulted in 3,207 cases included in this study (28% of all patients < 55 in the LROI).
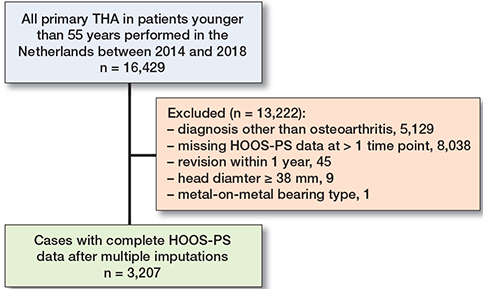
Figure 1. Flowchart of inclusion of patients.
Statistics
We performed multiple imputation by chained equations procedures using predictive mean matching to impute missing values. 10 imputed datasets were created. Missing data ranged from 0.03% for gender up to 16% for 12 months postoperative HOOS-PS score (Table 1).
To investigate whether different recovery patterns could be distinguished in the outcome of the HOOS-PS, we used Mplus (version 8.4; https://www.statmodel.com) to perform latent class growth analysis (LCGA). For the HOOS-PS, we performed a 1-class to 4-class latent class growth model for all 10 imputed datasets, as a model with more classes showing only small variations in the same recovery pattern would be less meaningful. As described by Ram and Grimm (20), we based our model selection on a combination of visual inspection of the plots, considering interpretability and clinical meaningfulness. Next, we examined the relative fit statistics Bayesian Information Criteria (BIC), Akaike Information Criteria (AIC), and Adjusted BIC, where a lower value indicates a better fit for all statistics. Lastly, we evaluated the entropy of the model, where a higher entropy (ranging from 0 to 1) indicates a higher confidence in the correct classification of individuals (20). On the basis of these considerations, we chose the model that had the lowest relative fit statistics of the models that still had good interpretability and clinical meaningfulness, as well as an adequate entropy (> 0.80) as our final model (20). A Lo–Mendell–Rubin (LMR) likelihood ratio test was performed to compare the improvement in model fit between 2 nested models. A significant LMR test indicates that the model with k classes fits better compared with the same model with k – 1 classes (21). The quality of the model in terms of average posterior probabilities for each trajectory class was also assessed. Model estimates were presented with their 95% confidence intervals (CI). Subsequently, we used the ‘r3step’ procedure in Mplus to perform both crude and adjusted multinomial logistic regression analyses on each of the 10 imputed datasets, to explore whether risk factors commonly associated with functional outcome were associated with class membership.
Risk factors were selected based on expert opinion and literature. Based on availability in the LROI, the following factors were included: age, sex, BMI, ASA score, smoking status, surgical approach, fixation method, head diameter, and bearing type. Directed acyclic graphs were used to depict a possible causal relationship between the risk factors selected, possible confounding variables, and the outcomes. Confounding variables, as given in Table 4 and Figure 2 (see Supplementary data), were selected based on the approach described by Shrier and Platt (22).
The resulting regression coefficients of the 10 imputed datasets were pooled using Rubin’s rule. Odds ratios were converted to the corresponding relative risks (RR) using the approach described by Zhang and Yu (23). Multiple imputation and pooling of regression coefficients was performed using R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) with package ‘mice’.
Ethics, funding, data sharing, and potential conflict of interest
Ethical approval was not applicable, as all data was received anonymously. This study was funded by the Van Rens Foundation, the Netherlands (VRF2017-009). The funding body had no role in the design of the study, data collection, analysis and interpretation, or in writing of the manuscript. Data is available from the LROI but restrictions apply to the availability of this data, which was used under license for the current study. The authors declare that they have no competing interests.
Results
An overview of imputed data is set out in Table 2. Model statistics of all imputed datasets are given in Table 3. The relative fit statistics (BIC, AIC, Adjusted BIC) continued to decrease up to the 4-class model, indicating a better fit with increasing number of classes. In all datasets, entropy was highest for the 2-class model, and comparable for the 3-class and 4 class model. However, group sizes became very small in the 4-class model. Additionally, the 4-class model showed only small variations in recovery patterns, and was therefore considered less clinically relevant and meaningful. Therefore, based on relative fit statistics, entropy, and group sizes, the 3-class model was chosen as the final model. In addition, when fitting models with increasing numbers of classes, the 3-class model showed the best balance between model fit and model complexity. This was confirmed by the LMR test (3- vs. 4-class model: –2 log likelihood (4) = 149.8, p = 0.1). Posterior probabilities (which measure classification accuracy) were high for the 3 classes, indicating good model fit. For this 3-class solution, the mean (SD) probability of membership was 0.96 (0.09) for class 1, 0.89 (0.14) for class 2, and 0.95 (0.10) for class 3, well over the recommended minimum for model adequacy of 0.7 (24).
| All cases | Optimal responders | Good responders | Poor responders | |||||
| n (%) a | range b | n (%) a | range b | n (%) a | range b | n (%) a | range b | |
| Cases, n | 3,207 | 2,391 (75) | 2,356–2,430 | 664 (21) | 631–699 | 152 (4.7) | 143–162 | |
| Age, mean (SD) | 49 (5) | 49–49 | 49 (5) | 49–49 | 49 (6) | 49–49 | 49 (5) | 48–49 |
| Sex | ||||||||
| Male | 1,509 (47) | 1,508–1,509 | 1,170 (49) | 1,144–1,187 | 267 (40) | 250–287 | 72 (47) | 68–78 |
| Female | 1,698 (53) | 1,698–1,699 | 1,221 (51) | 1,201–1,243 | 397 (60) | 377–421 | 81 (53) | 74–88 |
| BMI, mean (SD) | 28 (5) | 28 (5) | 28–28 | 28 (5) | 26–29 | 28 (5) | 28–29 | |
| ASA | ||||||||
| I | 1,395 (44) | 1,395–1,396 | 1,105 (46) | 1,092–1,116 | 245 (37) | 232–256 | 46 (30) | 39–50 |
| II | 1,607 (50) | 1,606–1,607 | 1,152 (48) | 1,126–1,183 | 366 (55) | 338–391 | 89 (59) | 82–95 |
| III–IV | 205 (6.4) | 205–205 | 134 (5.6) | 128–141 | 53 (8.0) | 47–61 | 18 (12) | 15–21 |
| Smoking | ||||||||
| No | 2,593 (81) | 2,587–2,595 | 1,977 (83) | 1,941–1,999 | 515 (78) | 495–544 | 100 (66) | 93–108 |
| Yes | 614 (19) | 612–620 | 414 (17) | 403–431 | 148 (22) | 136–162 | 52 (34) | 49–58 |
| Surgical approach | ||||||||
| Posterolateral | 1,713 (53) | 1,706–1,718 | 1,270 (53) | 1,250–1,284 | 360 (54) | 349–381 | 83 (55) | 77–90 |
| Anterior | 1,080 (34) | 1,077–1,085 | 832 (35) | 819–851 | 205 (31) | 187–215 | 43 (28) | 40–47 |
| Anterolateral | 159 (5.0) | 157–163 | 115 (4.8) | 110–119 | 35 (5.3) | 31–40 | 9 (5.9) | 7–11 |
| Direct lateral | 256 (8.0) | 253–285 | 174 (7.3) | 167–180 | 63 (9.5) | 58–72 | 18 (12) | 14–23 |
| Fixation method | ||||||||
| Uncemented | 2,778 (87) | 2,777–2,780 | 2,106 (88) | 2,064–2,144 | 548 (83) | 519–581 | 125 (82) | 116–132 |
| Cemented | 165 (5.1) | 164–167 | 104 (4.3) | 100–108 | 53 (8.0) | 47–58 | 8 (5.3) | 6–11 |
| Hybrid | 74 (2.3) | 74–75 | 55 (2.3) | 52–57 | 14 (2.1) | 12–18 | 5 (3.3) | 4–6 |
| Reversed hybrid | 190 (5.9) | 188 –191 | 126 (5.3) | 124–128 | 48 (7.2) | 44–51 | 15 (9.9) | 13–18 |
| Head diameter, mm | ||||||||
| 22–28 | 492 (15) | 486–498 | 332 (15) | 327–343 | 130 (20) | 123–139 | 30 (20) | 27–35 |
| 32 | 1,920 (60) | 1,911–1,927 | 1,457 (60) | 1,436–1,483 | 381 (57) | 358–401 | 81 (53) | 75–91 |
| 36 mm | 795 (25) | 789–800 | 602 (25) | 589–610 | 153 (23) | 146–162 | 41 (27) | 39–46 |
| Bearing type | ||||||||
| Ceramic-on-polyethylene | 2,012 (63) | 2,005–2,020 | 1,512 (63) | 1,474–1,533 | 412 (62) | 388–442 | 89 (59) | 82–95 |
| Ceramic-on-ceramic | 330 (10) | 324–335 | 247 (10) | 243–255 | 63 (9.5) | 59–67 | 19 (13) | 16–21 |
| Metal-on-polyethylene | 607 (19) | 601–610 | 431 (18) | 421–442 | 142 (21) | 128–154 | 34 (22) | 30–38 |
| Zirconium-on-polyethylene | 258 (8.0) | 254–262 | 201 (8.4) | 194–206 | 47 (7.1) | 43–52 | 10 (6.6) | 8–12 |
| Estimates are pooled across the 10 multiple imputed datasets. | ||||||||
| a Pooled cases are rounded to the nearest number. | ||||||||
| b Range (lowest and highest value) of all means across 10 multiple imputed datasets. | ||||||||
| Factor | Crude RR (95% CI) b | Adjusted a RR (95% CI) b |
| “Good responders” vs. “Optimal responders” | ||
| Age | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| Female sex (ref. male sex) | 1.1 (1.1–1.1) | 1.1 (1.1–1.1) |
| BMI | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| ASA (ref. ASA I) | ||
| II | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) |
| III–IV | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| Smoking (ref. no smoking) | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) |
| Approach (ref. posterolateral) | ||
| Anterior | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) |
| Anterolateral | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Direct lateral | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| Fixation (ref. uncemented) | ||
| Cemented | 1.2 (1.1–1.2) | 1.2 (1.1–1.2) |
| Reversed hybrid | 1.0 (0.7–1.2) | 1.0 (0.7–1.2) |
| Hybrid | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| Head diameter, mm (ref. 32) | ||
| 22–28 | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| 36 | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Bearing type (ref. ceramic-on-polyethylene) | ||
| Ceramic-on-ceramic | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Metal-on-polyethylene | 1.1 (1.0–1.1) | 1.0 (1.0–1.1) |
| Zirconium-on-polyethylene | 1.0 (0.8–1.1) | 1.0 (0.8–1.1) |
| “Poor responders” vs. “Optimal responders” | ||
| Age | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| Female sex (ref. male sex) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| BMI | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
| ASA (ref. ASA I) | ||
| II | 1.1 (1.1–1.2) | 1.1 (1.0–1.2) |
| III–IV | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) |
| Smoking (ref. no smoking) | 1.2 (1.1–1.2) | 1.2 (1.1–1.2) |
| Approach (ref. posterolateral) | ||
| Anterior | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) |
| Anterolateral | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) |
| Direct lateral | 1.1 (1.0–1.2) | 1.1 (0.9–1.2) |
| Fixation (ref. uncemented) | ||
| Cemented | 1.1 (0.8–1.2) | 1.0 (0.8–1.2) |
| Reversed hybrid | 1.1 (0.8–1.2) | 1.1 (0.7–1.2) |
| Hybrid | 1.2 (1.0–1.2) | 1.2 (1.0–1.2) |
| Head diameter, mm (ref. 32) | ||
| 22–28 | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| 36 | 1.1 (0.9–1.1) | 1.1 (0.9–1.1) |
| Bearing type (ref. ceramic-on-polyethylene) | ||
| Ceramic-on-ceramic | 1.1 (0.9–1.2) | 1.1 (0.9–1.2) |
| Metal-on-polyethylene | 1.1 (1.0–1.2) | 1.1 (0.9–1.2) |
| Zirconium-on-polyethylene | 1.0 (0.7–1.1) | 1.0 (0.7–1.1) |
| Estimates are pooled across the 10 multiple imputed datasets. | ||
| a Confounding variables were selected based on the directed acyclic graph depicted in Figure 2 (see Supplementary data): BMI was adjusted for smoking; ASA was adjusted for age, BMI, and smoking; Smoking was adjusted for age; Approach was adjusted for BMI; Fixation was adjusted for age and approach; Head diameter was adjusted for approach; Bearing type was adjusted for age, BMI, and head diameter; No adjustments were included in the analysis of age and sex. | ||
| b Odds ratios obtained from multinomial regression were converted to the corresponding relative risks using the methods described by Zhang and Yu (23). | ||
HOOS-PS trajectories
The identified recovery trajectories based on the pooled HOOS-PS data are shown in Figure 3. The largest class consisted of 2,391 cases (75%). The pooled estimated preoperative HOOS-PS score of this class was 48 (CI 47–49). This group showed a steep decline towards a pooled estimated HOOS-PS score of 14 (CI 13–15) at 3 months postoperatively. After the first 3 months, the estimated pooled HOOS-PS score declined even more towards a score of 6 (CI 5–7), indicating a further improvement of physical functioning up to 12 months postoperatively. We labelled this class as the “optimal responders.”
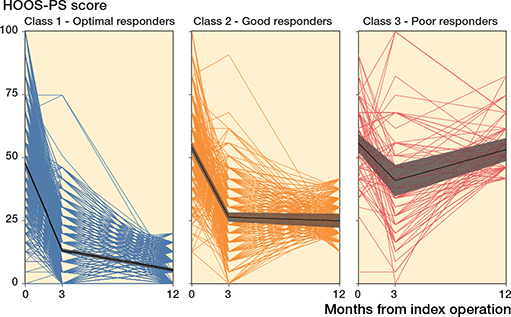
Figure 3. Recovery trajectories based on HOOS-PS score across class 1 (n = 2,391), class 2 (n = 664), and class 3 (n = 152). The mean trajectories with their 95% CI per class are shown in bold (black); individual trajectories are in color. Lower HOOS-PS scores indicate better physical functioning. For the purpose of plotting, individuals were assigned to classes based on their most likely class membership; it should be noted that individuals are in fact assigned a probability of class membership in the model.
The second class consisted of 664 cases (21%). The pooled estimated preoperative HOOS-PS score for this group was 54 (CI 52–55). Similar to the first class, also this group showed a steep decline in pooled HOOS-PS score during the first 3 months. The pooled estimated HOOS-PS score decreased to 29 (CI 26–31) at 3 months postoperatively, indicating an increase in physical functioning in this time period after surgery. However, this class showed no further improvement, as the pooled HOOS-PS score was 26 (CI 24–28) at 12 months postoperatively. Therefore, we labelled this class as the “good responders.”
The third class consisted of 152 cases (4.7%). Pooled estimated preoperative HOOS-PS score was with 58 (CI 55–62) the highest of all 3 groups. This group showed a small decrease in pooled HOOS-PS score within the first 3 months after surgery, where the pooled estimated score at 3 months postoperatively was 45 (CI 40–50). However, after this time-point, this group showed an increase in mean pooled HOOS-PS score up to 12 months postoperatively, where the pooled estimated HOOS-PS score was 56 (CI 51–60). This indicates a decrease in physical functioning of these patients after the first 3 months postoperatively. This class was labelled as the “poor responders” (Table 2 and Figure 3).
Factors associated with class membership
In both the crude and adjusted multinomial regression analysis, the “optimal responder” class was chosen as the reference group. In the adjusted analysis, female sex (RR 1.1), ASA II (RR 1.1), ASA III–IV (RR 1.1), smoking (RR 1.1), cemented fixation (RR 1.2), and a 22–28 mm head diameter (RR 1.1) were associated with “good responder” class membership. ASA II (RR 1.1), ASA III–IV (RR 1.2), smoking (RR 1.2), and hybrid fixation (RR 1.2) were associated with “poor responder” class membership (Table 4).
Discussion
We identified 3 different recovery trajectories in young patients according to their HOOS-PS score using data from the LROI.
Female sex, ASA score, smoking, type of fixation, and head diameter were associated with “good responder” or “poor responder” class membership versus “optimal responder” class membership. However, the total of 95% of young patients shows a favorable outcome after primary THA (optimal responders and good responders).
Several systematic reviews have examined the role of different factors on the response after primary THA; however, none of these reviews focused on young patients. A review of Lungu et al. showed poorer outcomes after primary THA in patients with higher levels of preoperative pain and physical functioning, higher BMI, and worse general health (5). A review by Buirs et al. (25) showed an association between BMI, age, comorbidity, preoperative physical functioning, and mental health with functional outcome after primary THA. This is in line with our study, where patients with worse general health (ASA II or ASA III–IV) were more likely to be a “good responder” or a “poor responder.” However, in contrast to these reviews, we found no association between a worse outcome and an increased BMI.
Our results showed that a cemented fixation was associated with a less optimal patient-reported outcome after primary THA, as these patients were more often members of the “good responders” class. This is in line with a study from the Swedish register which found that uncemented fixation was associated with better outcomes after primary THA when compared with cemented fixation, as patients reported better health-related quality of life, lower postoperative pain, and greater satisfaction after primary THA (26). Another study found similar results, with a higher decrease in patient-reported postoperative pain and increase in self-care after the use of uncemented fixation (27). Other studies reported contrasting findings, with no difference in patient-reported outcome between cemented and uncemented fixation (28), or even better results after cemented fixation (29). However, most of these studies did not use the HOOS-PS to assess patient-reported outcome, and did not focus solely on young patients. The association between cemented fixation and a worse patient-reported outcome we found might be influenced by the choice of fixation for certain patients. For example, patients with larger bone defects might more often be treated with a cemented prosthesis. Unfortunately, as data on these factors is not available in the LROI, this effect could not be examined within this study.
Only a few other studies used LCGA to determine different response patterns in patients after primary THA. Hesseling et al. (8) found also 3 distinct postoperative trajectories after analyzing the Oxford Hip Score (OHS) in a large cohort of patients with a mean age of 69 years. That study confirms our findings, as they also found that female sex, ASA II or ASA III–IV, and smoking were associated with suboptimal patient-reported outcomes after primary THA. Additionally, an association between obesity, age above 75 years, direct lateral approach, reversed hybrid fixation, and poorer scores in EQ-5D domains and worse patient-reported outcomes were reported. They also found a small portion of patients (8%) who showed initially a small increase in reported outcome, which decreased after 3 months postoperatively. Although that study did not focus on young patients, this might confirm the finding of the “poor responders” in our study, as we found a similar pattern. Other studies examining different response patterns after primary THA used predefined subgroups (30), had only short-term follow-up, and had small number of included patients (9), which made comparison of results difficult.
Psychological and mental factors were not taken into account in our analysis. The HOOS-PS aims to evaluate the physical outcome of patients. However, especially in young patients, the mental and psychological factors might be of importance in the outcome of patient-reported outcome. For example, preoperative expectations contribute strongly to the final degree of satisfaction after primary THA (31). Additionally, preoperative expectations were positively related to improvement in pain and function after THA (32). Especially in younger patients, keen to return to work, sports, and physical social activities in their daily life, preoperative expectations might be high. Therefore, it would be of interest to account for these factors in the analysis of patient-reported outcome in young patients. Unfortunately, this data is not registered within the LROI.
Identifying patients who are more likely to have a worse outcome can help in the management of expectations prior to primary THA. Additionally, this information is valuable for orthopedic surgeons, as this can aid in the process to determine which patients are at risk of a suboptimal outcome.
In this study, some limitations must be considered. First, although we used multiple imputation to complete the HOOS-PS score for cases with a missing score at one time point, we were able to include still only 28% of all patients younger than 55 years registered in the LROI. In addition, not all hospitals were collecting PROMs data since the start of registration of PROMS in the LROI, which may have influenced the completeness of our data. However, in 2018, 34% of all cases in the LROI had completed the preoperative, 3-, and 12 months postoperative PROMs. Additionally, this percentage was much lower in previous years, ranging from 8% to 32% (15). Therefore, when compared with the completeness of PROMS data within the LROI, the inclusion of cases in this study is acceptable. However, it must be noted that the overall completeness of PROMs within the LROI is low, as a response rate of 60% is advised by the International Society of Arthroplasty Registers (ISAR) (33). Therefore, the completeness of our data is a strong limitation of this study. The percentages of obtained HOOS scores were comparable to obtained EQ5D scores. The percentage of obtained Oxford Hip scores was lower as this score is not mandatory within the LROI.
Second, for ease of interpretation, we converted odds ratios to the corresponding relative risks using the methods described by Zhang and Yu (23). It should be noticed that these confidence intervals converted using this method could be slightly biased, leading one to believe that the relative risk estimate is more precise than is true (34). This bias appears to occur because the proposed calculation does not take into consideration the covariance between the estimated incidence and estimated odds ratio. Yu and Zhang (35) note that a “trade-off between simplicity and precision” is at issue with their method.
Third, motivated patients are more likely to complete the questionnaires at all time-points, whereas patients with postoperative complications are more likely to have incomplete PROMs (36), which might have affected our findings. Additionally, we found small differences in patients who were included in our analyses and patients who were excluded, as included patients were slightly older, were more often non-smokers, and had a lower ASA classification. However, included patients did not differ from excluded patients in sex and BMI. Despite these small differences, we think that the effect of these differences on the generalizability of our results to the entire cohort of young patients is small.
Finally, only patients who had a primary THA after primary osteoarthrosis were studied. In younger patients, the number of cases with secondary osteoarthrosis is higher, but there is limited PROM data available on these patients.
In conclusion, we found 3 distinct response patterns in young patients after primary THA, which could be classified as “optimal responders,” “good responders,” and “poor responders.” Female sex, ASA II or ASA III–IV classification, smoking, cemented fixation, and a small head diameter were associated with “good responder” class membership, whereas an ASA II or ASA III–IV classification, smoking, and hybrid fixation were associated with “poor responder” class membership. The “poor responders” showed almost no improvement in patient-reported outcome 1 year after primary THA. This information is valuable for both patients and orthopedic surgeons, as these findings can aid in the process to determine which patients are at risk of a suboptimal outcome. Additionally, this study may help young patients in management of their expectations prior to their surgery.
- Anakwe R E, Jenkins P J, Moran M. Predicting dissatisfaction after total hip arthroplasty: a study of 850 patients. J Arthroplasty 2011; 26(2): 209-13. doi: 10.1016/j.arth.2010.03.013.
- Judge A, Cooper C, Williams S, Dreinhoefer K, Dieppe P. Patient-reported outcomes one year after primary hip replacement in a European collaborative cohort. Arthritis Care Res (Hoboken) 2010; 62(4): 480-8. doi: 10.1002/acr.20038.
- Palazzo C, Jourdan C, Descamps S, Nizard R, Hamadouche M, Anract P, et al. Determinants of satisfaction 1 year after total hip arthroplasty: the role of expectations fulfilment. BMC Musculoskelet Disord 2014; 1553. doi: 10.1186/1471-2474-15-53.
- Hofstede S N, Gademan M G, Vliet Vlieland T P, Nelissen R G, Marang-van de Mheen P J. Preoperative predictors for outcomes after total hip replacement in patients with osteoarthritis: a systematic review. BMC Musculoskelet Disord 2016; 17212. doi: 10.1186/s12891-016-1070-3.
- Lungu E, Maftoon S, Vendittoli P A, Desmeules F. A systematic review of preoperative determinants of patient-reported pain and physical function up to 2 years following primary unilateral total hip arthroplasty. Orthop Traumatol Surg Res 2016; 102(3): 397-403. doi: 10.1016/j.otsr.2015.12.025.
- Nagin D S, Odgers C L. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6109-38. doi: 10.1146/annurev.clinpsy.121208.131413.
- Berlin K S, Parra G R, Williams N A. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J Pediatr Psychol 2014; 39(2): 188-203. doi: 10.1093/jpepsy/jst085.
- Hesseling B, Mathijssen N M C, van Steenbergen L N, Melles M, Vehmeijer S B W, Porsius J T. Fast starters, slow starters, and late dippers: trajectories of patient-reported outcomes after total hip arthroplasty: results from a Dutch nationwide database. J Bone Joint Surg Am 2019; 101(24): 2175-86. doi: 10.2106/JBJS.19.00234.
- Porsius J T, Mathijssen N M C, Klapwijk-Van Heijningen L C M, Van Egmond J C, Melles M, Vehmeijer S B W. Early recovery trajectories after fast-track primary total hip arthroplasty: the role of patient characteristics. Acta Orthop 2018; 89(6): 597-602. doi: 10.1080/17453674.2018.1519095.
- Kurtz S M, Lau E, Ong K, Zhao K, Kelly M, Bozic K J. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res 2009; 467(10): 2606-612. doi: 10.1007/s11999-009-0834-6.
- Bayliss L E, Culliford D, Monk A P, Glyn-Jones S, Prieto-Alhambra D, Judge A, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017; 389(10077): 1424-30. doi: 10.1016/S0140-6736(17)30059-4.
- NJR. 16th annual report: National Joint Registry for England, Wales, Northern Ireland and the Isle of Man; 2019.
- Walker R P, Gee M, Wong F, Shah Z, George M, Bankes M J, et al. Functional outcomes of total hip arthroplasty in patients aged 30 years or less: a systematic review and meta-analysis. Hip Int 2016; 26(5): 424-31. doi: 10.5301/hipint.5000376.
- van Steenbergen L N, Denissen G A, Spooren A, van Rooden S M, van Oosterhout F J, Morrenhof J W, et al. More than 95% completeness of reported procedures in the population-based Dutch arthroplasty register. Acta Orthop 2015; 86(4): 498-505. doi: 10.3109/17453674.2015.1028307.
- LROI. LROI annual report. 2020. Available from: https://www.lroi-report.nl/previous-reports/online-lroi-report-2020/.
- NOV. PROMs-advies Orthopedie. 2020. Available from: https://www.orthopeden.org/downloads/775/nov-proms-advies.pdf.
- Davis A M, Perruccio A V, Canizares M, Hawker G A, Roos E M, Maillefert J F, et al. Comparative, validity and responsiveness of the HOOS-PS and KOOS-PS to the WOMAC physical function subscale in total joint replacement for osteoarthritis. Osteoarthritis Cartilage 2009; 17(7): 843-7. doi: 10.1016/j.joca.2009.01.005.
- Nilsdotter A K, Lohmander L S, Klassbo M, Roos E M. Hip disability and osteoarthritis outcome score (HOOS): validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003; 410. doi: 10.1186/1471-2474-4-10.
- Davis A M, Perruccio A V, Canizares M, Tennant A, Hawker G A, Conaghan P G, et al. The development of a short measure of physical function for hip OA HOOS-Physical function Shortform (HOOS-PS): an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008; 16(5): 551-9. doi: 10.1016/j.joca.2007.12.016.
- Ram N, Grimm K J. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev 2009; 33(6): 565-76. doi: 10.1177/0165025409343765.
- Nylund K L, Asparouhov T, Muthén B O. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model 2007; 14(4): 535-69. doi: 10.1080/10705510701575396.
- Shrier I, Platt R W. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 870. doi: 10.1186/1471-2288-8-70.
- Zhang J, Yu K F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280(19): 1690-1. doi: 10.1001/jama.280.19.1690.
- Nagin D S. Group-based modeling of development. London, UK: Harvard University Press; 2005.
- Buirs L D, Van Beers L W, Scholtes V A, Pastoors T, Sprague S, Poolman R W. Predictors of physical functioning after total hip arthroplasty: a systematic review. BMJ Open 2016; 6(9): e010725. doi: 10.1136/bmjopen-2015-010725.
- Rolfson O, Donahue G S, Hallsten M, Garellick G, Kärrholm J, Nemes S. Patient-reported outcomes in cemented and uncemented total hip replacements. Hip Int 2016; 26(5): 451-7. doi: 10.5301/hipint.5000371.
- Bagaric I, Sarac H, Borovac J A, Vlak T, Bekavac J, Hebrang A. Primary total hip arthroplasty: health related quality of life outcomes. Int Orthop 2014; 38(3): 495-501. doi: 10.1007/s00264-013-2142-8.
- Rorabeck C H, Bourne R B, Mulliken B D, Nayak N, Laupacis A, Tugwell P, et al. The Nicolas Andry award: Comparative results of cemented and cementless total hip arthroplasty. Clin Orthop Relat Res 1996; (325): 330-44.
- Abdulkarim A, Ellanti P, Motterlini N, Fahey T, O’Byrne J M. Cemented versus uncemented fixation in total hip replacement: s systematic review and meta-analysis of randomized controlled trials. Orthop Rev (Pavia) 2013; 5(1): e8. doi: 10.4081/or.2013.e8.
- Lenguerrand E, Wylde V, Gooberman-Hill R, Sayers A, Brunton L, Beswick A D, et al. Trajectories of pain and function after primary hip and knee arthroplasty: the ADAPT cohort study. PLoS One 2016; 11(2): e0149306. doi: 10.1371/journal.pone.0149306.
- Neuprez A, Delcour J P, Fatemi F, Gillet P, Crielaard JM, Bruyere O, et al. Patients’ expectations impact their satisfaction following total hip or knee arthroplasty. PLoS One 2016; 11(12): e0167911. doi: 10.1371/journal.pone.0167911.
- Hafkamp F J, de Vries J, Gosens T, den Oudsten B L. High preoperative expectations precede both unfulfilled expectations and clinical improvement after total hip and total knee replacement. J Arthroplasty 2020; 35(7): 1806-12. doi: 10.1016/j.arth.2020.02.061.
- Rolfson O, Bohm E, Franklin P, Lyman S, Denissen G, Dawson J, et al. Patient-reported outcome measures in arthroplasty registries: report of the Patient-Reported Outcome Measures working group of the International Society of Arthroplasty Registries. Part II: Recommendations for selection, administration, and analysis. Acta Orthop 2016; 87(eSuppl.362): 19-23. doi: 10.1080/17453674.2016.1181816.
- McNutt L A, Hafner J P, Xue X. Correcting the odds ratio in cohort studies of common outcomes. JAMA 1999; 282(6): 529. doi: 10.1001/jama.282.6.529.
- Yu K F, Zhang J. Correcting the odds ratio in cohort studies of common outcomes (in reply). JAMA 1999; 282(6): 529. doi: 10.1001/jama.282.6.529.
- Patel J, Lee J H, Li Z, SooHoo N F, Bozic K, Huddleston J I 3rd. Predictors of low patient-reported outcomes response rates in the California joint replacement registry. J Arthroplasty 2015; 30(12): 2071-5. doi: 10.1016/j.arth.2015.06.029
Supplementary data
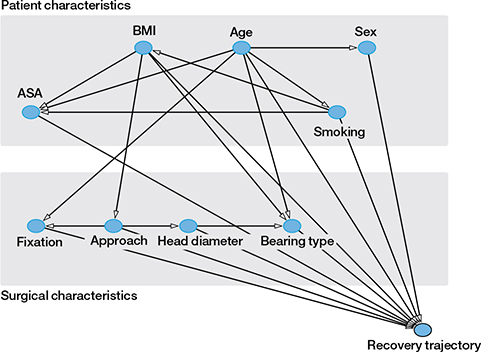
Figure 2. Directed acyclic graph showing possible relationship between risk factors and recovery trajectory after primary total hip arthroplasty. Confounding variables were selected based on the directed acyclic graph: BMI was adjusted for smoking; ASA was adjusted for age, BMI, and smoking; Smoking was adjusted for age; Approach was adjusted for BMI; Fixation was adjusted for age and approach; Head diameter was adjusted for approach; Bearing type was adjusted for age, BMI, and head diameter; No adjustments were included in the analysis of age and sex.