Bioactive glass granules versus standard autologous and allogeneic bone grafts: a randomized trial of 49 adult bone tumor patients with a 10-year follow-up
Hannu T ARO 1, Ville-Valtteri VÄLIMÄKI 1, Niko STRANDBERG 1, Petteri LANKINEN 1, Eliisa LÖYTTYNIEMI 2, Virva SAUNAVAARA 3, and Marko SEPPÄNEN 4
1 Department of Orthopaedic Surgery and Traumatology, Turku University Hospital and University of Turku, Turku; 2 Unit of Biostatistics, Department of Clinical Medicine, University of Turku, Turku; 3 Turku PET Center, University of Turku and Turku University Hospital, and Department of Medical Physics, Division of Medical Imaging, Turku University Hospital, Turku; 4 Department of Clinical Physiology, Nuclear Medicine and Turku PET Centre, University of Turku and Turku University Hospital, Turku, Finland
Background and purpose — As a synthetic bone void filler, bioactive glasses (BGs) may enhance angiogenesis and osteogenesis. In this randomized trial, we compared the clinical efficacy of BG granules and standard bone grafts in patients undergoing surgery for benign bone tumors.
Patients and methods — 49 recruited patients were randomized to receive BG granules or undergo conventional bone grafting to fill defects following tumor removal. As the standard of care, small-sized defects were filled with autologous graft, and large-sized defects were filled with allogeneic graft. The primary endpoint was treatment success at 1 year, defined by no reoperation, no tumor recurrence, and no device-related adverse events. Secondary endpoints included patient-reported outcomes (Rand-36 and pain scores) and quantitative assessment of blood flow and metabolic activity by means of 18F-fluoride PET/CT imaging. As an off-trial group, 15 children and adolescents (age < 18 years) underwent tumor removal and BG-filling, without randomization.
Results — At 1-year, 21 of 25 BG-treated patients (risk estimate 0.84, 95% confidence interval [CI] 0.70–0.98) and 20 of 24 patients in the standard of care group (0.83, CI 0.68–0.98) met the criteria for treatment success. The groups had similar Rand-36 scores. In patients with small defects, BG filling was associated with shorter operative time and less postoperative pain at 1 month. In patients with large defects, blood flow was similar, but BG-filled defects showed higher metabolic activity than allograft-filled defects at 1-year. The survey of the postoperative period ≥10 years revealed no BG-related adverse events.
Interpretation — BG granules had similar overall rates of treatment success compared with autografts and allografts, but large-scale trials are needed for the confirmation of clinical equivalence. The extended metabolic activity confirms the expected cellular responses of osseointegrated BG granules.
Citation: Acta Orthopaedica 2022; 93: 519–527. DOI http://dx.doi.org/10.2340/17453674.2022.2808.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-01-02. Accepted: 2022-05-02. Published: 2022-06-01.
Correspondence: hannu.aro@utu.fi
HTA: conceptualization, investigation, writing—original draft. V-VV: investigation. NS and PN: investigation. EL and VS: formal analysis. MS: methodology. All authors reviewed the manuscript.
Acta thanks Lars Nordsletten and Pietro Ruggieri for help with peer review of this study.
Among synthetic bone graft substitutes (1), silica-based bioactive glasses (BGs) have a unique mechanism for chemical bonding and continuous ionic interchange with new bone (2,3). As a result, the attached new bone undergoes high turnover with accelerated formation and resorption rates (4). Related to the dissolution products (5), BG granules may induce expression of angiogenic growth factors (6) and bone morphogenic protein-2 (7). In contrast with robust basic science reports, clinical evidence for enhanced angiogenesis and osteogenesis remains lacking.
Overall, published evidence of clinical efficacy has progressed slowly for all approved synthetic bone grafts. In bone tumor patients, most studies of bone graft substitutes remain observational and are rarely randomized (8-10). A randomized phase I trial compared BG granules (S53P4) with autogenous bone graft (11), but the clinical equivalence of the material has not been tested against allogeneic bone grafts.
Our randomized trial examined the clinical efficacy of BG granules and standard bone grafts in adult patients undergoing surgery for benign bone tumors. We hypothesized that BG granules (S53P4) are as good as traditional autogenous and allogeneic bone grafts in treatment of bone defects following tumor curettage. The primary endpoint was treatment success, defined by defect healing without surgical complications. Secondary endpoints included patient-reported outcome measures (PROMs) and quantitative assessment of blood flow and metabolic activity by means of hybrid dynamic 18F-fluoride positron emission tomography (PET) and computed tomography (CT) imaging.
Patients and methods
Trial design
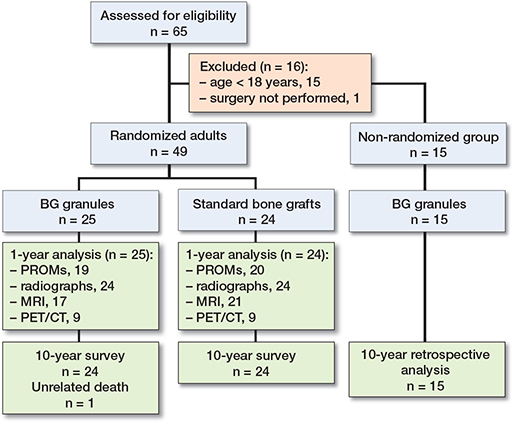
This single-center, investigator-initiated, randomized, active-controlled, open, phase II trial evaluated the efficacy of BG granules as a synthetic bone graft substitute. The subjects were recruited between January 1, 2007, and April 30, 2009. Patients were randomized to receive BG granules or undergo conventional bone grafting to fill surgically created defects following tumor removal (Figure 1). As the standard of care, small-sized control defects were filled with autologous bone grafts, and large-sized control defects were filled with allogeneic bone grafts. Postoperative recovery was evaluated at follow-up visits after 1, 6, and 12 months according to PROMs. 2-plane standard radiographs were used to evaluate defect healing. MRI, or supplemental CT for patients with metal-implant artifacts, was performed postoperatively and repeated at 1 year. Postoperative MRI images were used to evaluate the completeness of surgical curettage and defect filling, and 1-year MRI images were applied for the exclusion of tumor recurrence. In patients with large-sized defects, dynamic 18F-fluoride PET/CT was performed at 1 month and 1 year for quantitative assessment of local angiogenesis and osteogenesis. As the safety measure, the occurrence of adverse events, serious adverse events (SAEs), and adverse medical device reactions was recorded. SAEs were defined as tumor recurrence or any event that required inpatient hospitalization. The original 1-year follow-up was extended, because the prolonged persistence of synthetic bone-graft substitutes, such as ß-tricalcium phosphate (10) and BG granules (11,12), may serve as a potential stress riser. Long-term adverse events were evaluated based on the review of electronic medical records at 10 years.
Eligibility criteria, randomization, and stratification
Patients scheduled to undergo bone tumor surgery at Turku University Hospital were recruited. Adult patients (≥ 18 years) with a benign intraosseous bone tumor or a tumor-like skeletal disease were eligible to participate in the trial. Giant cell tumors of the long bones were not included due to the preferred use of polymethylmethacrylate filling. Exclusion criteria included a history of any malignancy, a medication affecting bone metabolism, or a contraindication for MRI.
Among eligible subjects, there were no refusals to participate in randomization. The enrolled patients were randomly allocated in a 1:1 ratio into 2 groups (Table 1) using a computer-generated random sequence, stratified by lesion size, with the planned inclusion of 24 subjects per group. Smallsized lesions were defined as lesions amenable to filling with autografts. No established standards exist to determine when a defect is sufficiently large to require allogeneic bone grafting. Therefore, the operating surgeon selected between autologous or allogeneic bone grafting according to subjective preoperative estimations of graft volume. This pragmatic selection was made on the day of surgery before the electronic opening of the randomization code performed by the external trial coordinator. This mechanism ensured the concealment of the random allocation sequence at the time of enrollment.
Non-randomized group
Children and adolescents younger than 18 years (n = 15) (Table 2) underwent surgery and defect filling with BG granules, without randomization (Figure 1), between December 21, 2006, and November 17, 2010. At 10 years, their clinical outcomes and postoperative radiographs were retrospectively reviewed from electronic medical records. In this review, the median postoperative follow-up time was 4.5 years (1–11). The success of treatment in children was defined by the absence of reported tumor residuals, recurrences, revision surgery for any reason, or BG-related adverse events.
Surgical technique
A standardized surgical technique was applied. Using a cortical bone window, the lesion was removed with a curette and a dental burr. Thin walls of bone cysts were scraped. No local adjuvants were used after curettage. After BG filling or bone grafting of the resultant defect, the cortical window was closed. Prophylactic internal fixation was performed when indicated (8 patients). The curetted tissue was sent for histopathologic examination. Surgery for a pathologic fracture (encountered exclusively in the hand) was delayed until the fracture healed (8 patients).
Autologous and allogeneic bone grafting
Autologous bone grafts were harvested from the anterior iliac crest using a standard technique (13). Using tissue bank protocols, allogeneic cancellous bone chips were obtained from fresh-frozen femoral heads retrieved during total hip arthroplasty. To reduce immunogenicity, allogeneic bone chips were defatted by pulse-lavage washing before implantation.
Synthetic bone graft substitute
The investigated BG (S53P4; BonAlive Biomaterials Ltd [formerly Vivoxid Ltd], Turku, Finland) is a bioresorbable, synthetic bone graft substitute, consisting of 53% SiO2, 23% Na2O, 20% CaO, and 4% P2O5. The product was approved in Europe in 2006 and in the Unites States in 2012 for intended use as a bone void filler. The BG composition was previously investigated in preclinical (4,14) and clinical (11,12,15) studies of bone defects. Granule sizes between 2.0 and 3.15 mm were used to fill large defects, and 0.8 to 1.0 mm granules were used to fill small defects. The cost of filling of a large defect (20 cm3) was €1,500 with BG granules and €980 with a bone banked allogeneic femoral head. Using prefilled applicators, granules were implanted without any vehicle or blood/bone marrow addition.
18F-Fluoride PET/CT imaging
Dynamic 18F-fluoride PET/CT (16) was performed using a hybrid scanner containing a PET and a 64-slice CT (Discovery VCT, General Electric Medical Systems, Milwaukee, WI, USA). The system produces 47 axial planes, with a slice thickness of 3.75 mm and a total transaxial field of view of 15.2 cm. The image matrix size was 128 × 128, with a 70-cm field of view. The PET study duration was 60 minutes, and data was reconstructed into 27 different dynamic frames (5 × 20 seconds, 5 × 40 seconds, 5 × 60 seconds, 5 × 180 seconds, and 7 × 300 seconds) using a 3D iterative method with 2 iterations and 28 subsets. All quantitative corrections, including attenuation, scatter, and random correction; detector normalization; and dead time, were included in the reconstruction. A diagnostic CT scan (120 kVp, 30–120 mA, noise index 15) was performed for PET attenuation correction. After the intravenous bolus injection of 18F-fluoride (318 [SD 47] MBq), the uptake data of the initial frames (5 minutes) was used as a blood-pool phase marker, and the last frame (300 seconds) was used as a static-phase marker of metabolic activity.
Reconstructed PET images, fused with CT images, provided a visual demonstration of the tracer uptake. Uptake was measured in regions of interest (ROIs), including the average values of 3 ROIs (ROI2D) and 3 reference ROIs (ROIref) from the reference intact bone (range of voxels, 25–30). An additional 3D ROI for the filled defect (ROI3D; 474–485 voxels) was analyzed, when feasible. ROIs were maintained constant by selecting the equivalent set of slices through the treated defects for 1-month and 1-year imaging. 18F-fluoride uptake was analyzed as standardized uptake values (SUVs), which were calculated as the measured tissue radioactivity divided by the relative injected dose, expressed per kilogram of bodyweight. Spatial and temporal differences in uptake were observed in the peripheral and central regions of healing defects. Therefore, both the peak (SUVmax) and mean (SUVmean) values were recorded for all ROIs. The ratios (SUVratio) between the SUVmean values of filled defects (ROI2D) and reference regions (ROIref) were also calculated.
Radiographs and MRI/CT images
Standard imaging procedures aimed for bone tumor patients (17) were adopted. Our contrast-enhanced MRI protocol included conventional signal sequences and dynamic imaging with intravenous gadolinium. Supplemental CT imaging was performed without contrast enhancement. Independent musculoskeletal radiologists examined the images for the success of tumor curettage and defect treatment.
Using preoperative radiographs and MRI images, the tumor volume was calculated using the measured anterior–posterior and mediolateral dimensions and the height (18). The success of tumor curettage and defect treatment was classified using the Neer Classification (19), modified to include the information provided by MRI/CT. Complete evacuation and defect healing were classified as Grade 1. Grade 2 represented successful defect healing with suspicion of static minor residua on MRI/CT. Grade 3 represented tumor recurrence without reoperation. Grade 4 represented patients with reoperation for any reason.
Statistics
The original primary endpoint was defined as defect healing examined by plain radiographs and MRI. This definition was aimed to represent radiological healing without complications. In order to emphasize this fact, the primary endpoint was renamed treatment success, defined by Grade 1–2 defect healing on the modified Neer Classification, with no reoperation for any reason, no tumor recurrence, and no devicerelated adverse events. The between-group difference for treatment success, together with 95% confidence interval (CI), was tested with a chi-square test.
Secondary endpoints included the physical (PCS) and mental (MCS) summary components of the Rand-36 survey (20) and pain assessments using the visual analog scale (VAS). Between-group differences of these PROMs were analyzed by a mixed-model repeated-measures analysis for the subcohorts of patients with small-sized and large-sized defects. The leastsquares mean differences with CI are presented.
SUVmean values of 18F-fluoride uptake for ROI2D were used to estimate blood flow and metabolic activity as secondary endpoints. Due to occasional non-normality, between-group differences and within-group changes were tested using a 2-sided Mann–Whitney U test and Wilcoxon signed rank test, respectively. Results are presented as mean differences with 95% confidence intervals (CI). Treatment group difference for the occurrence of adverse events and SAEs was tested with a chi-square test.
The frequency of missing data varied between 4% and 15% for 2-plane radiographs (number of examinations, n = 245), PROMs (n = 343), and MRI imaging (n = 147). 4 subjects did not undergo either of the 2 scheduled PET/CT sessions (n = 50), and 3 additional subjects missed either the first or second imaging session.
P < 0.05 was considered significant. Data was analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and SPSS Statistics version 25.0 (IBM Corp, Armonk, NY, USA).
Ethics, registration, data sharing plan, funding, and potential conflicts of interest
All participants provided written informed consent. The Ethics Committee of the Hospital District of South–West Finland approved the protocol (2/2003/49 and 4/2006/193). The trial is registered at clinicaltrials.gov (NCT01304121). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Data may be shared after ethical approval and consent of the principal investigator. Funding was received from the Finnish Government, Instrumentarium Foundation, Emil Aaltonen Foundation and the Finnish Medical Foundation. Individual potential conflicts of interests: V-VV, NS, PL, EL, VS, MS: none, HTA: founding partner of former Vivoxid Ltd, Finland.
Results
Patient characteristics
All 49 patients (25 in the BG group and 24 in the graft group) were available for the primary outcome analyses at 1 year (Figure 1). The baseline characteristics of the BG-treated patients and the standard-of-care group were similar (see Table 1). In patients with small-sized defects, the operative time was statistically significantly shorter in patients undergoing BG filling (Table 1) (mean difference 35 minutes, CI 8–61).
Primary endpoint
At 1 year, 21 of 25 BG-treated patients (risk estimate 0.84, CI 0.70–0.98) and 20 of 24 graft-treated patients (0.83, CI 0.68–0.98) met the criteria for treatment success (difference 0.01, CI -0.20–0.21).
Radiographs and MRI/CT images
Radiographs showed the rebuilding of bone structure in grafttreated defects and incorporation of granules in BG-filled defects within 1 year. The sharp margins of BG granules disappeared with time (Figure 2).
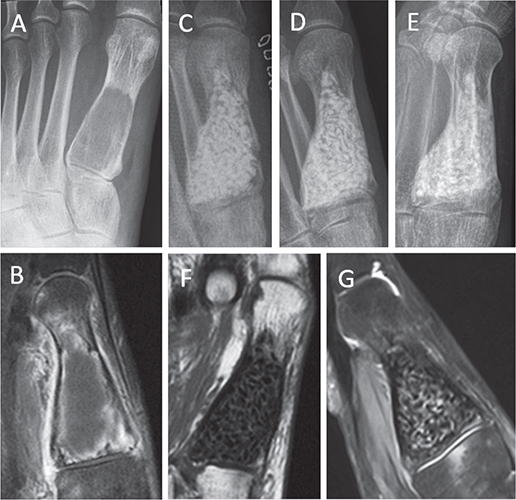
Figure 2. Giant cell granuloma of the first metatarsal bone with corticalbone thinning and an undisplaced pathologic fracture in a 42-year-old female (A–B). Radiographs immediately after tumor evacuation and filling with BG granules (C), at 1 year (D) and at 3 years (E) showed incorporation and gradual changes of BG granules. Postoperative MRI images demonstrated no signs of residuals (T1-weighted sequence) (F) and no tumor recurrence at 1-year (T2-weighted sequence with contrast enhancement) (G).
MR imaging gave accurate information regarding the completeness of tumor evacuation and the success in filling of the resultant defect (Figure 2). On postoperative MRI images, contrast enhancement helped to distinguish any residua of the primary tumor from postoperative oedema and enhancement of granular tissue at the periphery of the evacuated lesion. Static minor residua (Grade 2) were found in 10 patients (Table 3). These lesions did not progress with time.
At 1 year, MR images showed reconstitution of cancellous bone structure in autografted defects, incorporation of allogeneic grafts in large defects, and the healing of cortical bone windows independently of the filling material. MR images were not amenable to the measurement of new bone within narrow spaces between BG granules. BG granules showed low signals of T1, T2 weighted imaging and did not exhibit remarkable dissolution with time.
Patient-reported outcomes
No statistically significant differences in the PCS and MCS scores of Rand-36 were observed between the BG group and the autograft group or between the BG group and the allograft group (Figure 3). Compared with autografts, BG filling was associated with less pain at 1 month (p < 0.001; Figure 3).
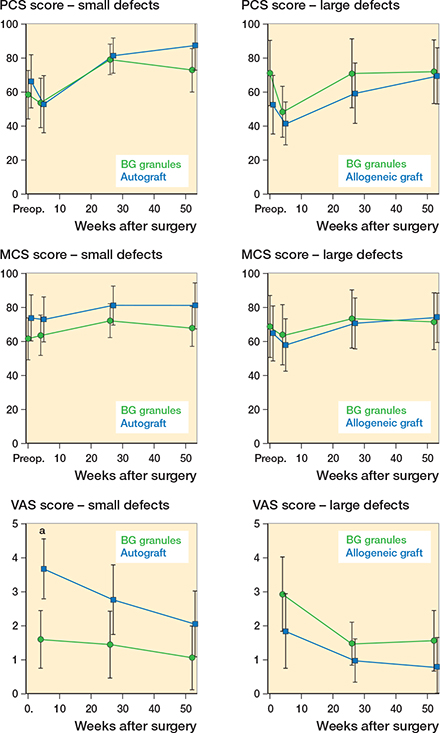
Figure 3. Patient-reported outcomes. PCS and MCS scores of Rand-36 and VAS scores in patients with small defects treated by BG filling or autografts and in patients with large defects treated by BG filling or allografts. The values represent the least-squares means with CI. a statistically significant.
Dynamic 18F PET/CT imaging
The treatment groups showed similar blood flow in filled defects. The result was independent of the ROI (ROI3D or ROI2D), the method of measurement (SUVmax or SUVmean), and the postoperative time point (1 month or 12 months). SUVmean values of blood flow were many times higher in treated defects than in intact bone regions (ROIref) at 1 month (Table 4). Based on SUVmean values of ROI2D, blood flow decreased statistically significantly by 1 year both in BGtreated and allograft-treated defects (Table 4). Compared with intact bone regions, the SUVratio values of blood flow were 4.2 (CI–1.2 to 9.D6) in BG-filled defects and 1.8 (CI 1.0–2.5) in allograft-filled defects at 1-year (difference 2.5, CI –2.5 to 7.5).
The 2 groups showed no statistically significant difference in metabolic activity at 1 month, assessed by SUVmean values of ROI2D for the 18F-fluoride uptake (Table 4). In BG-filled defects, the metabolic activity decreased slower and was higher (p = 0.01) than in allograft-filled defects at 1 year. Compared with metabolic activity of intact bone regions, BG-filled defects also showed higher SUVratio values (4.5, CI 3.0–6.0) than allograft-filled defects (2.6, CI 1.6–3.7) (difference 1.9, CI 0.2–3.6) (p = 0.04) at 1 year.
Intact bone regions (ROIref) did not show statistically significant time-related within-group changes or between-group differences in blood flow and metabolic activity (Table 4).
SAEs and adverse events
No reoperations were performed to enhance defect healing. Based on the categorization (Table 3), treatment failures (Grade 3 or 4) occurred in 8 patients. 3 patients from both groups experienced local recurrence. 2 additional patients, 1 in each group, underwent reoperation for postoperative fracture. The total number of SAEs and adverse events was 5 in the BG group and 10 in the graft group (p = 0.1) (Table 5, see Supplementary data). 3 patients who underwent autograft harvesting experienced donor-site pain for more than 1 month. No adverse BG-related medical device reactions were recorded. There were no late fractures of BG-filled bone regions.
Non-randomized group
Of 15 children and adolescents, 12 had complete clinical recovery without reoperation or residual symptoms. The successful outcomes included 2 children who were referred for treatment of recurrent aneurysmal bone cysts following allogeneic bone grafting. 3 children underwent reoperation. A 4-year-old boy with an aneurysmal bone cyst of the tibia underwent surgical exploration, but the postoperative radiographic change was not a recurrence (Figure 4). A 14-year-old girl with fibrous dysplasia of the proximal femur encountered treatment-resistant recurrence. A 9-year-old boy had a recurrence of an osteofibrous dysplasia of the anterior tibial cortex as a low-grade malignant osteofibrous dysplasia-like adamantinoma.
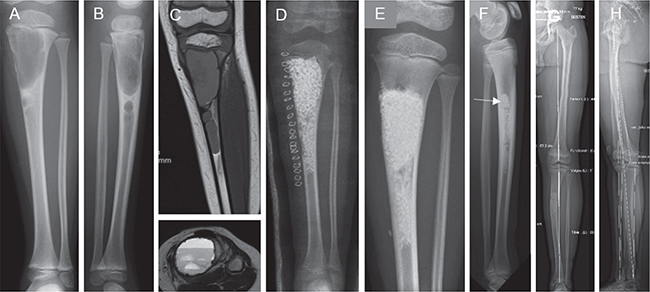
Figure 4. Large aneurysmal bone cyst of the proximal tibia in a 4-year-old boy. On admission (A–C), there was severe thinning of the medial cortex with an emerging pathologic fracture. The surgery involved thorough curettage and filling with BG granules close to the proximal growth plate (D). Control radiographs at 17 months showed solid regrowth of the cortical bone and bone growth (E). At the 10-year follow-up, the middle part of the tibia showed remnants of BG granules. The arrow indicates the site of surgical exploration performed to exclude disease recurrence (F). There was a slight overgrowth of the affected tibia (+3 mm) with mild medial bowing of the shaft. The mechanical loading axis was unaffected and the functional outcome was excellent (G–H).
Discussion
In our pragmatic active-controlled trial, synthetic BG granules had similar overall rates of success compared with autografts in the treatment of small defects and with allografts in the treatment of large defects, but the clinical equivalence could not be confirmed due to the large CI for the difference of treatment success. In dynamic 18F-fluoride PET/CT imaging, BG-filled defects showed higher metabolic activity than allograft-filled defects at 1 year, confirming the expected cellular responses of osseointegrated BG granules. There were no treatment group differences in blood flow.
This trial represents the first clinical evaluation of a synthetic bone graft by means of 18F-fluoride PET/CT imaging, extending previous studies of bone allografts (21-23). Using18F-PET/CT, the numeric estimation of blood flow requires a plasma clearance technique, whereas the measurement of SUV values provides a semiquantitative approach (16). A previous 18F-fluoride PET study of bone tumor patients showed a 60% to 65% postoperative decrease of metabolic activity in allogeneic cancellous bone grafts at 2 years (23). In our study, BG-filled and allograft-filled defects showed high blood flow and metabolic levels as early as 1 month after operation. The likely explanation is strong blood flow-dependent angiogenesis and osteogenesis from the surrounding healthy bone (24), overshadowing any angiogenic and osteopromotive effects of BG granules. Like the original observation, the metabolic activity remained elevated for BG-filled defects at 1 year, confirming preclinical results regarding high turnover of the attached new bone (4). The clinical significance of high metabolic activity in BG-filled defects remains open. High turnover of attached new bone is associated with slow dissolution of BG granules (4). Interestingly, as reported previously (11,12), slow replacement of BG granules particularly in large defects seems to promote the cortical bone to increase in thickness.
The slow dissolution of BG granules over many years is a safety feature to control the released ion levels, particularly silicon (Si). BG compositions with too high reactivity may cause toxic tissue reactions (2). Commercialized BG compositions have controlled surface activity. In line with the longterm results of the phase I trial (12), our survey of the postoperative period ≥ 10 years revealed no BG-related adverse events.
Despite the use of preoperative MRI to outline the lesion margins, our MRI monitoring revealed the presence of nonprogressive scanty residuals (Grade 2). Previous studies have rarely applied MRI to evaluate the success of intralesional tumor curettage. Our rate for reoperations and recurrences (8 of 49 patients) was in line with the reported rate in the literature (8,25,26). The recurrence of fibrous dysplasia in the proximal femur confirms recent evidence that the disease is resistant to surgical evacuation and bone grafting (27). The observed malignant transformation of osteofibrous dysplasia of the tibia is concordant with the complex nature of the disease (28).
We are reluctant to use allografts in children due to their high immunologic capacity to respond to histocompatibility antigen disparities. Our supervised use of BG granules in children was efficacious. We observed no recurrences of aneurysmal and simple bone cysts in children treated with BG granules, and even recurrent aneurysmal bone cysts responded to treatment. A retrospective multicenter study of 18 children showed a recurrence in BG-filled aneurysmal bone cysts of 2 children (15). The systemic release of Si ions from large BG-filled cysts may be a concern, although BG filling is not associated with increased serum Si levels in adults (29). BG granules may also have an influence on open physes. However, no growth arrest or major overgrowth were observed, even after BG granule implantation next to growth plates (Figure 4).
Limitations
The sample size selection was not based on a power calculation because the treatment effect of allogeneic bone graft was unknown. The limitations of this study included failure to perform all scheduled study assessments. Clinical noninferiority testing was not feasible due to the small study population with wide CI for the difference of treatment success. Therefore, definitive large-scale trials are necessary. Based on the current data and the recommended 10% noninferiority margin, the required sample size would be approximately 290 patients (145 per group). Our trial would have benefited from a control group of unfilled small-sized defects. Largesized defects carry an increased fracture risk if left empty after evacuation, but the absolute need for a bone-void filler for small-sized defects has been questioned (26,30). Our results are applicable only to the investigated graft substitute, as BG composition, particle size, and particle size range might have biological effects. High-resolution CT imaging allowed for the measurement of osteogenesis between BG granules in a preclinical model (14), but neither MRI nor CT was useful for the measurement of new bone volume as a reference for PET/CT results.
Conclusion
These results suggest the potential of BG granules in the treatment of contained bone defects, independent of defect size, but large-scale trials remain necessary to confirm the noninferiority compared with standard bone grafts. Especially in large defects with a need for large amounts of grafting material, not only the biologic efficacy and safety but also the cost-effectiveness is important. As a cost-saving feature, BG granule filling does not appear to require biologic augmentation, such as adjunctive use of bone marrow aspirate. Our study urges that further clinical trials with dynamic 18F-fluoride PET/CT imaging be undertaken to delineate biologic responses to synthetic bone grafts.
- Kurien T, Pearson R G, Scammell B E. Bone graft substitutes currently available in orthopaedic practice. Bone Joint J 2013; 95-B(5): 583-97. doi: 10.1302/0301-620X.95B5.30286.
- Hench L L. The story of Bioglass®. J Mater Sci Mater Med 2006; 17(11): 967-78. doi: 10.1007/s10856-006-0432-z.
- Jones J R. Review of bioactive glass: From Hench to hybrids. Acta Biomater 2013; 9(1): 4457-86. doi: 10.1016/j.actbio.2012.08.023.
- Välimäki V-V, Aro H T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand J Surg 2006; 95(2): 95-102. doi: 10.1177/145749690609500204.
- Hoppe A, Güldal N S, Boccaccini A R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011; 32(11): 2757-74. doi: 10.1016/j.biomaterials.2011.01.004.
- Gorustovich A A, Roether J A, Boccaccini A R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B Rev 2010; 16(2): 199-207. doi: 10.1089/ten.teb.2009.0416.
- Gao T, Aro H T, Ylänen H, Vuorio E. Silica-based bioactive glasses modulate expression of bone morphogenetic protein-2 mRNA in Saos-2 osteoblasts in vitro. Biomaterials 2001; 22(12): 1475-83. doi: 10.1016/S0142-9612(00)00288-X.
- Ogose A, Hotta T, Kawashima H, Kondo N, Gu W, Kamura T, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res 2005; 72B(1): 94-101. doi: 10.1002/jbm.b.30136.
- Kim J H, Oh J H, Han I, Kim H-S, Chung S W. Grafting using injectable calcium sulfate in bone tumor surgery: comparison with demineralized bone matrix-based grafting. Clin Orthop Surg 2011; 3(3): 191-201. doi: 10.4055/cios.2011.3.3.191.
- Damron T A, Lisle J, Craig T, Wade M, Silbert W, Cohen H. Ultraporous β-tricalcium phosphate alone or combined with bone marrow aspirate for benign cavitary lesions: comparison in a prospective randomized clinical trial. J Bone Joint Surg Am 2013; 95(2): 158-66. doi: 10.2106/JBJS.K.00181.
- Lindfors N C, Heikkilä J T, Koski I, Mattila K, Aho A J. Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res Part B Appl Biomater 2008; 90B(1): 131-6. doi: 10.1002/jbm.b.31263.
- Lindfors N C, Koski I, Heikkilä J T, Mattila K, Aho A J. A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater 2010; 94(1): 157-64. doi: 10.1002/jbm.b.31636.
- Shaw K A, Griffith M S, Shaw V M, Devine J G, Gloystein D M. Harvesting autogenous cancellous bone graft from the anterior iliac crest. JBJS Essent Surg Tech 2018; 8(3): e20. doi: 10.2106/JBJS.ST.17.00068.
- Välimäki V-V, Moritz N, Yrjans J J, Dalstra M, Aro H T. Peripheral quantitative computed tomography in evaluation of bioactive glass incorporation with bone. Biomaterials 2005; 26(33): 6693-703. doi: 10.1016/j.biomaterials.2005.04.033.
- Syvänen J, Nietosvaara Y, Kohonen I, Koskimies E, Haara M, Korhonen J, et al. Treatment of aneurysmal bone cysts with bioactive glass in children. Scand J Surg 2018; 107(1): 76-81. doi: 10.1177/1457496917731185.
- Blake G M, Puri T, Siddique M, Frost M L, Moore A E B, Fogelman I. Site specific measurements of bone formation using [18F] sodium fluoride PET/CT. Quant Imaging Med Surg 2018; 8(1): 47-59. doi: 10.21037/qims.2018.01.02.
- Woertler K. Benign bone tumors and tumor-like lesions: value of cross-sectional imaging. Eur Radiol 2003;13(8):1820-35. doi: 10.1007/s00330-003-1902-z.
- Dierselhuis E F, Gerbers J G, Ploegmakers J J W, Stevens M, Suurmeijer A J H, Jutte P C. Local treatment with adjuvant therapy for central atypical cartilaginous tumors in the long bones. J Bone Joint Surg 2016; 98(4): 303-13. doi: 10.2106/JBJS.O.00472.
- Neer C S, Francis K C, Marcove R C, Terz J, Carbonara P N. Treatment of unicameral bone cyst: a follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am 1966; 48-A(4): 731-45. doi: 10.2106/00004623-196648040-00006.
- Laucis N C, Hays R D, Bhattacharyya T. Scoring the SF-36 in orthopaedics: a brief guide. J Bone Joint Surg Am 2015; 97(19): 1628-34. doi: 10.2106/JBJS.O.00030.
- Piert M, Winter E, Becker G A, Bilger K, Machulla H, Müller-Schauenburg W, et al. Allogenic bone graft viability after hip revision arthroplasty assessed by dynamic [18F]fluoride ion positron emission tomography. Eur J Nucl Med Mol Imaging 1999; 26(6): 615-24. doi: 10.1007/s002590050429.
- Ullmark G, Sörensen J, Långström B, Nilsson O. Bone regeneration 6 years after impaction bone grafting: a PET analysis. Acta Orthop 2007; 78(2): 201-5. doi: 10.1080/17453670710013681.
- Brenner W, Vernon C, Conrad E U, Eary J F. Assessment of the metabolic activity of bone grafts with (18)F-fluoride PET. Eur J Nucl Med Mol Imaging 2004; 31(9): 1291-8. doi: 10.1007/s00259-004-1568-z.
- Kusumbe A P, Ramasamy S K, Adams R H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014; 507(7492): 323-8. doi: 10.1038/nature13145.
- Horstmann P F, Hettwer W H, Kaltoft N S, Petersen M M. Early clinical and radiological experience with a ceramic bone graft substitute in the treatment of benign and borderline bone lesions. Sci Rep 2018; 8(1): 15384. doi: 10.1038/s41598-018-33736-w.
- Sassoon A A, Fitz-Gibbon P D, Harmsen W S, Moran S L. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am 2012; 37(6): 1229-34. doi: 10.1016/j.jhsa.2012.03.019.
- Leet A I, Boyce A M, Ibrahim K A, Wientroub S, Kushner H, Collins M T. Bone-grafting in polyostotic fibrous dysplasia. J Bone Joint Surg 2016; 98(3): 211-19. doi: 10.2106/JBJS.O.00547.
- Most M J, Sim F H, Inwards C Y. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg 2010; 18(6): 358-66. doi: 10.5435/00124635-201006000-00008.
- Lindfors N C, Heikkilä J T, Aho A J. Long-term evaluation of blood silicon and ostecalcin in operatively treated patients with benign bone tumors using bioactive glass and autogenous bone. J Biomed Mater Res Part B Appl Biomater 2008; 87B(1): 73-6. doi: 10.1002/jbm.b.31070.
- Hirn M, de Silva U, Sidharthan S, Grimer R J, Abudu A, Tillman R M, et al. Bone defects following curettage do not necessarily need augmentation. Acta Orthop 2009; 80(1): 4-8. doi: 10.1080/17453670902804505.