Birthweight correlates to pubo-femoral distances and α angles in hip ultrasound of newborns at 6 weeks of age: a retrospective cohort study
Maria TIRTA 1, Michel Bach HELLFRITZSCH 2,3, Rikke Damkjær MAIMBURG 2,4-6, Mads HENRIKSEN 2,3, Natallia LAPITSKAYA 2,3, Søren KOLD 1,2, Bjarne MØLLER-MADSEN 2,7, Ole RAHBEK 1,2, and Hans-Christen HUSUM 1,2
1 Interdisciplinary Orthopaedics, Aalborg University Hospital, Aalborg; 2 Danish Paediatric Orthopaedic Research, Aarhus University Hospital, Aarhus; 3 Department of Radiology, Aarhus University Hospital, Aarhus; 4 Department of Midwifery, University College of Northern Denmark, Aalborg; 5 Department of Clinical Medicine, Aarhus University, Aarhus; 6 Department of Occupational Medicine, Danish Ramazzini Centre, Aarhus University Hospital, Aarhus; 7 Department of Children’s Orthopaedics, Aarhus University Hospital, Aarhus, Denmark
Background and purpose — There is inconsistency in the literature regarding the relationship between increased birthweight and risk of developmental dysplasia of the hip (DDH). We aimed to investigate the correlation between birthweight and pubo-femoral distance (PFD), as well as Graf’s α angle in newborns undergoing hip ultrasound examination at 6 weeks of age.
Patients and methods — Basic newborn characteristics and ultrasound measurements were retrospectively collected during a 1-year study period. We excluded multiple births, newborns born at less than 37 gestational weeks, and incomplete information. Simple and multiple linear regression analyses were performed to evaluate the correlation of birthweight and PFD, and, second, birthweight and α angles including a stratified regression analysis investigating the potential effect modification of sex.
Results — 707 newborns (1,414 hips) were included. Mean birthweight was significantly higher for male newborns (P < 0.001). Increased birthweight was positively correlated to PFD values (crude coefficient 0.21, 95% confidence interval [CI] 0.10–0.32) and the correlation was still present after adjusting for sex, family history, and breech presentation (adjusted coefficient 0.18, CI 0.07–0.29). The stratified α angle model for the males was significant for both the crude coefficient (–0.73, CI –1.28 to –0.19) and the adjusted (–0.59, CI –1.15 to –0.03), and also for the females (crude coefficient –1.14, CI –1.98 to –0.31 and adjusted coefficient –1.15, CI –1.99 to –0.31).
Conclusion — We found that increased birthweight positively correlated to PFD, and negatively correlated to α angle, but this was not of clinical significance.
Citation: Acta Orthopaedica 2023; 94: 594–599. DOI: https://doi.org/10.2340/17453674.2023.26188.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-09-13. Accepted: 2023-11-05. Published: 2023-12-13.
Correspondence: mariatirta96@gmail.com, m.tirta@rn.dk
Study design and conception: all co-authors. Data acquisition: MBH, MH, NL, and HCH. Data analysis: MT and HCH. Writing: MT. Review: all authors.
Handling co-editor: Ilkka Helenius
Acta thanks Sophie Moerma, Michael Sussman, and an anonymous reviewer for help with peer review of this manuscript.
Developmental dysplasia of the hip (DDH), a disorder due to abnormal development of the acetabulum with or without hip dislocation, is the most common pediatric hip disorder [1]. The etiology of DDH is complex, thus multiple “risk factors” are used as referral criteria, and include breech position, female sex, and positive family history, for follow-up hip ultrasound in DDH screening [2]. Oligohydramnios, multiple births, and birthweight have also been proposed as increasing the risk of DDH [3-5].
The Graf ultrasound screening method in newborns for DDH is widely implemented [6] but requires training [7]. The Graf ultrasound method measures the α-angle. The pubo-femoral distance (PFD) was proposed as an accessible supplement to the α-angle [8]. PFD is a less complex ultrasound measurement for DDH screening supported by a high sensitivity and specificity for DDH [9] and is reliable when used by radiologists or technicians with limited experience in ultrasound [10].
There is inconsistency in the literature as to the relationship between birthweight and risk of DDH. Some have reported no or increasing correlation of birthweight and DDH [11,12]. Others found that increased birthweight was negatively correlated to α angles in hip ultrasound performed 7 days after birth, but only in females [13].
We aimed to investigate the correlation between birth-weight and PFD in DDH ultrasound examinations. Second, we wished to investigate whether increased birthweight is correlated to α angle in DDH ultrasound, and the influence of sex on this correlation.
Patients and methods
Study design
This was a retrospective observational study. Reporting follows the STROBE guidelines for reporting on observational studies [14].
Participants
We included newborns born at Aarhus University Hospital from October 2021 to October 2022 within 37–42 gestational weeks who were referred for follow-up hip ultrasound based on presence of risk factors (breech presentation, family history, multiple births, oligohydramnios, or clubfeet), positive clinical examination, a PFD above 5.1 mm or a combination of these factors. PFD as a referral criterion was introduced as part of the Danish Hip Screening project (DHS) at Aarhus University Hospital. Referred newborns received specialized hip ultrasound, ideally within 6 weeks or 2 weeks if clinical instability was detected upon screening.
From patients’ files we included birthweight, gestational age, sex, age at ultrasound examination, and hip ultrasound measurements. We excluded multiple births as newborns from multiple births are usually lighter, as well as premature newborns born at less than 37 gestational weeks. Newborns with incomplete data, defined as missing information on one or more variables, were also excluded.
Ultrasound examination
Hip ultrasound examination of referred newborns was made by 1 of 3 senior musculoskeletal radiologists experienced in pediatric hip ultrasound. α angles were obtained according to the methods described by Graf [6] (Figure 1a), and PFD measurements, defined as the minimal measurable distance between the medial femoral epiphysis and the ossified pubic bone while applying lateralizing stress to the hip, according to the methods described by Treguier [8] but with the child in the lateral examination position (Figure 1b). Ultrasound was performed using a high-frequency linear transducer (Canon Aplio i800; Canon Medical Systems, Tokyo, Japan).
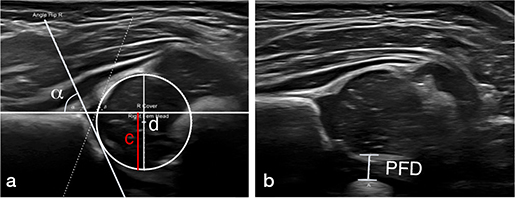
Figure 1. a. Ultrasound image of a newborn hip with annotated α angle and femoral head coverage (c/dx100) measurements, obtained according to the methods described by Graf [6] and Falliner [26]. b. Ultrasound image of a newborn hip with annotated PFD measurement, obtained according to the methods described by Tréguier et al. [8] but with the child in the lateral examination position.
Variables
Variables under investigation were sex (male/female), birthweight (gram), breech presentation (yes/no), family history of DDH (yes/no), positive clinical examination (yes/no), oligohydramnios (yes/no), congenital foot deformities (yes/no), referred based on primary PFD > 5.1 mm at first week after birth (yes/no), gestational age (days), and age at examination (days). Ultrasound measurements at 5–6 weeks were α angle (°) and second PFD measurement (mm), which were used for the correlation analysis.
Statistics
For descriptive statistics, continuous variables (birthweight, gestational age, age at examination, α angles, and PFD measurements) were reported as arithmetic mean and standard deviation (SD). For categorical variables (positive clinical examination, breech presentation, family history of DDH, oligohydramnios, congenital foot deformities), the number of observations and the relative percentages were reported. PFD and α-angle measurements from the follow-up hip ultrasound were used for the statistical analysis. As a first step, Pearson’s chi-square test and Fisher’s exact test (the latter when the expected count was < 5) were performed for categorical variables and t-test for the continuous variable to estimate possible differences between female and male newborns.
In a second step, simple linear regression analysis was performed to evaluate the potential association of birthweight (independent variable) and PFD (dependent variable), and, second, the potential association of birthweight (independent variable) and α angle (dependent variable). Multiple regression analysis was performed to evaluate the above-mentioned potential associations adjusted for 3 well-known risk factors (sex, breech presentation, and family history of DDH). As a third step, stratified regression analysis in male and female newborns was performed for birthweight and α angle, due to potential effect modification of sex between these 2 variables. The coefficient results are presented with 95% confidence interval (CI) and scatter plots were created to present the regression analysis results. To account for the bilaterality of data, a sensitivity analysis was performed using mixed-model analysis for all the previously mentioned simple and multiple linear regressions. As the analysis was not significantly different, independence between sides was assumed and the linear regression results are reported for simplicity.
Normal distribution of birthweight, PFD, and α angle measurements were inspected using QQ plots. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using Stata version 17.0 (StataCorp, College Station, TX, USA).
Ethics, registration, data sharing, funding, and disclosures
Written consent for data acquisition (prospectively collected data of PDF and α-angle measurements, as well as retrospective data such as birthweight) was obtained from both parents of the included newborns at the referred follow-up ultrasound. The study, the written patient information, and the protocol was approved by the institutional ethical committee (Ref no: N-20200051). This study was funded by the Independent Research Fund Denmark Grant no. 103000366B, Gigtforeningen, Health Professional Fund at Aarhus University, and Dagmar Marshall Fund. The authors declare no conflict of interest. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2023.26188
Results
Study population
Based on 4,694 newborns born during the 1-year study period, a cohort of 2,375 newborns (51%) participated in the Danish Hip Screening project following consent from their parents. Of these, 820 newborns (34%) were referred for ultrasound (Figure 2). 55 did not attend. Furthermore, 37 were multiple births and 13 had a gestational age < 37 weeks, leaving 707 newborns (1,414 hips) for inclusion in our study with equal distribution of males and females (348; 359). 431 newborns were referred based on their first PFD measurement witho having any other risk factor. Mean birthweight was significantly higher for male newborns at 3,689 g (SD 475) vs. 3,530 g (SD 439) (P < 0.001), while the total mean age at examination was 37 days (SD 11.9) (Tables 1 and 2). There was no difference between the 2 groups in the PFD measurements (PFD right side P = 0.7, PFD left side P = 0.4). The mean α-angle measurements for both left and right hip were significantly lower for female newborns (P < 0.001). Considering the risk factors, there was no difference between the groups (Table 2).
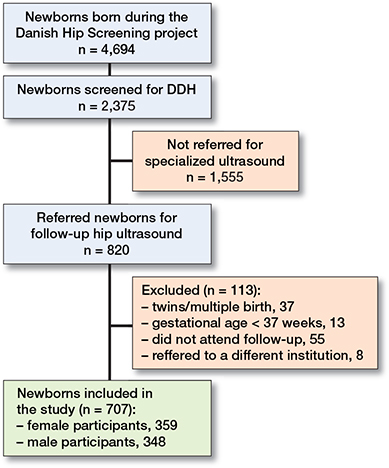
Figure 2. Flowchart of patients included in the study.
Birthweight and PFD
Increased birthweight was positively correlated to PFD values (crude coefficient 0.21, CI 0.10–0.32) and this correlation was still present when adjusting for sex, family history, and breech presentation (adjusted coefficient 0.18, CI 0.07–0.29). A regression model and a scatter plot were established according to the following: PFD = 3.27 (mm) + 0.18 (mm) × birthweight (kg) (Figure 3).
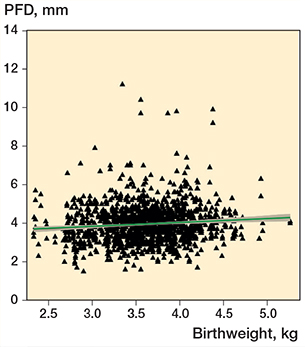
Figure 3. Scatter plot of birthweight and PFD values with fitted linear regression model on the effect of birthweight to PFD measurement (n = 707). PFD = pubo-femoral distance. The green line shows the fitted values, and the grey zone represents the CI.
Birthweight and α angle
Linear regression analyses showed a significant negative correlation between birthweight and α angles (crude coefficient –0.56, CI –1.06 to –0.07, P = 0.03), which was still present after adjusting for known risk factors and sex (adjusted coefficient –0.88, CI –1.38 to –0.38, P = 0.001), with sex being the largest contributor for this adjusted difference (Table 3). The regression analysis stratified by sex showed a significantly negative correlation between increased birthweight and α angles for both sexes but indicated a larger effect for females (Table 3). As a result, a regression model was established according to the following: females α angle = 69.3 – 1.15 × birthweight (kg), and males α angle = 69.0 – 0.59 × birthweight (kg). Figure 4 presents the scatter plot of these models.
| Linear regression for | Coefficient (CI) | P value | ||||||
| PFD and birthweight (per kg) | ||||||||
| Crude | 0.21 (0.09 to 0.32) | < 0.001 | ||||||
| Adjusted for sex | 0.22 (0.11 to 0.34) | < 0.001 | ||||||
| Adjusted for family history | 0.18 (0.08 to 0.29) | 0.001 | ||||||
| Adjusted for breech presentation | 0.20 (0.09 to 0.31) | < 0.001 | ||||||
| Adjusted for all above a | 0.18 (0.07 to 0.29) | 0.002 | ||||||
| α angle and birthweight (per kg) | ||||||||
| Crude | –0.56 (–1.06 to –0.07) | 0.03 | ||||||
| Adjusted for sex | –0.93 (–1.42 to –0.43) | < 0.001 | ||||||
| Adjusted for family history | –0.53 (–1.03 to –0.03) | 0.04 | ||||||
| Adjusted for breech presentation | –0.57 (–1.07 to –0.07) | 0.03 | ||||||
| Adjusted for all above a | –0.88 (–1.38 to –0.38) | 0.001 | ||||||
| α angle and birthweight (per kg): male | ||||||||
| Crude | –0.73 (–1.28 to –0.19) | 0.008 | ||||||
| Adjusted for family history and breech presentation | –0.59 (–1.15 to –0.03) | 0.04 | ||||||
| α angle and birthweight (per kg): female | ||||||||
| Crude | –1.14 (–1.98 to –0.31) | 0.007 | ||||||
| Adjusted for family history and breech presentation | –1.15 (–1.99 to –0.31) | 0.007 | ||||||
| a family history, breech presentation, and sex | ||||||||
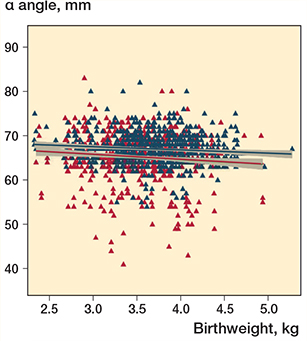
Figure 4. Scatter plot of regression model on the effect of birthweight to α-angle measurement for female (red) (n = 359) and male (blue) (n = 348) newborns. The red and blue lines show the fitted values, and the grey zones represents the CI.
Discussion
We aimed to investigate the correlation between birthweight and pubo-femoral distance (PFD), as well as Graf’s α angle in newborns undergoing hip ultrasound examination at 6 weeks of age. We found a positive correlation between increased birthweight and PFD measurements of newborns screened for DDH at 6 weeks of age, regardless of sex. Birthweight was found to be negatively correlated to α angles for both females and males. However, the correlations were too small to be considered clinically significant.
Although several risk factors have been examined in relation to DDH, birthweight still remains a controversial factor. The meta-analysis of De Hundt et al. [2], based on 4 studies [15-18], reported low birthweight (< 2,500 g) as being protective. This finding supported the concept of intrauterine crowding, as DDH may develop in part due to limited space for the fetus inside the uterus. 3 additional studies [11,19-20] have presented high birthweight (> 4 kg) as a risk factor, and Falliner et al. [21] found that children with dysplastic hips had markedly higher birthweight than children with normal hips. The cohort study by Schams et al. [20] suggested low birthweight as a protective factor. Hanratty et al. [12] found no correlation between increased birthweight and risk of DDH.
However, a dichotomous DDH diagnosis as an outcome measurement, while clinically relevant, might not be sensitive enough to show the possible contribution of birthweight, and as a result the use of α-angle measurement or PFD as an outcome seems more suitable for the investigation of birthweight’s effect. The positive correlation of increased birthweight to PFD values found in our study may partly be explained by the fact that the PFD is an absolute distance measurement that increases with the size of the child, similarly to the increase that is seen when the child ages [22]. But as PFD is highly sensitive for DDH [9], increased birthweight may be perceived as an effect modifier on the relationship between PFD and hip dysplasia. These findings may also indicate that a cut-off value for PFD should be assessed on a semi-individual basis, taking the child’s size (i.e., birthweight) into account, in order to decrease false-positive referrals based on PFD measurements.
Orak et al. [13] and Kolb et al. [23] used α-angle measurements as a main outcome. The findings of Kolb et al. seem to be in agreement with our results, as their univariate regression analysis showed a significant negative influence of increased birthweight per 100 grams on the α angle (coefficient –0.071, CI –0.096 to –0.046) [23]. Orak et al. found that increased birthweight negatively affects α angles in hip ultrasound but only for female newborns, without reporting the exact results of linear regression analysis for males. Our findings suggest that increased birthweight has a negative effect on α angle measurements for both sexes.
Orak et al. [13] performed the ultrasound in the first after birth and Kolb et al. [23] in the first 2 postnatal weeks, which may entail a higher prevalence of false-positive or false-negative results due to immaturity of the hips. The newborns included in this study were screened at 5–6 weeks post-partum, as we aimed to investigate the persistent effect of birthweight on PFD and α-angle measurements. In addition to the previous studies, these results highlight that maturity of the hips does not seem to change the correlation between birthweight and α angles.
Roposch et al. [24] proposed a prediction model generating absolute predicted risk of DDH within 8 weeks postpartum on the basis of each at-risk newborn’s individualized clinical risk profile. The risk model includes 4 predictors: female sex, first-degree family history of DDH, birthweight > 4,000 g and abnormal examination of hip. This prediction model suggests increased birthweight is regarded as a component of the total risk of DDH for each newborn rather than a stand-alone referral criterion, as a correlation between DDH status and increased birthweight cannot be detected. While the study by Hanratty et al. [12] suggests that increased birthweight does not sufficiently increase the risk associated with increased birthweight, to warrant its use as a referral criterion birthweight may be considered as a contributing factor to the overall DDH risk profile of newborns. This suggestion is supported by the existing literature, as well as by the results of our study.
Strengths
First, this is the first study that examined the influence of increased birthweight on PFD measurements, as well as on α-angle measurements in hip ultrasounds of newborns screened for DDH at 6 weeks postpartum. Second, we included ultrasound data of a non-selected cohort of newborns with family history of DDH and breech presentation performed by welltrained and experienced senior musculoskeletal radiologists. In contrast, Orak et al. [13] excluded newborns with these risk factors, which makes their population even more selective. In addition, premature newborns were defined as newborns born alive before 37 gestational weeks [25], while Orak et al. [13] included 38–42-week-old newborns and Kolb et al. [23] included both premature and full-term newborns.
Limitations
First, the results cannot be generalized because the population consist of screened newborns with risk factors and/or a pathological primary PFD measurement. However, as the PFD measurement was used as a stand-alone referral criterion, 61% of the included newborns had no risk factors for DDH, which makes the present results more generalizable than previous reports from selective screening programs that exclusively included children who had a risk factor for DDH. Second, no interobserver evaluation was performed on ultrasound examinations.
Conclusions
Increased birthweight was positively correlated to PFD measurements for both female and male at-risk newborns, and negatively correlated to α-angle measurements in newborns screened for DDH at 6 weeks of age, regardless of sex. The findings seem not to be of clinical significance due to small effect size. Increased birthweight may only be relevant in the context of a multiple-factor risk profile for DDH.
- Pollet V, Percy V, Prior H J. Relative risk and incidence for developmental dysplasia of the hip. J Pediatr 2017; 181: 202-7. doi: 10.1016/j.jpeds.2016.10.017.
- De Hundt M, Vlemmix F, Bais J M, Hutton E K, de Groot C J, Mol B W, et al. Risk factors for developmental dysplasia of the hip: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2012; 165(1): 8-17. doi: 10.1016/j.ejogrb.2012.06.030.
- Manoukian D, Rehm A. Oligohydramnios: should it be considered a risk factor for developmental dysplasia of the hip? J Pediatr Orthop B 2019; 28(5): 442-5. doi: 10.1097/BPB.0000000000000624.
- Barr L V, Rehm A. Should all twins and multiple births undergo ultrasound examination for developmental dysplasia of the hip?: a retrospective study of 990 multiple births. Bone Joint J 2013; 95-B(1): 132-4. doi: 10.1302/0301-620X.95B1.29927.
- Vaquero-Picado A, González-Morán G, Garay E G, Moraleda L. Developmental dysplasia of the hip: update of management. EFORT Open Rev 2019; 4(9): 548-56. doi: 10.1302/2058-5241.4.180019.
- Graf R. The diagnosis of congenital hip-joint dislocation by the ultrasonic Combound treatment. Arch Orthop Trauma Surg 1980; 97 (2): 117-33. doi: 10.1007/BF00450934.
- Quader N, Schaeffer E K, Hodgson A J, Abugharbieh R, Mulpuri K. A systematic review and meta-analysis on the reproducibility of ultrasound-based metrics for assessing developmental dysplasia of the hip. J Pediatr Orthop 2018; 38(6): 305-11. doi: 10.1097/BPO.0000000000001179.
- Tréguier C, Chapuis M, Branger B, Bruneau B, Grellier A, Chouklati K, et al. Pubo-femoral distance: an easy sonographic screening test to avoid late diagnosis of developmental dysplasia of the hip. Eur Radiol 2013; 23(3): 836-44. doi: 10.1007/s00330-012-2635-7
- Husum H C, Hellfritzsch M B, Hardgrib N, Møller-Madsen B, Rahbek O. Suggestion for new 4.4 mm pubo-femoral distance cut-off value for hip instability in lateral position during DDH screening. Acta Orthop 2019; 90(1): 88-93. doi: 10.1080/17453674.2018.1554404
- Husum H C, Bach Hellfritzsch M, Maimburg R D, Henriksen M, Lapitskaya N, Møller-Madsen B, et al. Pubo-femoral distances measured reliably by midwives in hip dysplasia ultrasound. Children (Basel) 2022; 9(9): 1345. doi: 10.3390/children9091345.
- Chan A, McCaul K A, Cundy P J, Haan E A, Byron-Scott R. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed 1997; 76(2): F94-100. doi: 10.1136/fn.76.2.f94.
- Hanratty C, Thyagarajan B, Clarke N M, Aarvold A. There is no link between birth weight and developmental dysplasia of the hip. Indian J Orthop 2021; 55(6): 1515-22. doi: 10.1007/s43465-021-00465-8.
- Orak M M, Karaman O, Gursoy T, Cagirmaz T, Oltulu I, Muratli H H. Effect of increase in birth weight in a newborn on hip ultrasonography. J Pediatr Orthop B 2015; 24(6): 507-10. doi: 10.1097/BPB.0000000000000206.
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13(Suppl. 1): S31-4. doi: 10.4103/sja.SJA_543_18.
- Azzopardi T, Van Essen P, Cundy P J, Tucker G, Chan A. Late diagnosis of developmental dysplasia of the hip: an analysis of risk factors. J Pediatr Orthop B 2011; 20(1): 1-7. doi: 10.1097/BPB.0b013e3283415927.
- Dogruel H, Atalar H, Yavuz O Y, Sayli U. Clinical examination versus ultrasonography in detecting developmental dysplasia of the hip. Int Orthop 2008; 32(3): 415-19. doi: 10.1007/s00264-007-0333-x.
- Hinderaker T, Daltveit A K, Irgens L M, Uden A, Reikeras O. The impact of intrauterine factors on neonatal hip instability: an analysis of 1,059,479 children in Norway. Acta Orthop Scand 1994; 65(3): 239-42. doi: 10.3109/17453679408995446.
- Yiv B C, Saidin R, Cundy P J, Tgetgel J D, Aguilar J, McCaul K A, et al. Developmental dysplasia of the hip in South Australia in 1991: prevalence and risk factors. J Paediatr Child Health 1997; 33(2): 151-6. doi: 10.1111/j.1440-1754.1997.tb01019.x.
- Bache C E, Clegg J, Herron M. Risk factors for developmental dysplasia of the hip: ultrasonographic findings in the neonatal period. J Pediatr Orthop B 2002; 11(3): 212-18. doi: 10.1097/00009957-200207000-00004.
- Schams M, Labruyère R, Zuse A, Walensi M. Diagnosing developmental dysplasia of the hip using the Graf ultrasound method: risk and protective factor analysis in 11,820 universally screened newborns. Eur J Pediatr 2017; 176(9): 1193-200. doi: 10.1007/s00431-017-2959-z.
- Falliner A, Hahne H J, Hassenpflug J. Sonographic hip screening and early management of developmental dysplasia of the hip. J Pediatr Orthop B 1999; 8(2): 112-17. PMID: 10218172
- Ban Y, Luan Q, Shi M, Sun B, Li T, Wang Y, et al. Establishing reference values for the pubofemoral distance in normal infant medial hips by ultrasound. Acta Radiol 2021; 62(4): 551-6. doi: 10.1177/0284185120933240.
- Kolb A, Schweiger N, Mailath-Pokorny M, Kaider A, Hobusch G, Chiari C, et al. Low incidence of early developmental dysplasia of the hip in universal ultrasonographic screening of newborns: analysis and evaluation of risk factors. Int Orthop 2016; 40(1): 123-7. doi: 10.1007/s00264-015-2799-2.
- Roposch A, Protopapa E, Malaga-Shaw O, Gelfer Y, Humphries P, Ridout D, et al. Predicting developmental dysplasia of the hip in at-risk newborns. BMC Musculoskelet Disord 2020; 21(1): 442. doi: 10.1186/s12891-020-03454-4.
- Howson C P, Kinney M V, Lawn J. March of Dimes, PMNCH, Save the Children, Born Too Soon: the global action report on preterm birth. WHO 2012.
- Falliner A, Hahne H J, Hassenpflug J. Sonographic investigation of anatomical specimens of infant hip joints. J Pediatr Orthop B 2002; 11(3): 192-203. doi: 10.1097/00009957-200207000-00002.