The incidence of late-detected developmental dysplasia of the hip and its functional outcomes: a 17-year cohort study using selective ultrasound screening
Øyvind HÅBERG 2,3, Olav Andreas FOSS 1,3, Trude GUNDERSEN 4, Øystein BJERKESTRAND LIAN 2,3, Myrthle SLETTVÅG HOEL 2, and Ketil J HOLEN 1,3
1 Department of Orthopedic Surgery, Trondheim University Hospital; 2 Department of Orthopedic Surgery, Kristiansund Hospital; 3 Institute of Neuro Medicine and Movement Science, NTNU, Trondheim; 4 Department of Orthopedic Surgery, Haukeland University Hospital, Norway
Background and purpose — We aimed to establish the incidence of late-detected developmental dysplasia of the hip (DDH) with a selective ultrasound (US) examination over 17 years using the femoral head coverage (FHC) as a US measurement. The secondary aim was to establish the everyday function using patient-reported outcome measures (PROMs).
Patients and methods — The incidence of late-detected DDH was based on 60,844 children. Patients diagnosed for the first time after 3 months and before the age of 8 years were included. In the second part of the study, consent to participate was mandatory. PROMIS-25 Pediatric, PROMIS-25 Parent, and EQ-5D-5L were used according to the patient’s age to assess everyday function.
Results — The incidence of late-detected DDH was 0.48/1,000. The median age at diagnosis was 8 months (range 4–41 months), with a tendency to require repeated treatment with open surgery if DDH was diagnosed later. Most children reported no or minor health problems with a mean of 18 years’ follow-up.
Conclusion — We found that selective US examination of the hips by measuring the FHC is a reliable method to examine newborns for DDH resulting in a low incidence of late-detected DDH amounting to 0.48/1,000 newborn children.
Citation: Acta Orthopaedica 2023; 94: 588–593. DOI https://doi.org/10.2340/17453674.2023.24579.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-07-04. Accepted: 2023-10-11. Published: 2023-12-11.
Correspondence: Oyvind.Haberg@helse-mr.no
ØH: Collecting of data, writing of the manuscript. OAF: Supervision, statistical analysis, writing of the manuscript. TG: PROMs analyses and revision of the manuscript. ØBL: Supervision, writing of the manuscript. MSH: Collecting of data, writing of the manuscript. KJH: Supervision, statistical analysis, writing of the manuscript.
The authors thank Dr Helge Rønningen, Dr Lars Fosse, and Dr Torarin Lamvik for performing some of the US examinations, and the late Ole Jacob Johansen for most of the pediatric examinations.
Handling co-editors: Ivan Hvid and Robin Christensen
Acta thanks Klaus Dieter Parsch and Jaakko Sinikumpu for help with peer review of this manuscript.
The incidence of late-detected cases is regarded as an important marker for the quality of screening for developmental dysplasia of the hip (DDH). The introduction of ultrasonographic hip examination has been a large step forward in attempts to eradicate the condition. However, a study in 2019 found no reduction in the incidence of late-detected DDH in England over the last 35 years [1]. Another study reported that 20% of the hips treated for late-detected DDH underwent a total hip arthroplasty with 30 years of follow-up [2].
Two Norwegian randomized studies found no significant reduction in late-detected DDH using universal US screening versus selective screening [3,4]. Consequently, we have used selective US examination since 1995.
There are 2 main screening regimes using ultrasound (US) examination of the hip in newborns, either universal, for all children, or selective, only for children at risk of having DDH. Both methods aim for early diagnosis and treatment, and both screening regimes have their supporters, but there is no consensus on which approach is the best [4-6]. Universal screening seems to result in some degree of overtreatment [3,4,6]. However, some authors claim that universal screening has reduced the incidence of late-detected cases to virtually zero [5].
Since there is no consensus on whether to use selective or universal US screening, the aim of our study was to describe the incidence of late-detected DDH with 17 years of selective US screening. Furthermore, we wanted to report the functional outcomes.
Patients and methods
All 60,844 children born between 1996 and 2012 at Trondheim university hospital were eligible for the first part of the study that described the incidence of late-detected DDH. Children with syndromes or neuromuscular diseases were excluded due to an increased risk of hip pathology. Additionally, children treated for late-detected DDH at our university hospital but with a residential address outside of our county were excluded.
One of the most used definitions of late-detected DDH is DDH diagnosed in children older than 3 months. We used this as the lower age of inclusion [7]. The upper age of inclusion was set to 8 years, which was similar to another comparable study, and the ability of the acetabulum to remodel reduces after 8 years [1,8].
The hospital patient administration system was used to identify children within the determined age, treated for latedetected DDH, and born between 1996 and 2012. The International Classification of Disease (ICD)-9 and ICD-10 diagnosing codes and procedure codes were used in the search. The study is reported according to STROBE guidelines.
For further examination of the functional outcome with patient-reported outcome measures (PROMs), the patients or caregivers provided consent. All patients identified in the first part of the study were contacted by mail. Only those who had provided consent were included in the second part. Further data were then retrieved from their medical charts and from our local registry of children examined for DDH. The registry contained data regarding clinical and US findings, demographic data, birth length, presentation and weight, gestational age, birth number, family history of DDH, sex, and Ortolani and Barlow test results.
Screening of newborns
All newborns were examined by a pediatrician on the first day of life, including Ortolani and Barlow test [9,10]. With positive or doubtful Ortolani and/or Barlow tests, reduced abduction, or one or more risk factors for DDH, the child was referred to an orthopedic surgeon for a US hip examination. The risk factors were having close relatives with DDH (parents or siblings), breech presentation, and foot deformity [11]. The pediatric orthopedic surgeon conducted the US examination of the children before discharge from the maternity ward (within 3 days after birth).
A Siemens Antares (Siemens AG, Munich, Germany) with an 8–16 MHz probe was used, and since 2007, a GE Logic 7 (GE Healthcare, Milwaukee, WI, USA) with a similar probe has been used. The US method has been described in detail previously [12]. Femoral head coverage (FHC) of > 50% was classified as normal, FHC of 40%–49% was classified as unstable, FHC of 30%–39% was classified as subluxated, and FHC of < 30% was classified as luxated (dislocated). With positive Ortolani and/or Barlow test and FHC < 50%, we classified the findings as DDH, and treatment was initiated (Figure 1).
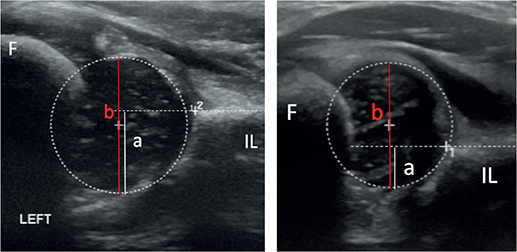
Figure 1. Ultrasound of a normal hip (left panel) with femoral head coverage of (a/b) x 100% = 61% and a hip with DDH (right panel) with femoral head coverage of (a/b) x 100% = 33%. IL = os ileum. Circle = femoral head.
Radiography of the pelvis, in addition to US examination, was always used to diagnose late-detected DDH. In the further follow-up of treated hips, radiography was the preferred examination for both early- and late-detected DDH. For children younger than 6–8 months, the US findings were used to classify the hips. The hips of older children were classified by radiography.
For a simplified classification, an acetabular index of < 30° at 1 year of age was classified as normal, and an acetabular index of < 24° and a center–edge angle of ≥ 15° at 5 years were classified as normal [13,14].
The hip joint was classified as dislocated if there was no contact between the articular surfaces [15]. In addition, the radiographs were examined for avascular necrosis (AVN) of the femoral head and classified according to the Bucholz Ogden classification [16].
US is a dynamic examination, and pathologic hips were classified into 3 categories: unstable, subluxated, or luxated. Older children were diagnosed by radiography, a static examination. Pathologic hips were classified to either subluxated or luxated. Since the introduction of US the US devices have markedly improved, and today we use US for even older children than previously (< 8 months), and thereby reduce radiation exposure.
Treatment and follow-up
Children diagnosed with late-detected DDH were treated with abduction orthosis, casting, and closed or open reduction, with or without soft tissue and/or bony procedures. Skin traction was often used prior to the main procedure in the long-persisting cases. However, all treatments were individualized according to the level of DDH, the child’s age, and the response to the treatment initiated. Some children treated with only abduction orthosis and those who achieved early normalization of the hips were followed up until 5 years of age. Most patients were followed up until skeletal maturity.
Functional outcomes
The results of PROMs of everyday function of children with hip pathology have not frequently been reported [17]. We used age-specific PROMs to describe the function of children and young adults with late-detected DDH. The patients and caregivers who agreed to participate in the study filled out agespecific questionnaires. All forms were translated into their first language, Norwegian.
Children aged 8 and up to 16 years filled out a 25-item PROM, the PROMIS-25 Pediatric, and their parents or caregivers completed the PROMIS-25 Pediatric Family Relationships measure [18]. These are generic and not disease-specific questionnaires. In the first part, 6 items were examined (mobility, anxiety, depression, fatigue, peer relationships, and pain). The score has a range of 4–20, with 20 being the best for mobility and peer relationships. A score of 4 is the best in the other categories. A T-score is also presented, where 50 is the average score of an age-matched population [19]. A score < 50 represents less anxiety, depression, fatigue, and pain than the average population, and a score > 50 represents more mobility and better peer relationships than the average population. In the second part, the pain intensity was rated from 0 (no pain) to 10 (worst pain).
Patients aged 16 years and older filled out the 2-part questionnaire EQ-5D-5L, which is also a generic questionnaire [20]. The first part assessed the patients’ health in 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), rated from 1 (no problem) to 5 (extreme problems). In the second part, a visual analog scale (VAS) was used, where the patient rated their perceived health from 0 (worst) to 100 (best).
Statistics
The statistical calculations were performed with the SPSS statistical software released in 2020 (IBM SPSS Statistics for Windows, Version 27.0, IBM Corp, Armonk, NY, USA). Results were presented with descriptive statistics of the mean, median, standard deviation (SD), and ranges (min–max). The results in the questionnaires are presented descriptively as the sample size is small.
Ethics, funding, and disclosures
The study was approved by the Regional Committee Central for Medical Health Research Ethics (Dnr 2016/1161). The children and parents included were given written information on the study and patients or parents signed consent for participation in the study. ØH received a PhD scholarship from the local hospital trust. None of the authors had any financial benefit from the paper. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.24578
Results
60,817 children, born in Norway during 1996–2012 were included, and 8,709 (14%) newborns were examined with US during these 17 years. 29 children were diagnosed with latedetected DDH, providing an incidence of 0.48/1,000 among newborn children (Figure 2). The median age at diagnosis was 8.0 months (range 4–41 months). 4 patients were boys, and 25 patients were girls. The mean time of follow-up after diagnosis was 18 years (SD 4.3, range 9–25 years). 3 children had a US examination at birth classified as normal. The US was conducted due to risk factors, i.e., 1 case of a close relative with DDH, 1 case of breech presentation, and 1 case of a close relative and breech presentation. The FHC (right/left hip) was, respectively, 55/54%, 60/58%, and 59/58% and classified as normal.
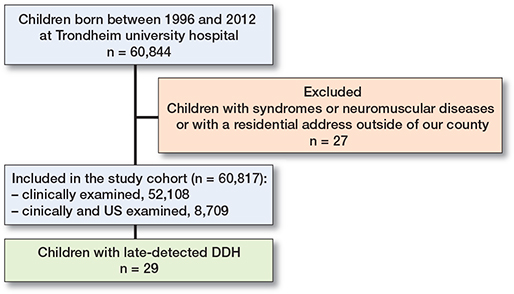
Figure 2. Patient flowchart. US = ultrasound. DDH = developmental dysplasia of the hip.
All 29 included patients gave consent to examine their medical charts and radiographs and were also requested to submit PROMs and 24 did so.
12 had right-sided, 17 left-sided, and no patients had bilateral late-detected DDH. Of the hips diagnosed before 12 months, 5 were luxated and 16 were subluxated. Of the hips diagnosed after 12 months, all 8 were luxated. 3 children had AVN, 1 patient had stage 2 AVN in the treated hip diagnosed at 6 years (treated with open reduction and casting), 1 patient had stage 1 AVN in the contralateral hip diagnosed at 18 months, and 1 patient had stage 1 AVN in the treated hip for DDH diagnosed at 30 months.
6 children were diagnosed with late-detected DDH because the parents raised the question. 16 children were diagnosed due to suspicion at the regular examination by the primary healthcare providers, 6 children were diagnosed with unknown or missing information in the medical chart, and 1 child was diagnosed incidentally. The reason for referral was either stiffness in a hip or limping gait. 7 children had a risk factor for DDH, but only 3 of them were examined by US at birth. The 4 children missed in the screening were 2 children with breech birth, 1 with close relatives treated for DDH, and 1 with a foot deformity.
The treatment given comprised orthosis only for 12 children, and all were younger than 12 months when DDH was first diagnosed. 9 children required repeated treatment, and 6 of these were treated with surgery. Those treated with surgery were 12 months or older when DDH was first diagnosed. 12 children were treated with skin traction before casting. The treatments given are presented in Table 1.
6 children and caregivers filled out PROMIS-25 Pediatric and PROMIS-25 Parent, respectively (Table 2). The mean age at follow-up for these patients was 13 years and all of them were girls. The mean score for pain intensity during the last 7 days was 0.7 for both children and caregivers.
18 patients filled out the EQ-5D-5L questionnaire. The mean age was 21 years at follow-up; 4 were boys and 14 were girls. The results are presented in Table 3. The mean EQ-VAS score was 74 (SD 19, range 30–100).
Discussion
We aimed to establish the incidence of late-detected DDH and found it to be 0.48/1,000 newborns after selective US screening.
The incidence of late-detected DDH is regarded as an important marker for the quality of screening for DDH. Before our hospital included US as part of the screening regime, the incidence of late-detected DDH was 2.6/1,000 [21]. Shortly after the introduction of US in the mid-1980s, the incidence decreased to 0.65/1000 [22]. Both studies defined late-detected DDH as DDH diagnosed 1 month after birth.
Many countries perform selective US screening of children to diagnose DDH, as in Norway, but with no consensus on whether universal or selective US screening is the best method. A recent systematic review and meta-analysis compared universal and selective screening, reporting a significantly increased treatment rate for those using universal screening with no significant difference in the rate of late-detected DDH and operative treatment between the 2 methods [6]. Universal US screening might detect more DDH cases, but cost-effectiveness studies have not identified an advantage with universal screening [23,24].
Studies have described hips with borderline pathology on US at birth often spontaneously resolving within the first months of life [22,25]. As a consequence, the timing of US examination has been altered to decrease the number of false-positive cases. We now conduct US examination of children with only a risk factor for DDH at 4 weeks after birth [26]. Children with pathologic or borderline clinical findings at birth are examined with US before departure from the hospital.
With several definitions of late-detected DDH, e.g., DDH diagnosed 3, 6, or 12 months after birth, comparing results can be difficult [1,21,27]. Setting the lower age for late-detected DDH can have a large impact on the incidence of late-detected cases. Studies defining 3 months as the lower threshold for late-detected DDH have reported an incidence of 0.57/1,000, 0.28/1,000, and 0.15/1,000, respectively [7,28,29]. Another study reported an incidence of 0.2/1,000 with late-detected DDH using 6 months of age as the threshold value [27], while a further study reported an incidence of 1.28/1,000 with 1 year of age as the threshold [1]. In general, studies of late-detected DDH include a small sample size; consequently, only a few cases will have a large impact on the reported incidence.
Selective screening carries the risk of losing children who should have been included.
We emphasize that all children with a risk factor for DDH must be examined with US of their hips. Safe selective screening depends on reliable screening routines. Because 4 children with late-detected DDH were missed, we have since then improved our screening routines.
Late-detected DDH may be a separate entity and thereby in need of a different approach for diagnosis [30,31]. Recent studies have described promising results with genetic analyses, and, it is hoped, the incidence of late-detected cases can be decreased in the future [32,33].
The later DDH is diagnosed and treatment initiated, the worse the prognosis for the hip [2]. A possible trend towards a worse prognosis has been suggested after 18 months [34,35]. In our study, the median age of the children diagnosed with late-detected DDH was 8 months. We found that a threshold for requiring more comprehensive treatment more likely to be at 12 months when DDH is first diagnosed. Interestingly, the majority of children with a dislocated hip were diagnosed late. We do not have a good explanation for this observation, but it is reasonable to believe that a more severe condition should have been diagnosed earlier.
Limitations
To report the clinical outcome of children with late-detected DDH we used generic questionnaires. The reason was that hip diseases would probably negatively influence their quality of life [17]. Reported problems in everyday life also could be due to other health conditions, as the questionnaires are not organ- or disease-specific. The overall impression is that most patients had minor problems at follow-up.
This retrospective study has limitations due to the potential loss of cases. We made an effort to reduce the risk as we searched with diagnosis and procedure codes in the hospital patient administration system, including the outpatient charts. The number of children with a milder degree of late-detected DDH could be higher as we do not know whether there were more missing asymptomatic cases during the first 8 years of life. Due to a small number of late-detected cases we have chosen not to perform subanalyses.
Conclusion
We found that selective US examination of the hips by measuring the FHC is a reliable method to examine newborns for DDH resulting in a low incidence of late-detected DDH amounting to 0.48/1,000 newborn children.
- Broadhurst C, Rhodes A M L, Harper P, Perry D C, Clarke N M P, Aarvold A. What is the incidence of late detection of developmental dysplasia of the hip in England?: a 26-year national study of children diagnosed after the age of one. Bone Joint J 2019; 101-b: 281-7. doi: 10.1302/0301-620x.101b3.Bjj-2018-1331.R1.
- Young E, Regan C, Milbrandt T A, Grigoriou E, Shaughnessy W J, Stans A A, et al. Predictors of total hip arthroplasty following pediatric surgical treatment of developmental hip dysplasia at 20-year follow-up. J Clin Med 2022; 11. doi: 10.3390/jcm11237198.
- Rosendahl K, Markestad T, Lie R T. Ultrasound screening for developmental dysplasia of the hip in the neonate: the effect on treatment rate and prevalence of late cases. Pediatrics 1994; 94: 47-52.
- Holen K J, Tegnander A, Bredland T, Johansen O J, Saether O D, Eik-Nes S H, et al. Universal or selective screening of the neonatal hip using ultrasound? A prospective, randomised trial of 15,529 newborn infants. J Bone Joint Surg Br 2002; 84: 886-90. doi: 10.1302/0301-620x.84b6.12093.
- Biedermann R, Eastwood D M. Universal or selective ultrasound screening for developmental dysplasia of the hip? A discussion of the key issues. J Child Orthop 2018; 12: 296-301. doi: 10.1302/1863-2548.12.180063.
- Kuitunen I, Uimonen M M, Haapanen M, Sund R, Helenius I, Ponkilainen V T. Incidence of neonatal developmental dysplasia of the hip and late detection rates based on screening strategy: a systematic review and meta-analysis. JAMA Netw Open 2022; 5: e2227638. doi: 10.1001/jamanetworkopen.2022.27638.
- Talbot C, Adam J, Paton R. Late presentation of developmental dysplasia of the hip: a 15-year observational study. Bone Joint J 2017; 99-B: 1250-5. doi: 10.1302/0301-620X.99B9.BJJ-2016-1325.R1.
- Weinstein S L, Mubarak S J, Wenger D R. Developmental hip dysplasia and dislocation: Part I. Instr Course Lect 2004; 53: 523-30.
- Barlow T G. Early diagnosis and treatment of congenital dislocation of the hip. Proc R Soc Med 1963; 56: 804-6.
- Ortolani M. Congenital hip dysplasia in the light of early and very early diagnosis. Clin Orthop Relat Res 1976: 6-10.
- Håberg Ø, Foss O A, Lian Ø B, Holen K J. Is foot deformity associated with developmental dysplasia of the hip? Bone Joint J 2020; 102-b: 1582-6. doi: 10.1302/0301-620x.102b11.Bjj-2020-0290.R3.
- Holen K J, Terjesen T, Tegnander A, Bredland T, Saether O D, Eik-Nes S H. Ultrasound screening for hip dysplasia in newborns. J Pediatr Orthop 1994; 14: 667-73. doi: 10.1097/01241398-199409000-00022.
- Tönnis D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin Orthop Relat Res 1976: 39-47.
- Håberg Ø, Bremnes T, Foss O A, Angenete O, Lian Ø B, Holen K J. Children treated for developmental dysplasia of the hip at birth and with normal acetabular index at 1 year: how many had residual dysplasia at 5 years? J Child Orthop 2022; 16: 183-90. doi: 10.1177/18632521221106376.
- Musielak B, Idzior M, Jóźwiak M. Evolution of the term and definition of dysplasia of the hip: a review of the literature. Arch Med Sci 2015; 11: 1052-7. doi: 10.5114/aoms.2015.52734.
- Bucholz R. Patterns of ischemic necrosis of the proximal femur in non-operatively treated congenital hip disease. In: Hip proceedings of the Sixth Open Scientific Meeting of the Hip Society. St Louis, MO: CV Mosby, 1978. p. 43-63.
- Leo D G, Perry D C, Abdullah B, Jones H. PROMIS Paediatric Mobility tool is correlated with accelerometer-measured physical activity in children with hip diseases. Bone Joint J 2021; 103-b: 405-10. doi: 10.1302/0301-620x.103b2.Bjj-2020-0744.R1.
- Cox E D, Connolly J R, Palta M, Rajamanickam V P, Flynn K E. Reliability and validity of PROMIS® pediatric family relationships short form in children 8–17 years of age with chronic disease. Qual Life Res 2020; 29: 191-9. doi: 10.1007/s11136-019-02266-x.
- Irwin D E, Varni J W, Yeatts K, DeWalt D A. Cognitive interviewing methodology in the development of a pediatric item bank: a patient reported outcomes measurement information system (PROMIS) study. Health Qual Life Outcomes 2009; 7: 3. doi: 10.1186/1477-7525-7-3.
- Group T. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199-208. doi: 10.1016/0168-8510(90)90421-9.
- Tegnander A, Terjesen T, Bredland T, Holen K J. Incidence of latediagnosed hip dysplasia after different screening methods in newborns. J Pediaatr Orthop B 1994; 3: 86-8.
- Holen K J, Tegnander A, Eik-Nes S H, Terjesen T. The use of ultrasound in determining the initiation of treatment in instability of the hip in neonates. J Bone Joint Surg Br 1999; 81: 846-51. doi: 10.1302/0301-620x.81b5.9502.
- Rosendahl K, Markestad T, Lie R T, Sudmann E, Geitung J T. Cost-effectiveness of alternative screening strategies for developmental dysplasia of the hip. Arch Pediatr Adolesc Med 1995; 149: 643-8.
- Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet 2007; 369: 1541-52. doi: 10.1016/s0140-6736(07)60710-7.
- Lussier E C, Sun Y T, Chen H W, Chang T Y, Chang C H. Ultrasound screening for developmental dysplasia of the hip after 4 weeks increases exam accuracy and decreases follow-up visits. Pediatr Neonatol 2019; 60: 270-7. doi: 10.1016/j.pedneo.2018.07.008.
- Tan S H S, Wong K L, Lim A K S, Hui J H. The earliest timing of ultrasound in screening for developmental dysplasia of the hips. Ultrasonography 2019; 38: 321-6. doi: 10.14366/usg.18075.
- Pollet V, Percy V, Prior H J. Relative risk and incidence for developmental dysplasia of the hip. J Pediatr 2017; 181: 202-7. doi: 10.1016/j.jpeds.2016.10.017.
- Kamath S, Mehdi A, Wilson N, Duncan R. The lack of evidence of the effect of selective ultrasound screening on the incidence of late developmental dysplasia of the hip in the Greater Glasgow Region. J Pediatr Orthop B 2007; 16: 189-91. doi: 10.1097/01.bpb.0000236229.44819.43.
- Sharpe P, Mulpuri K, Chan A, Cundy P J. Differences in risk factors between early and late diagnosed developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed 2006; 91: F158-62. doi: 10.1136/adc.2004.070870.
- Wynne-Davies R. Acetabular dysplasia and familial joint laxity: two etiological factors in congenital dislocation of the hip. A review of 589 patients and their families. J Bone Joint Surg Br 1970; 52: 704-16.
- Haasbeek J F, Wright J G, Hedden D M. Is there a difference between the epidemiologic characteristics of hip dislocation diagnosed early and late? Can J Surg 1995; 38: 437-8.
- Wen J, Ping H, Kong X, Chai W. Developmental dysplasia of the hip: a systematic review of susceptibility genes and epigenetics. Gene 2023; 853: 147067. doi: 10.1016/j.gene.2022.147067.
- Harsanyi S, Zamborsky R, Kokavec M, Danisovic L. Genetics of developmental dysplasia of the hip. Eur J Med Genet 2020; 63: 103990. doi: 10.1016/j.ejmg.2020.103990.
- Malvitz T A, Weinstein S L. Closed reduction for congenital dysplasia of the hip: functional and radiographic results after an average of thirty years. J Bone Joint Surg Am 1994; 76: 1777-92. doi: 10.2106/00004623-199412000-00004.
- Terjesen T. Long-term outcome of closed reduction in late-detected hip dislocation: 60 patients aged six to 36 months at diagnosis followed to a mean age of 58 years. J Child Orthop 2018; 12: 369-74. doi: 10.1302/1863-2548.12.180024.