Incidence and risk factors of intraoperative periprosthetic femoral fractures during primary total hip arthroplasty: 218,423 cases reported to the Norwegian Arthroplasty Register between 1987 and 2020
Heinrich BRÜGGEMANN 1, Ingvild DALEN 2, Lena Kristin BACHE-MATHIESEN 2,3, Anne Marie FENSTAD 4, Geir HALLAN 4,5,6, and Lars FOSSE 1
1 Department of Orthopaedic Surgery, Stavanger University Hospital, Stavanger; 2 Department of Research, Stavanger University Hospital, Stavanger; 3 Oslo Sports Trauma Research Centre, Department of Sports Medicine, Norwegian School of Sport Sciences, Oslo; 4 The Norwegian Arthroplasty Register, Department of Orthopaedic Surgery, Haukeland University Hospital, Bergen; 5 Department of Orthopaedic Surgery, Haukeland University Hospital, Bergen; 6 Department of Clinical Medicine, University of Bergen, Bergen, Norway
Background and purpose — Intraoperative periprosthetic femoral fractures (IPFFs) can occur during primary total hip arthroplasty (THA). We describe the incidence of IPFFs during THA in Norway and estimate potential risk factors that could be associated with IPFF
Patients and methods — Data from the Norwegian Arthoplasty Register (1987–2020) was used: 2,268 IPFFs from 218,423 primary THAs in 172,598 patients. The following factors were analyzed: sex, age, diagnosis, previous operation on the same hip, surgical approach, and stem fixation technique. Association of these factors with IPFF risk was assessed using multivariable Poisson regression.
Results — IPFF occurred during 2,268 operations with an incidence of 1.0% among all primary THAs. The risk of IPFF was associated with female sex (relative risk 1.8; 99% CI 1.5–2.1), age 80–90 years and age over 90 years (compared with age 60–70 years: 1.3; CI 1.0–1.6 and 2.6; CI 1.6–4.3, respectively), non-osteoarthritis diagnoses (2.2; CI 1.9–2.6), previous surgery to the same hip (1.8; CI 1.5–2.2), lateral approach (compared with the posterior approach: 1.5; CI 1.1–2.0), and cementless stem fixation (2.7; CI 2.0–3.6).
Interpretation — Surgeons should be aware of the factors associated with an increased risk of IPFF: female sex, age above 80 years, non-osteoarthritis diagnoses, and previous surgery to the same hip. Cemented stem fixation and posterior approach should be favored in high-risk patients, such as elderly women.
Citation: Acta Orthopaedica 2022; 93: 405–412. DOI http://dx.doi.org/10.2340/17453674.2022.2431.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-02-01. Accepted: 2022-03-06. Published: 2022-04-06.
Correspondence: hbru@sus.no
HB, GH, and LF conceptualized the study. All authors were involved in the study design. AMF retrieved and prepared the data. ID, AMF, and LKBM performed the statistical analyses. HB wrote the manuscript, and all authors contributed with critical appraisal of the text.
The authors would like to thank all surgeons for conscientiously reporting total hip arthroplasties to the Norwegian Arthroplasty Register (NAR), and the NAR staff for facilitating this work.
Acta thanks Antti Eskelinen, Maziar Mohaddes and Claus Varnum for help with peer review of this study.
Intraoperative periprosthetic femoral fracture (IPFF) is a well-known iatrogenic complication of total hip arthroplasty (THA) (1).
Most IPFFs are nondisplaced, and simple fractures such as cracks in the calcar and trochanteric region can occur during preparation of the femoral canal, during impaction of the femoral stem or during forceful repositioning of the implanted stem (2). These fractures are the focus of the present paper. The consequences of minor IPFFs in terms of implant performance have not been thoroughly investigated, but they may be associated with an increased risk of revision surgery (3,4). Although less frequent, more serious IPFFs, such as shaft fractures, femoral perforations, or displaced trochanteric fractures, regularly require immediate intraoperative reduction and fixation and/or the use of revision implants to achieve a stable implant (2). Despite these concerns, the incidence of IPFF varied widely between 0.1% to 27.8% in previous studies. This wide range is suspected to be due to small study sizes and different outcome measures (5).
Some studies have identified risk factors for IPFF, including cementless stem fixation, female sex, and age (3,5,6). Most of these studies were based on moderately sized populations, included both primary and revision THAs, or followed specific cohorts (i.e., cementless THAs only). The findings are somewhat conflicting, i.e., studies found that younger and older age is associated with fractures (7), whereas others linked these fractures only to older age (8).
Understanding the extent of the IPFF problem would quantify the importance of targeting this complication in quality improvement endeavors. Ideally, THA surgeons can correctly identify patients predisposed to IPFF and adjust operation procedures accordingly. We describe the incidence of IPFFs during THA and estimate potential risk factors that could be associated with IPFF.
Patients and methods
Study population
This study was performed with data from the Norwegian Arthroplasty Register (NAR). All hospitals performing THA surgery in Norway report data to the NAR with patient consent. The register was established in 1987 by the Norwegian Orthopaedic Association and receives data on more than 97% of primary THAs and 93% of revision surgeries (9). The data for this study was collected from September 1987 to December 2020.
218,423 THAs were performed on 172,598 patients in 91 different units during this 33-year period (Figure 1). Some units have since stopped performing such procedures, whereas others have started. At any given year, 55–73 units were active. The completeness of the primary THAs reported to our register is high (> 97%).
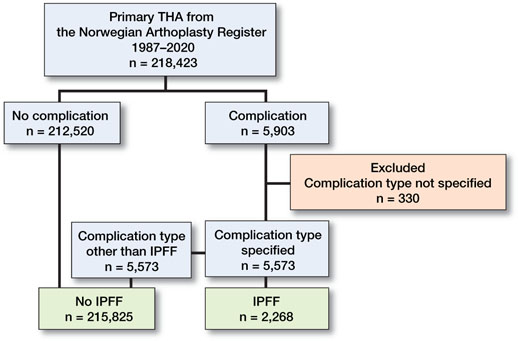
Figure 1. Categorization process of perioperative complications in 218,423 THAs registered in the Norwegian Arthroplasty Registry from 1987 to 2020. THA = total hip arthroplasty; IPFF = intraoperative periprosthetic femoral fracture.
Variables and outcomes
Immediately after each THA surgery, the surgeon filled out a standardized hip form. The form included a question on whether there were any intraoperative complications (Yes/No). If yes, the surgeon was prompted to describe the complications in a free-text field. These intraoperative complications were categorized and numerically coded in the database (Figure 1), and subsequently validated by 2 orthopedic surgeons. IPFF was defined as any intraoperative fracture of the proximal femur (calcar and trochanteric fracture) that was reported to NAR. The other intraoperative complication categories were medical complications (e.g., heart attack, respiratory or circulatory problems), technical issues (e.g., problems with cement pump, cup or stem revision), other fractures (e.g., pelvic or femoral shaft fractures), and other complications (e.g., break in sterility, assistant’s collapse into surgical field). Bilateral staged and simultaneous THA were reported on two separate forms.
Based on clinical rationale and availability of data, we evaluated the following factors as potential risk factors of IPFF: sex, age, diagnosis, previous operation on the ipsilateral hip, surgical approach, and stem fixation technique. Age was stratified into 6 groups: < 50, 50–59, 60–69, 70–79, 80–89, and 90 years and older. Diagnosis was dichotomized into primary OA and non-OA (including all other diagnoses: inflammatory joint disease, secondary OA, acute or sequelae after fracture collum femoris avascular necrosis of the femoral head and developmental dysplasia). The surgical approach was divided into lateral (direct lateral Hardinge/transgluteal), posterior, anterior (direct anterior Smith-Petersen and anterolateral Watson-Jones combined) and other approaches. A directed acyclic graph was drawn to consider adjustment strategies for the potential risk factors respectively (Figure 2, see Supplementary data). The operation year of the primary THA surgery was included as a confounder (Figure 2, see Supplementary data), with time stratified into five-year periods, except for the first period, which was longer.
Statistics
Demographic and clinical variables were presented as counts and percentages (%). The distribution of intraoperative complications was calculated, and the yearly incidence of IPFF was plotted in a line graph with 99% confidence intervals (CIs). An area chart was used to illustrate the use of different fixation techniques within age groups over time.
IPFFs were estimated using univariable and multivariable Poisson regression, from which relative risks (RRs) with 99% CIs based on robust standard errors were determined (10). The correlation among THAs at the same treatment facility was accounted for by adding random intercept terms to the models. The correlation among bilateral THAs was disregarded (11). RRs for sex and age were adjusted for operation year; RRs for diagnosis were adjusted for sex, age, and operation year; RRs for previous surgery were adjusted for sex, age, diagnosis, and operation year; whereas RRs for fixation technique and approach were adjusted for all the aforementioned variables (Figure 2, see Supplementary data).
The statistical significance of individual effects and interactions was tested with 2-sided chi-square tests. P-values ≤ 0.01 were considered statistically significant. THAs with missing data on any of the risk factors or outcome variables (n = 8,167, 3.7%) were excluded from the regression analyses (Table 1, see Supplementary data).
We repeated Poisson regression to explore the potential nonlinear relationship between age and the risk of IPFF. In this analysis, age was treated as a continuous variable and modeled with restricted cubic splines (12). The analyses were repeated separately for men and women. The resulting predicted probabilities with 99% confidence bands are presented in plots.
Regression analyses were performed in STATA v.17 with the function mepoisson. All statistical figures were made using R version 4.1.0 (R Core team, R Foundation for Statistical Computing, Vienna, Austria, 2019) using the rms package for splines (13) and ggeffects (14) to estimate predicted probabilities.
Ethics, funding, data sharing, and potential conflicts of interest
Ethical approval for this study was obtained from the Norwegian Data Protection Authority (03/00058-20/CGN). Registration of the data and study was performed confidentially with patient consent and according to Norwegian and EU data protection rules. This paper was written according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (15) and the Reporting of studies Conducted using Observational Routinely-collected Data (RECORD) guidelines (16). Data may be accessible upon application to the NAR. There was no external funding for this study.
The authors declare there were no conflicts of interest.
Results
218,423 THAs were performed on 172,598 patients (66% women, mean age 69 years, SD 11; Table 2).
5,573 THAs had a specified intraoperative complication, among which IPFF was the most frequent complication type (n = 2,268, 41%). The other intraoperative complications were medical complications (19%), technical issues (18%), other fractures (11%), and all other complications (11%).
The mean incidence of IPFF over the whole study period (1987–2020) was 1.0%, with some temporal variation (Figure 3).
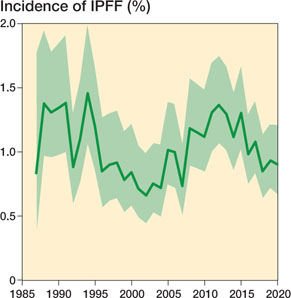
Figure 3. Annual incidence of intraoperative periprosthetic femoral fracture (IPFF) from 1987 to 2020. There were 218,423 total hip arthroplasties without missing data on perioperative complications. Error zone represent 99% confidence intervals.
The use of cementless stem fixation increased during the study period. Starting in approximately 2008, this press-fit technique became more common than cemented fixation in all age groups, except for patients over 80 years of age (Figure 4).
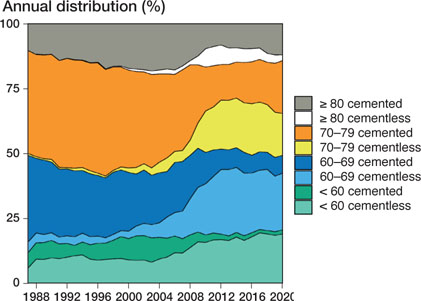
Figure 4. Type of stem fixation (cemented or cementless) for different age groups during the study period. Based on 216,317 total hip arthroplasties with available information on stem-fixation technique.
The fixation technique was strongly associated with the risk of IPFF, with an almost 3 times higher risk with cementless stem fixation, also after adjusting for all measured confounders (RR 2.7, CI 2.0–3.6; Table 3).
| IPFF n (%) | Unadjusted RR (99% CI) | Adjusted a RR (99% CI) | Adjusted b RR (99% CI) | Adjusted c RR (99% CI) | Adjusted d RR (99% CI) | |
| Sex | ||||||
| Male | 469 (0.7) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| Female | 1693 (1.2) | 1.8 (1.5-2.1) | 1.8 (1.5-2.1) e | 1.9 (1.6-2.2) | ||
| Age | ||||||
| < 50 | 268 (2.1) | 2.2 (1.7-2.9) | 2.3 (1.8-2.9) e | 1.2 (1.0-1.5) | ||
| 50-59.99 | 384 (1.4) | 1.5 (1.2-2.0) | 1.6 (1.2-2.0) e | 1.1 (1.0-1.4) | ||
| 60-69.99 | 561 (0.9) | 1 (ref) | 1 (ref) | 1 (ref) | ||
| 70-79.99 | 598 (0.8) | 0.9 (0.7-1.0) | 0.9 (0.7-1.1) e | 1.0 (0.9-1.2) | ||
| 80-89.99 | 320 (1.1) | 1.2 (1.0-1.5) | 1.3 (1.0-1.6) e | 1.4 (1.2-1.7) | ||
| ≥ 90 | 31 (2.4) | 2.5 (1.5-4.2) | 2.6 (1.6-4.3) e | 2.3 (1.4-3.6) | ||
| Diagnosis | ||||||
| Primary OA | 1209 (0.8) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Other | 953 (1.9) | 2.5 (2.1-2.8) | 2.5 (2.2-2.9) | 2.2 (1.9-2.6) e | 1.7 (1.4-2.1) | |
| Previous surgery | ||||||
| No | 1624 (0.9) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 538 (2.5) | 2.8 (2.3–3.3) | 2.8 (2.4–3.3) | 2.5 (2.1–3.0) | 1.8 (1.5–2.2) e | 1.8 (1.5–2.2) |
| Surgical approach | ||||||
| Lateral | 1289 (1.2) | 1.5 (1.1-1.9) | 1.6 (1.1-2.3) | 1.5 (1.1-2.2) | 1.5 (1.1-2.1) | 1.5 (1.1-2.0) e |
| Posterior | 618 (0.8) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Anterior | 248 (1.2) | 1.5 (1.0-2.2) | 1.4 (0.9-2.1) | 1.4 (0.9-2.0) | 1.5 (1.0-2.1) | 1.3 (0.9-1.8) e |
| Other | 7 (3.1) | 3.9 (1.2-13.1) | 4.1 (1.2-14) | 4.0 (1.2-14) | 3.6 (1.1-12) | 3.0 (1.0-8.9) e |
| Fixation technique | ||||||
| Cemented | 908 (0.7) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Cementless | 1254 (1.6) | 2.2 (1.5-3.2) | 2.7 (1.9-3.8) | 2.7 (2.0-3.7) | 2.7 (2.0-3.7) | 2.7 (2.0-3.6) e |
| Operation year | ||||||
| 1987-1995 | 462 (1.2) | 1 (ref) | 1 (ref) | |||
| 1996-2000 | 226 (0.9) | 0.7 (0.5-1.0) e | 0.7 (0.5-1.0) | |||
| 2001-2005 | 247 (0.8) | 0.7 (0.4-1.0) e | 0.7 (0.4-1.0) | |||
| 2006-2010 | 341 (1.0) | 0.9 (0.6-1.2) e | 0.7 (0.5-1.0) | |||
| 2011-2015 | 470 (1.3) | 1.0 (0.7-1.6) e | 0.8 (0.5-1.1) | |||
| 2016-2020 | 416 (0.9) | 0.8 (0.5-1.1) e | 0.7 (0.5-1.1) | |||
| N = 210,256 (of which 2,162 IPFF). a Adjusted for operation year (in groups) b Adjusted for operation year, age (groups), and sex. c Adjusted for operation year, age, sex, and diagnosis. d From full model, all variables included. e Indicate estimates of total effect after adjustment for measured confounders (Figure 2, see Supplementary data). IPFF = intraoperative periprosthetic femoral fracture; OA = osteoarthritis; RR = relative risk. |
||||||
THAs performed with a lateral approach have 50% increased risk (RR 1.5, CI 1.1–2.0) of IPFF compared with the posterior approach. The anterior approach had a seemingly increased risk of IPFF compared with the posterior approach (unadjusted RR 1.5, CI 1.0–2.2), but some of the risk was explained by previous surgery, and adjusted RR was reduced to 1.3 and confidence intervals overlapped with 1 (0.9 to 1.8), which does not exclude clinical relevance.
Female sex and age above 80 years and below 60 were associated with higher risks of IPFF (Table 3). Pathways through diagnosis, previous surgery, approach, and fixation technique (Table 3) accounted for most of the increased risk for the younger age groups, in particular diagnosis and fixation technique (data not shown). Possible age–sex interaction was tested in the model without adjustment, the model with adjustment for operation year, and in the full model, but deemed not statistically significant (p > 0.01).
Nonlinear modeling revealed that the youngest patients appeared to have the highest risk of IPFF in the confounder-adjusted models. In the fully adjusted models, the oldest patients were at highest risk. The age association was again similar between men and women (p > 0.01 for age–sex interaction) (Figure 5).
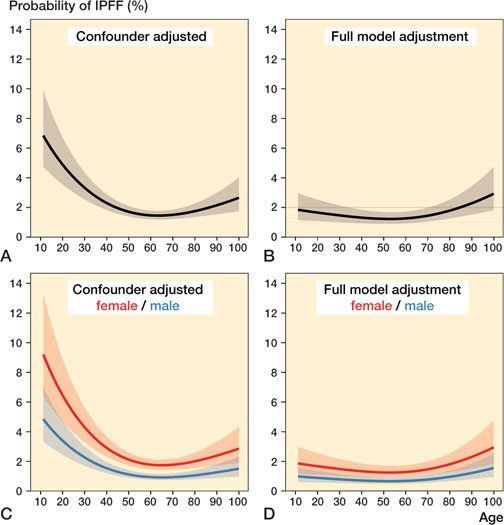
Figure 5. Nonlinear relationship between age and probability of IPFF based on 210,256 total hip arthroplasties without missing data. Figures A and C are adjusted for operation year, whilst B and D are adjusted for all the studied potential risk factors of IPFF. For A–B, no stratification, and C–D, stratified on sex. Adjustment variables were set to the following fixed levels: cementation type = cemented; previous operation = no; sex = female; diagnosis = osteoarthritis; and operation year group = 2016–2020. IPFF = intraoperative periprosthetic femoral fracture.
Patients with non-OA diagnosis and previous surgery to the ipsilateral hip were also at higher risk of IPFF (Table 3), and fixation technique and/or approach explained some but not all of the risk increase.
The risk increase for cementless vs. cemented stem fixation was relatively stable across different age and sex strata (Table 4). Corail was by far the most common cementless stem prosthesis, used in 61,864, i.e., 74% of all cementless THAs. Of the 132,706 cemented THAs, Charnley was the most frequently used stem prosthesis (32%), then Exeter (27%), Titan (9.1%), Lubinus SP II (8.3%), and Spectron (8.0%).
| Sex Age group | IPFF/n | Unadjusted RR (99% CI) | Adjusted a RR (99% CI) |
| Women | |||
| < 50 | 190/6,848 | 1.6 (0.7-3.7) | 1.9 (0.9-3.9) |
| 50-59 | 279/16,869 | 2.7 (1.4-5.4) | 3.3 (1.8-5.8) |
| 60-69 | 428/41,268 | 2.4 (1.6-3.7) | 3.1 (2.0-4.8) |
| 70-79 | 487/53,761 | 2.0 (1.4-2.7) | 2.1 (1.5-2.9) |
| 80-89 | 285/21,536 | 2.0 (1.3-2.9) | 2.3 (1.5-3.5) |
| ≥ 90 | 24/1,007 | 2.7 (0.8-9.5) | 3.3 (1.0-11) b |
| Men | |||
| < 50 | 78/5,665 | 1.7 (0.8-3.8) | 2.0 (0.9-4.5) |
| 50-59 | 105/10,791 | 1.7 (0.7-4.2) | 1.9 (0.7-5.0) |
| 60-69 | 133/21,644 | 2.2 (1.3-3.6) | 2.2 (1.1-4.5) |
| 70-79 | 111/23,186 | 1.5 (0.7-3.3) | 2.0 (0.9-4.1) |
| 80-89 | 35/7,384 | 2.4 (1.0-5.6) | 1.7 (0.6-4.7) b |
| ≥ 90 | 7/297 | 3.2 (0.5-22) b | 3.3 (0.3-38) b |
| a Adjusted for diagnosis (OA), previous surgery, approach, and operation year (in groups). b Result unreliable due to few cases in stratum. IPFF = intraoperative periprosthetic femoral fracture. CI = confidence interval. |
|||
Discussion
To our knowledge, this is one of the largest studies on IPFFs. These fractures were the most frequently reported intraoperative complication during primary THA over a 33-year period, with an incidence of 1.0%.
The incidence of IPFF during all primary THAs in Norway was 1.0% (2,268/215,825), and our data showed no systematic change in the incidence of IPFF over 33 years despite the evolution of new techniques and implants. This is relatively low compared with the range between 0.1% and 27.8% from a previous review of Sidler-Maier and Waddell (5). The wide range of incidence in IPFF studies suggests uncertainty in previous estimates, and our findings improve the knowledge of IPFF incidence in Norway.
However, epidemiological studies can be difficult to compare due to variations in methodology, classification of fractures, between-country differences in choice of implants, fixation, surgical approach, and so on. Ponzio et al. (8) investigated the incidence of IPFF in a selected cohort treated with different cementless stem designs and found a fracture incidence of 4.4%. Other studies that included only cementless stems reported incidences of 2.1%–3.7% (3,17,18).
The Danish Hip Arthroplasty Register reveal an overall IPFF incidence of 2.1%: 2.4% for cementless stems and 0.9% for cemented stems. However, they included fractures that appeared within 90 days from the date of surgery. Therefore, the incidence numbers are not comparable (19).
In our study, female sex was associated with IPFF; women had a nearly 2 times higher risk of IPFF than men, also after accounting for differences in diagnosis, previous surgery, surgical approach, and fixation technique. This finding is consistent with the literature (3,5,7,8). Some authors have found an even higher risk, i.e., that women are up to 8 times more likely to develop fractures (6,20). We hypothesize that the higher risk in female patients may be due to poor bone quality related to postmenopausal osteoporosis and changes in bone structure, factors not registered in the NAR.
Age has previously been explored as a risk factor for IPFF; however, the results have been conflicting. Some studies found that female patients over 65 years of age were at highest risk for such fractures (2,21). In contrast, other studies found that patients aged younger than 59 years (6,19,21) or younger than 50 years ( 7 ) were at high risk. We found increased risk of IPFF both for patients < 60 years and patients > 80 years. However, most of the risk increase for the younger patients was explained by diagnosis and fixation technique. Incidentally, cementless fixation has been prioritized for younger patients in the last decade. Bone morphology changes as seen in younger patients with secondary OA, avascular necrosis of the femoral head, and developmental dysplasia of the hip may challenge fitting of a standard cementless stem design.
We found no statistically significant interaction between age and sex in any analysis, meaning we found no evidence that the risk of IPFF for each sex depended on the age level. This was also found by Moroni et al. (20).
Patients with a non-OA diagnosis had over twice the risk of IPFF as compared with patients with other diagnoses, and still 70% higher risk after accounting for previous surgery on the hip, fixation technique, and approach. This finding agrees with several studies (5). Diagnoses such as inflammatory joint disease, secondary OA, avascular necrosis of the femoral head, and developmental dysplasia of the hip have all been shown to be associated with an increased risk of IPFF (6,7).
Another predisposing factor was previous surgery to the same hip, which led to a 1.8-fold higher risk for IPFF in this study. Ricioli et al. (21) and Moroni et al. (20) also found an increased risk in these patients, and the authors of the latter paper suggested using preventive measures such as cerclage wires to avoid or limit IPFFs.
Miettinen et al. (3) and Lamb et al. (7) found that IPFF occurred most frequently with the lateral approach (Hardinge). Zhao et al. (18) found a substantially increased risk of fracture in primary cementless THA procedures performed through an anterolateral (modified Hardinge) approach to the hip (4.8%) compared with cementless THA procedures performed through a posterior approach (1.4%). This finding agrees with our result of a 50% increased risk with the lateral approach, compared with the posterior approach. Future research may consider investigating whether anterior approach increases risk of IPFF.
The most important factor affecting IPFF was cementless stem fixation, leading to a 2.7-fold increase in risk for IPFF compared with cemented stems. This finding is comparable to those obtained with data from National Joint Registry for England and Wales and the Isle of Man (RR 2.4) (7). In the Danish Hip Arthroplasty Register, an even higher RR of 4.1 was observed (19), and Abdel et al., from the Mayo Clinic found that IPFF occurred 14 times more often with cementless stems than with cemented stems (2). In the review from Sidler-Maier and Waddell (5), the rate of IPFF for cementless primary THA ranged from 3% to 18%, compared with 0.3 to 1.0% for cemented stem fixation. The incidence in our data was 1.6% for cementless stems and 0.7% for cemented stems.
We found that the risk related to the use of cementless stem fixation was evident for most age and sex strata (though some strata had too few cases to get a reliable estimate of the risk). In that perspective, the increase in the use of cementless fixation that we have seen over the past 33 years, for all age groups, may not have been beneficial. We speculate that other advances in surgical technique etc. that would have lowered the incidence of IPFF may have been counteracted by this increased use of cementless stem fixation.
The strengths of this register study include the high number of cases and long observational period. A number of different implants, surgeons, and techniques were used. Therefore, the overall results are representative of the average surgeon applying average implants and instrumentation, and the external validity of the findings is presumably high. Another strength of our study is that due to the large sample size, we were able to apply a low probability of type 1 error (1%) and a high coverage probability of the CIs (99%).
However, there were also some limitations to our study. One limitation was that NAR only recorded fractures that were discovered during the operation. Schwartz et al. reported that almost 50% of their IPFFs were diagnosed only on postoperative radiographs (22). In addition, some IPFFs occur a few days postoperatively without trauma. These latter fractures were mainly reported and classified as postoperative periprosthetic fractures but are related to intraoperative events (pre-existing stress risers, fissures, cracks) and should be classified as IPFFs (23,24). There is therefore reason to believe IPFF is underreported, and the real frequency of IPFF was thus difficult to determine.
Another limitation was the validation of the outcomes. The intraoperative complications were reported as free text, often with keywords without further information, specification, or standardization. In addition, a number of factors could not be evaluated. No data on surgical factors such as broaching techniques and calcar preparation were available. Therefore, we could not assess whether cemented vs. uncemented fixation of the stem was associated with IPFF risk, mainly due to the fixation technique itself or to surgical factors related to the fixation technique. The most frequently used cementless stems throughout the study period were used with different broaches (aggressive vs. impacting), and this could potentially have an effect on the risk of IPFF. Furthermore, there was no bone morphology or bone mineral density data, and no radiological findings or surgical findings data. Surgeons are probably more inclined to choose cemented fixation for patients with poor bone structure and mineral density to reduce IPFF risk. Hence, adjusting for these factors would likely increase the difference in IPFF risk between cemented and cementless stem fixation we found. We did not know the exact timing of the fracture, or whether it occurred during femoral preparation, calcar reaming, implantation, or repositioning of the prosthesis.
We could not categorize the fractures more accurately. The complications from each surgery were reported in free text in each case, and the precision of each registration was insufficient to categorize the fractures accurately according to location and/or dislocation. Furthermore, we do not know if the IPFF was treated with additional interventions, such as cerclage wiring. Comorbidity data such as ASA score and BMI have only been registered since 2005 and 2019, respectively, and were therefore not evaluated.
For ease of presentation, we used categorized age in the main analyses, even though categorization of continuous exposure variables has been shown to reduce statistical power (25), is suboptimal for exploring nonlinear effects (26), and unreasonably assumes that the relationship between the exposure and the outcome is flat within intervals. Data-driven categorization such as quartile cut-offs exacerbates the issue, and we therefore chose intervals based on clinical rationale. Secondary analyses with nonlinear effects modeled using splines were, however, consistent with our findings from the main analysis.
Conclusion
This register study reveals that IPFF is the most frequent intraoperative complication and should be considered in future THA quality improvement programs. Patients predisposed to IPFF are female, aged above 80 years, have a non-OA diagnosis, and/or have had previous surgery on the same hip. Direct lateral approach and cementless fixation of the stem further increase risk of IPFF. To reduce IPFF occurrence, cemented stem fixation and posterior approach should be favored in high-risk patients, such as elderly women.
Further studies with standardized data on IPFFs, their subtypes, and their association with implant survival and clinical outcomes are needed to improve preoperative risk assessments and protect patients who are at particularly high risk of this complication.
- Greidanus N V, Mitchell P A, Masri B A, Garbuz D S, Duncan C P. Principles of management and results of treating the fractured femur during and after total hip arthroplasty. Instructional Course Lectures 2003; 52: 309-22.
- Abdel M P, Watts C D, Houdek M T, Lewallen D G, Berry D J. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J 2016; 98-B: 461-7.
- Miettinen S S, Makinen T J, Kostensalo I, Mäkelä K, Huhtala H, Kettunen J S, et al. Risk factors for intraoperative calcar fracture in cementless total hip arthroplasty. Acta Orthop 2016; 87: 113-19.
- Thillemann T M, Pedersen A B, Johnsen S P, Søballe K. Inferior outcome after intraoperative femoral fracture in total hip arthroplasty: outcome in 519 patients from the Danish Hip Arthroplasty Registry. Acta Orthop 2008; 79: 327-34.
- Sidler-Maier C C, Waddell J P. Incidence and predisposing factors of periprosthetic proximal femoral fractures: a literature review. Int Orthop 2015; 39: 1673-82.
- Nowak M, Kusz D, Wojciechowski P, Wilk R. Risk factors for intraoperative periprosthetic femoral fractures during the total hip arthroplasty. Polish Orthop Traumatol 2012; 77: 59-64.
- Lamb J N, Matharu G S, Redmond A, Judge A, West R M, Pandit H G. Risk factors for intraoperative periprosthetic femoral fractures during primary total hip arthroplasty: an analysis from the National Joint Registry for England and Wales and the Isle of Man. J Arthroplasty 2019; 34: 3065-73, e3061.
- Ponzio D Y, Shahi A, Park A G, Purtill J J. Intraoperative proximal femoral fracture in primary cementless total hip arthroplasty. J Arthroplasty 2015; 30: 1418-22.
- Furnes O G J-E, Hallan G, Visnes H, Gudnersen T, Kvinnesland I A, Fenstad A M, et al. Norwegian National Advisory Unit on Arthroplasty and Hip Fractures Yearly Report. 2020. Helse Bergen HF, Department of Orthopaedic Surgery, Haukeland University Hospital; 2020.
- Zou G Y, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013; 22: 661-70.
- Lie S A, Engesaeter L B, Havelin LI , Gjessing H K, Vollset S E. Dependency issues in survival analyses of 55,782 primary hip replacements from 47,355 patients. Stat Med 2004; 23: 3227-40.
- Gauthier J, Wu Q V, Gooley T A. Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant 2020; 55: 675-80.
- Harrell Jr F E. rms: Regression modeling strategies. R package version 6.2-0.; 2021.
- Lüdecke D. ggeffects: tidy data frames of marginal effects from regression models. J Open Source Software 2018; 3: 772.
- von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P C, Vandenbroucke J P, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344-9.
- Benchimol E I, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12: e1001885.
- Timmer C, Gerhardt D, de Visser E, de Kleuver M, van Susante J L C. High incidence of intraoperative calcar fractures with the cementless CLS Spotorno stem. Eur J Orthop Surg Traumatol 2018; 28: 1291-6.
- Zhao R, Cai H, Liu Y, Tian H, Zhang K, Liu Z. Risk factors for intraoperative proximal femoral fracture during primary cementless THA. Orthopedics 2017; 40: e281-e287.
- Lindberg-Larsen M, Jorgensen C C, Solgaard S, Kjersgaard A G, Kehlet H, Lunbeck Foundation Centre for Fast-track H, et al. Increased risk of intraoperative and early postoperative periprosthetic femoral fracture with uncemented stems. Acta Orthop 2017; 88: 390-4.
- Moroni A, Faldini C, Piras F, Giannini S. Risk factors for intraoperative femoral fractures during total hip replacement. Ann Chir Gynaecol 2000; 89: 113-18.
- Ricioli W Jr, Queiroz M C, Guimaraes R P, Honda E K, Polesello G, Fucs P M. Prevalence and risk factors for intra-operative periprosthetic fractures in one thousand eight hundred and seventy two patients undergoing total hip arthroplasty: a cross-sectional study. Int Orthop 2015; 39: 1939-43.
- Schwartz J T Jr, Mayer J G, Engh C A. Femoral fracture during noncemented total hip arthroplasty. J Bone Joimnt Surg Am 1989; 71: 1135-42.
- Hailer N P, Garellick G, Kärrholm J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop 2010; 81: 34-41.
- Pedersen A B, Mehnert F, Havelin L I, Furnes O, Herberts P, Kärrholm J, et al. Association between fixation technique and revision risk in total hip arthroplasty patients younger than 55 years of age: results from the Nordic Arthroplasty Register Association. Osteoarthritis Cartilage 2014; 22: 659-67.
- Bennette C, Vickers A. Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med Res Methodol 2012; 12: 21.
- Bache-Mathiesen L K, Andersen T E, Dalen-Lorentsen T, Clarsen B, Fagerland M W. Not straightforward: modelling non-linearity in training load and injury research. BMJ Open Sport Exerc Med 2021; 7: e001119.
Supplementary data
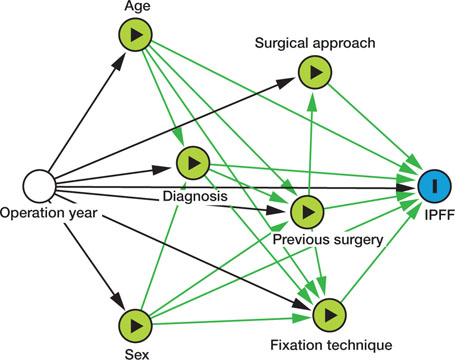
Figure 2. A directed acyclic graph (DAG) showing the theoretical pathways from the potential risk factors of interest—previous surgery, sex, age, diagnosis, fixation technique, surgical approach—to the outcome of interest, intraoperative periprosthetic femoral fracture (IPFF). The minimum set of required confounder adjustments varied between the risk factors of interest, operation year age and sex. An unbiased estimate of the association of diagnosis on the risk of IPFF required adjusting for operation year, sex, and age, whilst previous surgery also had to be adjusted for diagnosis. Confounders on the effect of surgical approach were operation year and previous surgery. Finally, for an unbiased estimate of the effect of fixation technique on the risk of IPFF, operation year, age, sex, previous surgery, and diagnosis had to be adjusted for.