Dose reduction does not impact the precision of CT-based RSA in tibial implants: a diagnostic accuracy study on precision in a porcine cadaver
Frank-David ØHRN 1,2, Lars H W ENGSETH 3,4, Are H PRIPP 5, Stephan M H RÖHRL 3,4, and Anselm SCHULZ 6
1 Orthopaedic Department, Kristiansund Hospital, Møre and Romsdal Hospital Trust, Kristiansund; 2 Faculty of Medicine and Health Sciences, Department of Neuromedicine and Movement Science (INB), NTNU Norwegian University of Science and Technology; 3 Division of Orthopaedic Surgery, Oslo University Hospital Ullevål, Oslo; 4 Faculty of Medicine, University of Oslo, Oslo; 5 Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo; 6 Department of Radiology and Nuclear Medicine, Oslo University Hospital Ullevål, Oslo, Norway
Background and purpose — Radiostereometric analysis (RSA) is the gold standard for evaluation of migration of implants. CT-RSA has been shown to have precision at the level of RSA in hip, shoulder, and knee joint replacements. We aimed to assess the impact of dose reduction on precision of CT-RSA on tibial implants, comparing it with previously published data on precision of standard dose CT-RSA on tibial implants.
Material and methods — We performed a total knee arthroplasty on a porcine knee cadaver, and subsequent CT-RSA with low effective doses (0.02 mSv). We compared the results with previously published CT-RSA data with standard (0.08 mSv) dose. The primary outcome variable was the difference in precision of the maximum total translation (MTT). Secondary variables included ratios of variances and standard deviations, and precision of peripheral point translations, center-of-mass translations, and rotations. A difference of more than 0.1 mm in precision was defined as clinically relevant. Our hypothesis was that precisions of low and standard CT-RSA doses were equal.
Results — Low dose (mean 0.07, 95% confidence interval [CI] 0.06–0.08) and standard dose CT-RSA (0.08, CI 0.07–0.09) achieve similar precision, with difference in precision of MTT of 0.01, CI 0.00–0.02 mm. The F-statistic (0.99, CI 0.63–1.55) and sdtest (1.05, CI 0.43–2.58) also supported this.
Conclusion — We conclude that the precision of low dose CT-RSA for tibial implants on a porcine cadaver is equal to standard dose CT-RSA. However, these findings should be confirmed in clinical trials.
Citation: Acta Orthopaedica 2023; 94: 550–544. DOI: https://doi.org/10.2340/17453674.2023.24022.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-07-04. Accepted: 2023-10-08. Published: 2023-10-31.
Correspondence: frank-david.ohrn@helse-mr.no
All authors contributed to the conceptualization, proofreading, and critical revision of the manuscript. FDØ wrote the paper. AHP, LHWE, and FDØ performed the statistical analyses. LHWE performed the CT-RSA analyses.
The authors would like to thank physicist Kristin Jensen for help with the CT protocol and performing CT scans, and radiographers Mona Risdal and Alexis Hinojosa. They are also grateful to Møre and Romsdal Hospital Trust for funding.
Handling co-editors: Ivan Hvid and Robin Christensen
Acta thanks Leif Rydfor help with peer review of this manuscript.
Total knee arthroplasty (TKA) is in most, but not all, cases successful in treating osteoarthritis of the knee. Thus, the industry is developing new designs in order to increase the satisfaction of patients and the function of their artificial joints. There are many examples of failed implants in terms of aseptic loosening or suboptimal migration patterns when we introduce new implants or alter their attachment to bone [1-3]. Hence, all new implants or surgical techniques should follow a stepwise introduction to the market [4-6]. Radiostereometric analysis (RSA) has been the gold standard for detecting increased early migration as a surrogate for aseptic loosening for more than 40 years [7]. The introduction of CT-RSA (CT-based RSA) represents a change of paradigm in the study of early implant loosening. In CT-RSA, we use CT scans and assess the migration of implants using a special software program, e.g., CT-based Micromotion Analysis (CTMA, Sectra AB, Linköping, Sweden) [8-10]. Instead of using computer-aided design (CAD) models, or reverse engineered models, as in model-based RSA (MBRSA) [11], models are created in the software based on the CT scans. There is no need for bone markers.
CT-RSA provides precision at or above the level of RSA in hip and shoulder implants [9,12-13]. The aim of our study was to assess the impact of dose reduction on precision of CT-RSA on tibial implants with the objective to compare the results of low dose CT-RSA with previously published data on precision of standard dose CT-RSA on tibial implants [10]. Our hypothesis (H0) was that the precision of low (0.02 mSv) doses would be equal to standard (0.08mSv) doses. The corresponding alternative hypothesis (H1) was that dose reduction results in inferior precision.
Methods and materials
This is an equivalence study that adheres to the STARD and ARRIVE guidelines [10,14-15]. For details of the methods and materials, please see our previously published paper on this topic [10]. A NexGen CR size 4 (ZimmerBiomet, Warsaw, IN, USA) was inserted in a hind knee of a porcine cadaver (Figure 1). The phantom was stored at –18℃, until it was thawed prior to testing. We used a GE Revolution (GE Healthcare, Chicago, IL, USA) CT scanner with the following standard scan protocol: tube voltage 120 kV, tube current 100 mAs, slice thickness 0.625 mm, rotation time 0.5 s, pitch 1.0, and field-of-view 200 x 200 mm. An iterative reconstruction algorithm (ASiRV50) with metal artifact reduction (MAR) was used for image reconstruction (Figure 1). The CT dose index volume (CTDIvol) of the standard dose scan was 6.34 mGy, and 1.59 mGy for low dose. The dose length product (DLP) of the standard CT was 197.8 mGy×cm, corresponding to an effective dose (ED) of 0.08 mSv, while the figures were 48.3 mGy×cm and 0.02 mSv for the low-dose scan. EDs were estimated by multiplying the DLP by the knee conversion factor of 0.0004 [16]. The CT-RSA analyses were performed using the CTMA software. During CT-RSA analysis, peripheral corresponding points on the tip, and lateral, medial, anterior, and posterior on the tibial implant were created (Figure 2) [10]. 7 exposures were performed with the phantom for the CT scanner. Between each CT-RSA exposure, the phantom was repositioned. LHWE performed the CT-RSA analyses in both studies, for which he has been certified by Sectra [10]. During CT-RSA analysis, each individual investigation was compared with the next, i.e., #1 and #2, #1 and #3 up to #7, and then #2 and #3 and so on, and given the name Sample 1, Sample 2 etc., thus yielding a total of 21 double examinations or samples, for both standard and lowdose CT-RSA. Sample 1 standard dose was compared with Sample 1 low dose. Assuming there would be no migration between any of the exposures, any deviation from zero represents a measure of the precision of the method. The higher the deviation, the larger the bias and the lower the precision. In the CTMA software, it is currently not possible to calculate the maximum total point motion (MTPM), a very important factor in RSA analysis. MTPM is a vector representing the highest motion on the implant from one time point to another. The closest we can get to the MTPM using CTMA is the highest total translation among each of the peripheral points. We called this the maximum total translation (MTT). The primary outcome of this study was the difference in precision of MTT between standard and low-dose CT-RSA. We also compared the motions and rotations at the center of mass (COM). This corresponds to the segmental motions and rotations of RSA. Finally, we compared the translations of the corresponding peripheral points on the CT-RSA model. The data for standard dose CT-RSA and RSA was presented in a previous publication. For further details, please see that paper [10].
Figure 1. CT-RSA model of the NexGen CR TKA.
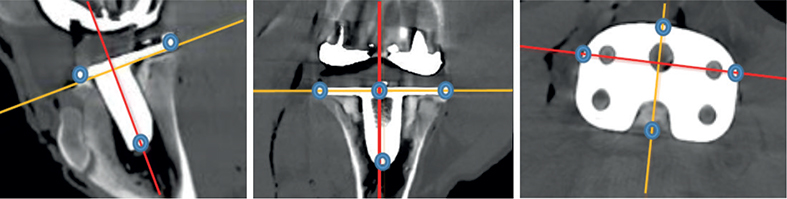
Figure 2. CT-RSA images with corrected axes and blue dots indicating the placement of the peripheral points on the tip, anterior, posterior, lateral, and medial part of tibial implant.
Statistics
All calculations were performed using STATA version 17 (StataCorp LLC, College Station, TX, USA). In RSA, precision can either be presented as means with 95% confidence intervals (CI) if normally distributed, or as standard deviations. As our data was normally distributed, the precisions were presented as means with 95% CI. We tested the equality of standard deviations (variances) using the sdtest command in Stata. The corresponding F statistic, the ratio of the variances, was also calculated.
The primary endpoint of this study was the difference in precision of MTT between the standard and low-dose CT-RSA. This was calculated with a mixed-model analysis using dose as fixed factor and sample number as random effect. Previous studies show that the precision of MTPM in RSA in TKA is larger than 0.14 mm [17-20]. On the other hand, an increase in MTPM of more than 0.2 mm from 1–2 years is shown to predict early loosening of the implants [7]. Our point of view is therefore that a clinically meaningful equivalence margin would be 0–0.10 mm. Thus, if the difference in precision of standard-dose and low-dose CT-RSA including CI was outside this interval, the H0 hypothesis would be rejected.
The Digital Imaging and Communications in Medicine (DICOM) coordinate system of CT-RSA was aligned to the RSA coordinate system in order to compare the methods, and was renamed medial, proximal and anterior translation, and transversal, internal, and varus rotation [10].
Ethics, data sharing, funding and disclosures
This study was not a clinical study. As such, approval from the Norwegian Ethical Committee was therefore not required. It was, however, approved by the local research committee of Oslo University Hospital on December 13, 2021. Data will be shared upon request to the main author. The study was funded by Møre and Romsdal Hospital Trust and Oslo University Hospital. The authors declare no conflict of interests. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.24022
Results
This study shows that the precision data of MTT of the low-dose CT-RSA (mean 0.07, CI 0.06–0.08) are comparable with standard-dose CT-RSA (mean 0.08, CI 0.07–0.09) (Table, Figure 3) with an F-statistic of 0.99 (CI 0.63-1.55), P = 0.9 and a corresponding sdtest of 1.05 (CI 0.43–2.58), P = 0.9. The mean difference in precision was 0.01 (CI 0.00–0.02) (Table). The precision and mean difference of precision for COM translations and rotations, and peripheral point translations are also given in the Table.
| Variable | Standard dose CT-RSA | Low dose CT-RSA | Difference |
| Maximum total translation (MTT) | |||
| MTT, mm | 0.08 (0.07–0.09) | 0.07 (0.06–0.08) | 0.01 (0.00–0.02) |
| Segmental translation, mm | |||
| Medial | 0.00 (–0.01 to 0.01) | 0.00 (–0.01 to 0.01) | 0.00 (–0.02 to 0.01) |
| Proximal | 0.02 (–0.00 to 0.04) | 0.00 (–0.01 to 0.01) | 0.02 (0.00 to 0.04) |
| Anterior | –0.01(–0.02 to 0.00) | 0.01 (0.01 to 0.02) | –0.02 (–0.03 to –0.01) |
| Segmental rotation, ° | |||
| Transversal | 0.01 (0.00 to 0.02) | –0.01 (–0.02 to 0.00) | 0.02 (0.01 to 0.03) |
| Internal | 0.02 (0.00 to 0.04) | 0.00 (–0.01 to 0.01) | 0.02 (0.00 to 0.04) |
| Varus | –0.01 (–0.02 to –0.00) | 0.01 (0.01 to 0.02) | –0.02 (–0.03 to –0.01) |
| Translations of the peripheral points, mm | |||
| Tip | |||
| Medial | –0.01 (–0.01 to v) | 0.01 (0.01 to 0.02) | –0.02 (–0.03 to –0.01) |
| Proximal | 0.02 (0.00 to 0.03) | 0.00 (–0.01 to 0.01) | 0.02 (0.00 to 0.04) |
| Anterior | 0.00 (–0.01 to 0.00) | 0.01 (–0.00 to 0.01) | –0.01 (–0.02 to –0.01) |
| Medial | |||
| Medial | 0.00 (–0.02 to 0.01) | 0.00 (–0.01 to 0.01) | 0.00 (–0.02 to 0.01) |
| Proximal | 0.02 (0.00 to 0.04) | 0.01 (–0.01 to 0.03) | 0.01 (–0.01 to 0.03) |
| Anterior | –0.03 (–0.04 to 0.01) | 0.02 (0.04 to 0.04) | –0.05 (–0.06 to –0.03) |
| Lateral | |||
| Medial | 0.00 (–0.02 to 0.01) | 0.00 (–0.01 to 0.01) | 0.00 (–0.02 to 0.01) |
| Proximal | 0.02 (0.00 to 0.04) | –0.01 (–0.03 to 0.01) | 0.03 (0.01 to 0.05) |
| Anterior | 0.01 (0.00 to 0.02) | 0.01 (0.00 to 0.02) | 0.00 (–0.01 to 0.01) |
| Anterior | |||
| Medial | –0.01 (–0.03 to 0.00) | 0.00 (–0.01 to 0.01) | –0.02 (–0.03 to 0.00) |
| Proximal | 0.02 (0.00 to 0.03) | 0.04 (–0.01 to 0.01) | –0.02 (–0.03 to –0.01) |
| Anterior | 0.02 (0.00 to 0.03) | 0.01 (0.01 to 0.01) | 0.01 (–0.01 to 0.03) |
| Posterior | |||
| Medial | 0.01 (–0.01 to 0.03 | –0.01 (–0.02 to 0.01) | 0.02 (0.00 to 0.03) |
| Proximal | 0.02 (0.01 to 0.04) | 0.00 (–0.02 to 0.01) | 0.03 (0.00 to 0.05) |
| Anterior | –0.01 (–0.02 to 0.00) | 0.01 (0.01 to 0.02) | –0.02 (–0.03 to –0.01) |
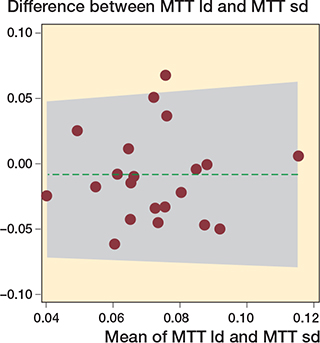
Figure 3. Bland–Altman plot of the mean maximum total translation (MTT) precisions (X-axis) and difference of precision (Y-axis). 1/21 (4.8%) outside the limits of agreement. MTT ld = low dose, 0.02 mSv and MTT sd = standard dose, 0.08 mSv.
Discussion
Our aim was to assess the impact of dose reduction on precision of CT-RSA on tibial implants comparing it with previously published data on precision of standard dose CT-RSA on tibial implants [10].
We showed that implementing a low-dose protocol with 75% reduction in radiation dosage can maintain precision comparable to that of a standard CT dose (Table). Furthermore, our results indicate that the precision achieved through the low-dose protocol surpasses that of RSA when assessing tibial implants [10]. The results in the Table also indicate that the lower precision in out-of-plane motions that RSA studies may be subject to is hardly relevant to CT-RSA [21-22].
CT-RSA is a novel method. It has been shown to have high accuracy and precision on migration analysis in shoulder and hip arthroplasty [9,12-13]. The documentation on tibial implants is, however, still rather sparse. We recently published a phantom study, which showed that CT-RSA had higher precision than RSA [10]. To our knowledge, this is the only publication on tibial implants using CT-RSA. Clinical documentation of this is therefore still lacking.
CT is known to have a much higher radiation dose than RSA, where we use a combination of 2 simultaneous X-ray images. In total hip arthroplasty, published effective doses of radiation vary between 0.2 and 0.8 mSv using CT-RSA [8,23-24], and 0.10–0.15 mSv using RSA [25]. Our group recently published a study showing that we can achieve high precision using CT-RSA on tibial implants with an ED of 0.08 mSv [10]. The use of low-dose CT-RSA would hold clinical significance if it could effectively reduce radiation exposure while maintaining precision, thereby mitigating the risk of cancer associated with higher radiation doses [26]. RSA, on the other hand, is a difficult procedure to analyze, and for radiographers to implement. The images must also quite frequently be retaken to show a sufficient number of tantalum markers for analysis. In CT-RSA, we need no bone markers, implant markers, or CAD models. CT scanners are also available at all hospitals, with no need for extra equipment. The main argument against CT-RSA, as opposed to RSA, has been the radiation dose. As there is a dose–response relationship between radiation dose from CT scans and risk of cancer, lowering the radiation dose is of paramount importance [26]. In our previous study, we were able to show that the ED was only 16 times higher with CT-RSA than with RSA [10]. Thus, if we can reduce the ED by 75%, and if we bear in mind the occasional retakes of the RSA, the 2 methods require approximately the same ED levels. With the even higher precision of CT-RSA, the fact that CT scanners are readily available in hospitals worldwide, and that the method is both easier to perform for radiographers and to analyze for surgeons/radiologists, CT-RSA has the potential to take over the role of RSA, without the risk of higher radiation doses.
Limitations of the study
The main limitation of our study is that it is a porcine cadaver study and not a clinical study. However, we believe that to use this method in a clinical study, it is imperative to show in advance that it works in a cadaver study. Despite the use of very low EDs in our study, for clinical verification we would still have to add this radiation dose to any other radiation exposure. We are of the opinion that it is a more ethical and clinically safe procedure to perform a phantom study in advance of a clinical study. Further, we used a porcine rather than a human cadaver. Human and porcine anatomy and bone structure are, however, quite similar [27]. Another limitation is that we used only 1 cadaver. This was done for practical purposes. We are aware that this makes the samples dependent on another, and in an ideal world we should have used 21 unique samples. On the other hand, similar study designs have been used in other cadaver studies previously [9-10,28-30]. The MTPM, a vector with no direction, representing the point of an implant that moves the most from one time point to another, is very important in migration and precision research [7,31-32]. However, MTPM is not currently available for calculation in the CTMA software. Despite this, the 5 peripheral points cover the surface of the implant, and thus also address this shortcoming. The peripheral point with the highest translation has previously been shown to be a good estimate of MTPM [33]. Nevertheless, it is likely that MTPM will soon be available in the CTMA software.
Strengths of the study
The study also has several strengths. First, this is the first study to show that CT-RSA with as low an ED as 0.02 mSv provides sufficient precision on tibial implants. CT-RSA only focuses on surface models of bones and implants, not soft tissue. This is probably the reason why we can reduce the radiation without sacrificing precision. Although the conversion factor is much higher in the hip (0.011) than the knee (0.0004) [16], the principles shown in this study are likely to apply to several other joints and implants. This, however, still remains to be proven. Second, we compared our results with previously published data on CT-RSA, and could thus compare the results directly, because all the data was collected at the same time and under the same circumstances.
CT-RSA will probably be the future measuring tool for migration studies. This study therefore adds important knowledge to this field of research.
Conclusion
The precision of low-dose CT-RSA for tibial implants on a porcine cadaver is equal to standard dose CT-RSA. However, these findings should be confirmed in clinical trials.
- Gothesen O, Lygre S H L, Lorimer M, Graves S, Furnes O. Increased risk of aseptic loosening for 43,525 rotating-platform vs. fixed-bearing total knee replacements. Acta Orthop 2017; 88(6): 649-56. doi: 10.1080/17453674.2017.1378533.
- Nilsson K G, Dalén T. Inferior performance of Boneloc bone cement in total knee arthroplasty: a prospective randomized study comparing Boneloc with Palacos using radiostereometry (RSA) in 19 patients. Acta Orthop Scand 1998; 69(5): 479-83. doi: 10.3109/17453679808997782.
- Kaptein B L, den Hollander P, Thomassen B, Fiocco M, Nelissen R. A randomized controlled trial comparing tibial migration of the ATTUNE cemented cruciate-retaining knee prosthesis with the PFC-sigma design. Bone Joint J 2020; 102-b(9): 1158-66. doi: 10.1302/0301-620x.102b9.Bjj-2020-0096.R1.
- Malchau H. Introducing new technology: a stepwise algorithm. Spine (Phila Pa 1976) 2000; 25(3): 285. doi: 10.1097/00007632-200002010-00004.
- Nelissen R G, Pijls B G, Kärrholm J, Malchau H, Nieuwenhuijse M J, Valstar E R. RSA and registries: the quest for phased introduction of new implants. J Bone Joint Surg Am 2011; 93(Suppl. 3): 62-5. doi: 10.2106/jbjs.k.00907.
- Hasan S, Marang-van de Mheen P J, Kaptein B L, Nelissen R, Pijls B G. RSA-tested TKA implants on average have lower mean 10-year revision rates than non-RSA-tested designs. Clin Orthop Relat Res 2020; 478(6): 1232-41. doi: 10.1097/corr.0000000000001209.
- Ryd L, Albrektsson B E, Carlsson L, Dansgard F, Herberts P, Lindstrand A, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br 1995; 77(3): 377-83. PMID: 7744919.
- Angelomenos V, Mohaddes M, Itayem R, Shareghi B. Precision of low-dose CT-based micromotion analysis technique for the assessment of early acetabular cup migration compared with gold standard RSA: a prospective study of 30 patients up to 1 year. Acta Orthop 2022; 93: 459-65. doi: 10.2340/17453674.2022.2528.
- Brodén C, Giles J W, Popat R, Fetherston S, Olivecrona H, Sandberg O, et al. Accuracy and precision of a CT method for assessing migration in shoulder arthroplasty: an experimental study. Acta Radiol 2020; 61(6): 776-82. doi: 10.1177/0284185119882659.
- Engseth L H W, Schulz A, Pripp A H, Röhrl S M H, Øhrn F D. CT-based migration analysis is more precise than radiostereometric analysis for tibial implants: a phantom study on a porcine cadaver. Acta Orthop 2023; 94: 207-14. doi: 10.2340/17453674.2023.12306.
- Kaptein B L, Valstar E R, Stoel B C, Rozing P M, Reiber J H C. A new model-based RSA method validated using CAD models and models from reversed engineering. J Biomech 2003; 36(6): 873-82. doi: 10.1016/s0021-9290(03)00002-2.
- Brodén C, Olivecrona H, Maguire G Q, Jr, Noz M E, Zeleznik M P, Sköldenberg O. Accuracy and precision of three-dimensional low dose CT compared to standard RSA in acetabular cups: an experimental study. Biomed Res Int 2016; 2016: 5909741. doi: 10.1155/2016/5909741.
- Brodén C, Sandberg O, Olivecrona H, Emery R, Sköldenberg O. Precision of CT-based micromotion analysis is comparable to radiostereometry for early migration measurements in cemented acetabular cups. Acta Orthop 2021; 92(4): 419-23. doi: 10.1080/17453674.2021.1906082.
- STAndards for the Reporting of Diagnostic accuracy studies (STARD) checklist. Equator network. 2015. Available from: https://www.equator-network.org/wp-content/uploads/2015/03/STARD-2015-checklist.pdf. Date assessed September 8, 2023.
- The ARRIVE Guidelines. Available from: https://arriveguidelines.org/. Date assessed September 8, 2023.
- Saltybaeva N, Jafari M E, Hupfer M, Kalender W A. Estimates of effective dose for CT scans of the lower extremities. Radiology 2014; 273(1): 153-9. doi: 10.1148/radiol.14132903.
- Sporer S, MacLean L, Burger A, Moric M. Evaluation of a 3D-printed total knee arthroplasty using radiostereometric analysis: assessment of highly porous biological fixation of the tibial baseplate and metal-backed patellar component. Bone Joint J 2019; 101-b(7_Supple_C): 40-7. doi: 10.1302/0301-620x.101b7.Bjj-2018-1466.R1.
- Øhrn F D, Lian Ø B, Tsukanaka M, Röhrl S M. Early migration of a medially stabilized total knee arthroplasty: a radiostereometric analysis study up to two years. Bone Jt Open 2021; 2(9): 737-44. doi: 10.1302/2633-1462.29.Bjo-2021-0115.R1.
- Winther N S, Jensen C L, Jensen C M, Lind T, Schroder H M, Flivik G, et al. Comparison of a novel porous titanium construct (Regenerex(R)) to a well proven porous coated tibial surface in cementless total knee arthroplasty: a prospective randomized RSA study with two-year follow-up. Knee 2016; 23(6): 1002-11. doi: 10.1016/j.knee.2016.09.010.
- Turgeon T R, Gascoyne T C, Laende E K, Dunbar M J, Bohm E R, Richardson C G. The assessment of the stability of the tibial component of a novel knee arthroplasty system using radiostereometric analysis. Bone Joint J 2018; 100-B(12): 1579-84. doi: 10.1302/0301-620X.100B12.BJJ-2018-0566.R1.
- Petersen E T, Vind T D, Jürgens-Lahnstein J H, Christensen R, de Raedt S, Brüel A, et al. Evaluation of automated radiostereometric image registration in total knee arthroplasty utilizing a synthetic-based and a CT-based volumetric model. J Orthop Res 2023; 41(2): 436-46. doi: 10.1002/jor.25359.
- Trozzi C, Kaptein B L, Garling E H, Shelyakova T, Russo A, Bragonzoni L, et al. Precision assessment of model-based RSA for a total knee prosthesis in a biplanar set-up. Knee 2008; 15(5): 396-402. doi: 10.1016/j.knee.2008.05.001.
- Brodén C, Sandberg O, Sköldenberg O, Stigbrand H, Hänni M, Giles J W, et al. Low-dose CT-based implant motion analysis is a precise tool for early migration measurements of hip cups: a clinical study of 24 patients. Acta Orthop 2020; 91(3): 260-5. doi: 10.1080/17453674.2020.1725345.
- Eriksson T, Maguire G Q, Jr., Noz M E, Zeleznik M P, Olivecrona H, Shalabi A, et al. Are low-dose CT scans a satisfactory substitute for stereoradiographs for migration studies? A preclinical test of low-dose CT scanning protocols and their application in a pilot patient. Acta Radiol 2019; 60(12): 1643-52. doi: 10.1177/0284185119844166.
- Blom I F, Koster L A, Brinke B T, Mathijssen N M C. Effective radiation dose in radiostereometric analysis of the hip. Acta Orthop 2020; 91(4): 390-5. doi: 10.1080/17453674.2020.1767443.
- Cao C F, Ma K L, Shan H, Liu T F, Zhao S Q, Wan Y, et al. CT scans and cancer risks: a systematic review and dose-response meta-analysis. BMC Cancer 2022; 22(1): 1238. doi: 10.1186/s12885-022-10310-2.
- Cone S G, Warren P B, Fisher M B. Rise of the pigs: utilization of the porcine model to study musculoskeletal biomechanics and tissue engineering during skeletal growth. Tissue Eng Part C Methods 2017; 23(11): 763-80. doi: 10.1089/ten.TEC.2017.0227.
- Kibsgard T J, Roise O, Stuge B, Rohrl S M. Precision and accuracy measurement of radiostereometric analysis applied to movement of the sacroiliac joint. Clin Orthop Relat Res 2012; 470(11): 3187-94. doi: 10.1007/s11999-012-2413-5.
- Fraser A N, Tsukanaka M, Fjalestad T, Madsen J E, Röhrl S M. Model-based RSA is suitable for clinical trials on the glenoid component of reverse total shoulder arthroplasty. J Orthop Res 2018; 36(12): 3299-307. doi: 10.1002/jor.24111
- Bojan A J, Bragdon C, Jönsson A, Ekholm C, Kärrholm J. Three-dimensional bone-implant movements in trochanteric hip fractures: precision and accuracy of radiostereometric analysis in a phantom model. J Orthop Res 2015; 33(5): 705-11. doi: 10.1002/jor.22822.
- Pijls B G, Valstar E R, Nouta K A, Plevier J W, Fiocco M, Middeldorp S, et al. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop 2012; 83(6): 614-24. doi: 10.3109/17453674.2012.747052.
- Pijls B G, Plevier J W M, Nelissen R. RSA migration of total knee replacements. Acta Orthop 2018; 89(3): 320-8. doi: 10.1080/17453674.2018.1443635.
- Niesen A E, Garverick A L, Hull M L. Maximum total point motion of five points versus all points in assessing tibial baseplate stability. J Biomech Eng 2021; 143(11). doi: 10.1115/1.4051347.