Comparison of early migration patterns between a ceramic and polyethylene liner in uncemented Trabecular Titanium cups: a 2-year randomized controlled trial of 52 hips using radiostereometric analysis
Amanda D KLAASSEN 1,*, Elisabeth A SCHÄFFER 1, Nienke W WILLIGENBURG 1, Loes W A H VAN BEERS 1, Vanessa A B SCHOLTES 1, Victor P M VAN DER HULST 2, Lennard A KOSTER 3, Bart L KAPTEIN 3, Dirk Jan F MOOJEN 1, and Rudolf W POOLMAN 1,4
1Department of Orthopedic Surgery, OLVG, Amsterdam; 2Department of Radiology, OLVG, Amsterdam; 3Department of Orthopedic Surgery, RSAcore, LUMC, Leiden; 4Department of Orthopedic Surgery, LUMC, Leiden, The Netherlands<
Background and purpose: Ceramic liners may reduce early stability of uncemented acetabular components due to higher stiffness. However, the bone ingrowth capacities of porous trabecular titanium might compensate for this effect. This prospective randomized trial quantifies migration patterns of the Delta-TT cup, and compares polyethylene and ceramic liners.
Patients and methods: Patients undergoing primary uncemented total hip arthroplasty with the Delta-TT cup and femoral stem with ceramic head were randomized to a polyethylene (n = 25) or ceramic (n = 28) liner. Radiostereometric analysis (RSA) radiographs, patient-reported hip function (HOOS-PS, OHS), and quality of life (EQ5D) were collected at baseline and 1.5, 3, 6, 12, and 24 months postoperatively. Model-based RSA was used to calculate 3D cup translation and rotation, and mixed models were used to compare effects over time between groups.
Results: At 2 years follow-up, Delta-TT cups showed similar mean proximal translation of 0.56 mm (95% CI 0.38– 0.75) in the ceramic (CE) group and 0.54 mm (0.30–0.77) in the polyethylene (PE) group, with a between group effect of 0.02 mm (–0.20–0.23). Most cup migration occurred in the first 1.5 to 3 months, stabilizing within 6 months. Any between-group effects were ≤ 0.30 mm for translation and ≤ 0.45° for rotation. Improvements in patient-reported hip function and quality of life were similar in both groups.
Interpretation: Regardless of liner type, Delta-TT cups showed some initial migration and stabilized within 6 months, which seems promising for longterm fixation in both cup-liner constructs.
Citation: Acta Orthopaedica 2022; 93: 2267–333. DOI http://dx.doi.org/10.2340/17453674.2022.2267.
Copyright: © 2022 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2021-09-24. Accepted: 2022-02-22. Published: 2022-04-28.
Correspondence: a.d.klaassen@olvg.nl
The authors would like to thank their OLVG colleagues from the radiology department for their tremendous help in obtaining excellent RSA X-rays, the investigators at JointResearch for logistic support, and all patients for their willingness and time to participate.
All co-authors (AK, ES, NW, LvB, VS, VvdH, LK, BK, DJM, RP) contributed to the set-up and execution of this randomized clinical trial. AK, ES, NW, and RP contributed to the writing process of the manuscript. All co-authors revised and approved the manuscript.
Acta thanks Thomas Baad-Hansen and Geir Hallan for help with peer review of this study.
Despite continuous developments to ensure long-term fixation of cementless hip implants, aseptic loosening of the acetabular component remains one of the main causes of implant failure and revision (1-3).
Early radiostereometric analysis (RSA) migration patterns at 2 years postoperatively can predict implants at risk of future aseptic loosening (4,5). Therefore, RSA has become the recommended means in evidence-based phased introduction of new implants (4-6).
Trabecular Titanium is a recently developed biomaterial, with osseointegration-promoting characteristics (7-10). This biomaterial was used to create the highly porous cementless press-fit hemispheric Delta-TT cup (LimaCorporate, Villanova San Daniele del Friuli, Italy). Various clinical studies have demonstrated promising short- and mid-term outcomes of the Delta-TT cup (11-13). However, this is the first study to assess implant fixation of this uncemented Trabecular Titanium cup by means of RSA.
An elasticity modulus similar to native bone results in reduced stress-shielding and improved bone remodeling (14-16), which is beneficial to osseointegration (17-19). Titanium cups with a polyethylene liner have been shown to generate a more physiological load transfer to the periprosthetic bone than less compliant cup-liner constructs (15,20). It is speculated that besides cup stiffness, the stiffness of the liner material affects the stability of the acetabular-liner construct (21).
We evaluated early migration patterns of uncemented Delta-TT cups with a ceramic liner compared with Delta-TT cups with a polyethylene liner. The hypothesis is that there will be more migration in the first 2 years postoperatively for Delta-TT cups with a ceramic liner because of the higher stiffness of the cup-liner construct. Secondary aims were to provide insight into migration patterns of this uncemented Trabecular Titanium cup and evaluate the improvement in patientreported outcome measures (PROMs).
Patients and methods
Patients and design
In this single-center, patient-blinded, randomized controlled trial patients scheduled to undergo hip arthroplasty were screened for eligibility. Criteria for inclusion were males or non-pregnant females between 18 and 75 years of age scheduled to undergo unilateral primary total hip arthroplasty. Exclusion criteria were BMI > 40, systemic or metabolic disorders leading to progressive bone deterioration, bone stock compromised by disease or infection, and comorbidities affecting the hip or lower extremity limiting their recovery. Surgeries were performed between October 2014 and February 2016 in OLVG hospital, Amsterdam, the Netherlands. All participating patients provided formal written consent prior to randomization. Patients were randomized using an online randomization program to receive the Delta-TT cup with either a polyethylene liner (PE group) or a ceramic liner (CE group). Data was collected preoperatively and at 6 weeks, 3 months, 6 months, 1 year, and 2 years after surgery.
Surgical technique
All surgeries were performed by 2 senior orthopedic surgeons (DJM and RP) using a posterolateral approach with lateral decubitus position. Both surgeons performed a minimum of 5 THAs with Delta-TT cups prior to any study-related surgeries. A straight cementless press-fit metaphyseal fixating stem (H-MAX S stem, LimaCorporate) was used, consisting of titanium alloy with a macro-textured hydroxyapatite coating. All femoral heads were sized 32 mm and made of BIOLOX Delta ceramic (CeramTec GmbH, Plochingen, Germany). Patients received an uncemented Delta-TT cup (LimaCorporate, Villanova San Daniele del Friuli, Italy), size 50–62 mm (Figure 1). The cup consists of a 3D-printed titanium alloy acetabular shell (22). With an average porosity of 65% and a mean pore diameter of 640 μm, the porous surface is expected to enhance osseointegration and eventually create a full cup surface force transmission, promoting strong fixation to the bone (11,22). Delta-TT cups were implanted aiming at 45° inclination and 15° anteversion. As defined by randomization, the cup was combined with either a cross-linked ultra-high molecular weight polyethylene (UHMWPE X-Lima, LimaCorporate) liner or a BIOLOX Delta ceramic liner (CeramTec GmbH, Plochingen, Germany).

Figure 1. Cementless press-fit Delta TT (Trabecular Titanium) cup.
RSA radiographs
During surgery, 9 1.0-mm spherical tantalum markers were inserted in the surrounding acetabular bone with a marker insertion tool (BAAT Medical, Hengelo, the Netherlands). Baseline RSA imaging was before weightbearing, 1–3 days postoperatively. Follow-up imaging was at 1.5, 3, 6, 12, and 24 months after surgery. Radiographs were acquired with the patient in supine position over a uniplanar calibration cage (Cage 18, Halifax Biomedical, Mabou, Canada), using a ceiling-mounted tube (43 x 35 cm Canon DR detector; Canon Medical Systems Corp, Tochigi, Japan) and a mobile tube (43 x 35 cm AGFA CR detector; AFGA HealthCare, Mortsel, Belgium).
RSA modeling
Anonymized RSA radiographs were analyzed using model-based RSA (Model-based RSA software, version 4.11, RSAcore, Leiden University Medical Center, Leiden, the Netherlands) with an Elementary Geometrical Shape (EGS) hemisphere model (23). Implant migration was defined as 3D translations and rotations over time with respect to the bone markers (Figure 2) and expressed as for a right-sided hip (24). Rotations about the 3 axes were calculated using the rotations of the Y-axis of the EGS model itself (25).
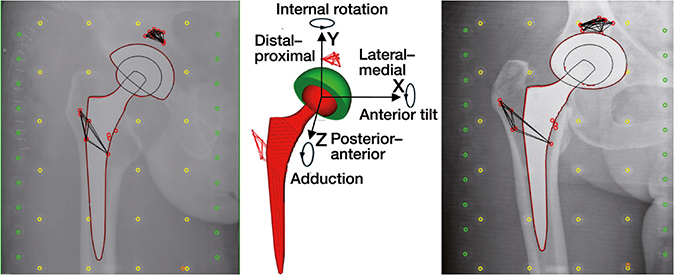
Figure 2. Model used for model-based RSA and coordinate system used to present migration along and about the X-, Y-, and Z-axis. Directions as presented in the figure indicate positive migration values. Migration results of left-sided hips were converted by changing the sign to comply with anatomical directions (24).
RSA images with at least 3 visible bone markers were analyzed. Criteria for marker stability and distribution were applied with a mean error of rigid body fitting (ME) < 0.35 mm and condition number (CN) < 150 m–1 (24). If possible, a marker configuration model (MC-model) was used to prevent data loss (26). Precision of RSA technique was measured by performing double RSA examinations at 1 year follow-up (Table 1).
Clinical outcomes
To evaluate patient-reported hip function and quality of life, patients completed the Hip disability and Osteoarthritis Outcome Score Physical function Short form (HOOS-PS), the Oxford Hip Score (OHS), and the EuroQol 5-Dimensions (EQ5D-3L). Questionnaires were completed using an online application or on paper at the same follow-up moments as the RSA radiographs. Demographics, medical history, and regular radiographs were collected preoperatively, and postoperative complications were registered.
Sample size
We aimed for 25 participants in each group, to be able to detect a difference in migration between groups with a large effect size (Cohen’s d = 0.8) at a 0.05 significance level with 80% power. Power calculations were based on a SD of 0.3 mm cup translation and 0.8° cup rotation (27).
Statistics
Migration and PROMs were assessed using mixed modeling, with group (CE vs. PE) as primary independent value of interest. For analysis of PROMs, preoperative baseline scores were added as covariate. Primary outcomes were the group effects over the 2-year follow up period. By including time as a categorical factor variable and a time-by-group interaction term, we also evaluated group differences at each time point. The repeated measurements were clustered within patients and the only random effect was a random intercept. SPSS Statistics software version 22.0 (IBM Corp, Armonk, NY, USA) was used for the statistical analysis.
Ethics, registration, funding, data sharing, and potential conflicts of interest
The trial was approved by the local medical ethics committee (registration number NL44230.100.13). The study was conducted according to the Declaration of Helsinki (2008) and registered at ClinicalTrials.gov (NCT03093038). The cohort study that is also described under this trial number will be reported separately. Data is available upon request. LimaCorporate was involved in the conception and design of the study and provided financial support. LimaCorporate provided information on their medical devices that the researchers used in the introduction and discussion of the manuscript. Lima-Corporate was not involved in the collection, analysis, and interpretation of the data.
Results
Patients
To ensure a minimum of 25 patients with acceptable baseline RSA in each study group, 58 patients were randomized (Figure 3). Patients were randomized 1 week before surgery in order to provide enough time to order the required materials and prepare surgery logistics. Patient demographics and PROM scores at baseline are presented in Table 2. For 3 cups, an MC-model was used. At 2 years postoperatively, 51 patients completed follow-up and 49 patients had RSA images suitable for analysis.
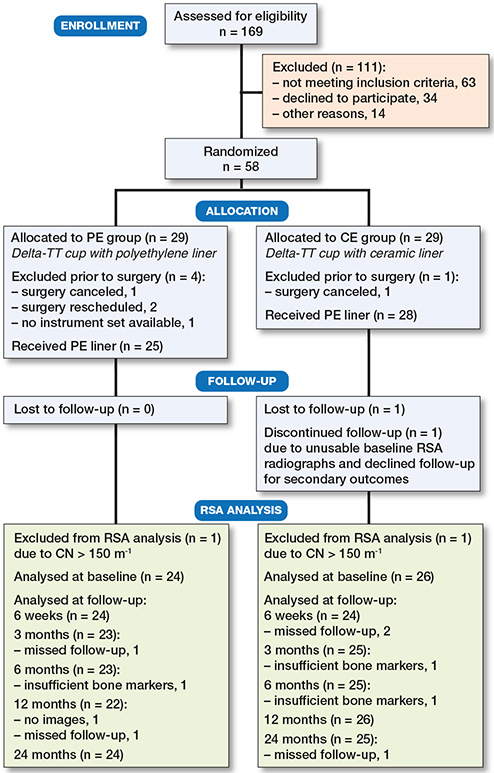
Figure 3. Flowchart of patient enrollment.
Radiostereometric analysis
At 2 years’ follow-up, Delta-TT cups showed mean proximal translation of 0.56 mm in the CE group and 0.54 mm in the PE group. For translation, between-group effects over time were 0.23 mm (95% CI –0.10–0.57), 0.05 mm (–0.14–0.25), and –0.01 mm (–0.25–0.24) on the X-, Y-, and Z axis respectively. For rotation, between-group effects over time were 0.20° (–0.25–0.65), 0.17° (–0.48–0.83), and 0.37° (–0.39–1.1) about the X-, Y-, and Z-axis respectively (Figure 4).
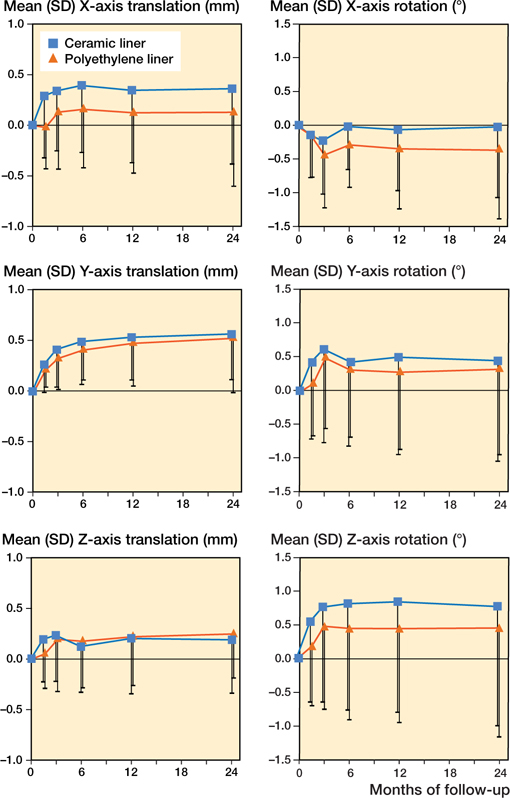
Figure 4. Mean (SD) cup translation (mm) and rotation (°) of the 2 study groups throughout the 2-year follow-up period.
Irrespective of liner type, migration occurred mainly in the first 6 weeks to 3 months, stabilizing within 6 months (Figure 4). Table 3 reports the group effects of the PE and CE liners with time and time-by-group interaction on all migration parameters. Group effects were ≤0.30 mm translation and ≤0.45° rotation. Mean proximal translation, which has been associated with aseptic loosening, was similar for cups in the CE and the PE group, with a between-group effect of 0.02 mm at 2 years.
None of the patients had clinical symptoms of cup loosening or needed revision at 2 years. 1 Delta-TT cup in the PE group showed continuous migration along the Y-axis, with a proximal translation of 2.0 mm at 1 year and 2.8 mm at 2 years that did not seem to stabilize. Detailed individual migration patterns are presented in Figures 5 and 6 (see Supplementary data).
Clinical outcomes
Patient-reported hip function improved substantially from preoperative to 2 years postoperative for both the PE and CE group, with mean HOOS improvement of 32 (27–37) and 30 (24–36) points, mean OHS improvement of 20 (17–23) and 20 (17–22) points, and mean EQ5D index improvement of 0.26 (0.17–0.36) and 0.34 (0.20–0.48) points, respectively. Mixed modeling showed a main effect of group over time for all 3 PROMs: 6.6 points on the HOOSPS (1.6–12); –2.7 points on the OHS (–4.8 to –0.7) and –0.1 on the EQ5D index (–0.2 to –0.0). Despite randomization, patients in the PE group had better PROM scores at baseline as well as throughout follow-up. When adding time and the interaction between group and time to the mixed models, 1 interaction effect was observed. Despite this one interaction term (OHS at 6 weeks: 3.8; 0.4–7.3), Figure 7 visualizes that improvements in patient-reported outcomes were similar over time.
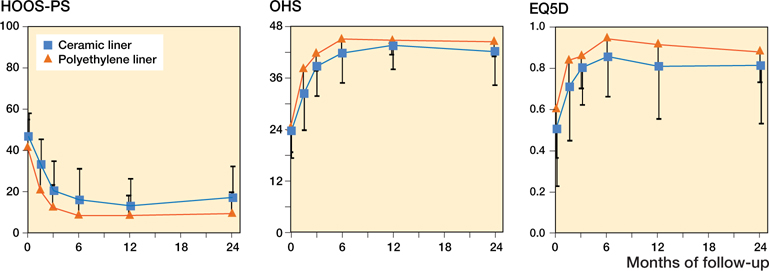
Figure 7. Mean (SD) improvement in patient-reported outcome (PROMs) scores of the 2 study groups throughout the 2-year follow-up period.
Adverse events included 2 patients with a dislocated hip; both were repositioned successfully. Early infection in 2 patients was treated with antibiotics; 1 patient required additional joint lavage and revision of the femoral head and liner to successfully resolve the infection.
Discussion
In this randomized controlled trial, we observed no substantial differences in migration patterns between cups with a polyethylene liner and cups with a ceramic liner throughout the 2-year follow-up period. While cups with a ceramic liner showed more migration on some parameters, between-group effects over time were small and all corresponding confidence intervals included 0. Proximal translation, which is associated with aseptic loosening (5), was similar for polyethylene and ceramic liners, with a between-group effect of 0.02 mm. Improvements in patient-reported hip function and quality of life over time were also similar between groups.
This is the first RSA study on early stability of the Delta-TT cup and our results are in line with an earlier clinical study on the performance of the Delta-TT cup. In their prospective non-randomized study of 142 Delta-TT cups with both ceramic-on-ceramic bearings and ceramic-onpolyethylene bearings, Perticarini et al. (11) reported good clinical and radio-graphic outcomes with a survivorship of 99% at 5 years’ follow-up.
To date, the threshold for clinical relevance of implant migration remains a topic of debate. Pijls and colleagues (5) demonstrated an association between proximal migration and aseptic loosening, with thresholds of < 0.2 mm for acceptable migration and > 1.0 mm for unacceptable migration. However, these thresholds were based on mean migration values in a large group of acetabular components of which the majority were cemented. According to these thresholds, the Delta-TT cup would be classified as ‘at risk’ (proximal migration > 0.2 mm and < 1.0 mm in both groups). However, the interpretation of early migration patterns of uncemented implants seems more complicated, due to the required settling period (28). Given the differences in fixation strategy between cemented and uncemented implants, more early migration could be expected in uncemented cups. Long-term follow-up of this and other RSA studies with uncemented cups is needed in order to substantiate the relevance of RSA in these uncemented components and develop more specific thresholds for clinical relevance on a group level. As a next step, guidelines for interpretation of individual migration patterns will help to inform patients and clinicians what amount of migration is acceptable on a patient level.
Our 2-year follow-up results on this highly porous Trabecular Titanium cup are comparable to the results of 3 recent randomized prospective RSA studies on cementless cups with similar surface properties. Baad-Hansen et al. compared migration of cups with a trabecular tantalum surface to cups with a titanium fiber-mesh surface. With mean proximal translation of 0.08 mm (CI –0.22–0.38), they found promising results with regard to early fixation of trabecular tantalum cups, with less rotation along the transverse axis and similar migration on all other migration parameters as compared with titanium fibermesh cups at 2 years postoperatively (27). Naudie et al. (29) analyzed migration patterns of 2 cups with porous ingrowth surfaces: 1 cup with a 45% low-porosity sintered bead porous surface versus a newer cup with a 61% high-porosity asymmetric titanium porous surface. They found good fixation of both cups, with mean proximal translation of 0.15 mm (SD 0.17) for the 45% low-porosity cup and 0.04 mm (SD 0.18) for the 61% high-porosity cup, respectively, at 2 years postoperatively. They observed no statistically significant differences between groups, except for a larger proximal translation of the cup with lower porosity (p = 0.04). In evaluating migration of a titanium plasma-sprayed porous surface cup with and without hydroxyapatite-coating, Munzinger et al. (30) found good stability at 2 years and no advantage of coating, with mean proximal translation below 0.12 mm in both groups. Similar to our study, these previous publications also describe that migration occurred mainly in the first 6 weeks and stabilized within 6 months after surgery, indicating a settling phase of uncemented acetabular components.
1 other RSA study has analyzed the influence of liner type on early stability of uncemented implants. Zhou et al. (31) compared early stability of 61 porous-coated hemispherical titanium alloy cups with a ceramic-on-ceramic bearing with cups with a metal-on-cross-linked polyethylene bearing. Similar to our results, they found no increased migration in cups with a ceramic liner at 2 years’ follow-up (all p > 0.3).
The study design is a major strength of this study. It is a randomized trial comparing 2 different liner types combined with the same acetabular cup and femoral stem, employing the same surgical technique. Patients were randomized, blinded for liner type, and there was no patient dropout at 2 years, minimizing the risk for bias. However, despite randomization a difference in preoperative PROMs was observed between the 2 groups. The small sample size, powered for RSA analysis, could be the cause of this difference. Furthermore, RSA is a highly precise technique that has proved to be a reliable method for a phased evidence-based introduction of new implants (4-6).
Our study is not without limitations. First, the difficulty in interpreting the clinical relevance of migration data of uncemented implants (as described above) hampers accurate sample size calculation for RSA studies. We observed substantial individual variation in migration patterns, which makes it harder to detect differences between groups. However, patient-reported outcomes, also in patients with increased migration, were good and none of them had symptoms of cup loosening at 2 years. Nevertheless, all patients will be invited for a follow-up at 5 years after surgery and will be monitored in the Dutch registry for orthopedic implants (LROI) to assess implant survival.
Importantly, migration is not the only mechanism involved in secondary loosening of implants (2). Therefore, for a true phased evidence-based introduction of the Delta-TT cup, future research should also focus on other factors, such as wear and osteolysis. Nevertheless, this study is a promising first step in the phased evidence-based introduction of Delta-TT cups in clinical practice.
In conclusion, regardless of liner type, Delta-TT cups showed some initial migration, stabilizing, however, within 6 months. These results confirm results of clinical studies on early performance of Delta-TT cups and are promising for cup survival combined with both a polyethylene and a ceramic liner, but longer follow-up is necessary to evaluate the longterm performance of this cementless cup.
- NJRR. Hip, Knee and Shoulder Arthroplasty Annual Report 2017. Available from: https://aoanjrr.sahmri.com/documents/10180/397736/Hip%2C%20Knee%20%26%20Shoulder%20Arthroplasty; 2017. Australian Orthopaedic Association National Joint Replacement Registry (AOA NJRR).
- LROI. Dutch Arthroplasty Register (LROI) Annual Report 2020. Available from: https://www.lroi-report.nl/app/uploads/2021/03/PDF-LROI-annual-report-2020.pdf; 2020.
- NJR. National Joint Registry (NJR)—18th Annual Report 2021.pdf . Available from: https://reports.njrcentre.org.uk/Portals/0/PDFdown-loads/NJR%2018th%20Annual%20Report%202021.pdf;. 2021.
- Nieuwenhuijse M J, Valstar E R, Kaptein B L, Nelissen R G. Good diagnostic performance of early migration as a predictor of late aseptic loosening of acetabular cups: results from ten years of follow-up with Roentgen stereophotogrammetric analysis (RSA). J Bone Joint Surg Am 2012; 94: 874-80. doi: 10.2106/jbjs.K.00305.
- Pijls B G, Nieuwenhuijse M J, Fiocco M, Plevier J W, Middeldorp S, Nelissen R G, et al. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survival studies. Acta Orthop 2012; 83: 583-91. doi: 10.3109/17453674.2012.745353.
- Swierstra B A, Vervest A M, Walenkamp G H, Schreurs B W, Spierings P T, Heyligers I C, et al. Dutch guideline on total hip prosthesis. Acta Orthop 2011; 82: 567-76. doi: 10.3109/17453674.2011.623575.
- Benazzo F, Botta L, Scaffino M F, Caliogna L, Marullo M, Fusi S, et al. Trabecular titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. J Biomed Mat Res Part A 2014; 102: 2061-71. doi: 10.1002/jbm.a.34875.
- Devine D, Arens D, Burreli S, Bloch H R, Boure L. In vivo evaluation of the osteointegration of new highly-porous Trabecular Titanium™. J Bone Joint Surg Br 2012; 94: 201.
- Massari L, Bistolfi A, Grillo P P, Borre A, Gigliofiorito G, Pari C, et al. Periacetabular bone densitometry after total hip arthroplasty with highly porous titanium cups: a 2-year follow-up prospective study. Hip Int 2017; 27: 551-7. doi: 10.5301/hipint.5000509.
- Bondarenko S, Dedukh N, Filipenko V, Akonjom M, Badnaoui A A, Schwarzkopf R. Comparative analysis of osseointegration in various types of acetabular implant materials. Hip Int 2018; 28: 622-8. doi: 10.1177/1120700018759314.
- Perticarini L, Zanon G, Rossi S M, Benazzo F M. Clinical and radiographic outcomes of a trabecular titanium acetabular component in hip arthroplasty: results at minimum 5 years follow-up. BMC Musculoskelet Disord 2015; 16: 375. doi: 10.1186/s12891-015-0822-9.
- Bistolfi A, Ravera L, Graziano E, Collo G, Malino D, Giordano A, et al. A Trabecular Titanium cup for total hip arthroplasty: a preliminary clinical and radiographic report. Minerva Ortop Traumatol 2014; 65: 199-205.
- Dlaska C E, Jovanovic I A, Grant A L, Graw G, Wilkinson M P, Doma K, et al. Short-term results of a new self-locking cementless femoral stem: a prospective cohort study of the Lima Master(SL). Musculoskelet Surg 2020; 10.1007/s12306-020-00651-1. doi: 10.1007/s12306-020-00651-1.
- Meneghini R M, Ford K S, McCollough C H, Hanssen A D, Lewallen D G. Bone remodeling around porous metal cementless acetabular components. J Arthroplasty 2010; 25: 741-7. doi: 10.1016/j.arth.2009.04.025.
- Small S R, Berend M E, Howard L A, Tunc D, Buckley C A, Ritter M A. Acetabular cup stiffness and implant orientation change acetabular loading patterns. J Arthroplasty 2013; 28: 359-67. doi: 10.1016/j.arth.2012.05.026.
- Moran M M, Ross R D, Virdi A S, Hallab N J, Sumner D R. Bone biology of implant failure. In: Zaidi M, editor. Encyclopedia of bone biology. Oxford: Academic Press; 2020. p 136-45.
- Orlik J, Zhurov A, Middleton J. On the secondary stability of coated cementless hip replacement: parameters that affected interface strength. Med Eng Phys 2003; 25: 825-31. doi: 10.1016/s1350-4533(03)00099-7.
- Shultz T R, Blaha J D, Gruen T A, Norman T L. Cortical bone visco-elasticity and fixation strength of press-fit femoral stems: finite element model. J Biomech Eng 2006; 128: 7-12. doi: 10.1115/1.2133765.
- Marin E, Fedrizzi L, Regis M, Pressacco M, Zagra L, Fusi S. Stability enhancement of prosthetic implants: friction analysis of Trabecular Titanium. Hip Int 2012; 22: 427-8.
- Manley M T, Ong K L, Kurtz S M. The potential for bone loss in acetabular structures following THA. Clin Orthop Relat Rees 2006; 453: 246-53. doi: 10.1097/01.blo.0000238855.54239.fd.
- van Loon J, Vervest A, van der Vis H M, Sierevelt I N, Baas D C, Opdam K T M, et al. Ceramic-on-ceramic articulation in press-fit total hip arthroplasty as a potential reason for early failure, what about the survivors: a ten year follow-up. Int Orthop 2021; 45: 1447-54. doi: 10.1007/s00264-020-04895-1.
- Marin E, Fusi S, Pressacco M, Paussa L, Fedrizzi L. Characterization of cellular solids in Ti6Al4V for orthopaedic implant applications: trabecular titanium. J Mech Behav Biomed Mat 2010; 3: 373-81. doi: 10.1016/j.jmbbm.2010.02.001.
- Kaptein B L, Valstar E R, Spoor C W, Stoel B C, Rozing P M. Model-based RSA of a femoral hip stem using surface and geometrical shape models. Clin Orthop Relat Res 2006; 448: 92-7. doi: 10.1097/01.blo.0000224010.04551.14.
- Valstar E R, Gill R, Ryd L, Flivik G, Borlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76: 563-72. doi: 10.1080/17453670510041574.
- Valstar E R, Spoor C W, Nelissen R G, Rozing P M. Roentgen stereophotogrammetric analysis of metal-backed hemispherical cups without attached markers. J Orthop Res 1997; 15: 869-73. doi: 10.1002/jor.1100150612.
- Kaptein B L, Valstar E R, Stoel B C, Rozing P M, Reiber J H. A new type of model-based Roentgen stereophotogrammetric analysis for solving the occluded marker problem. J Biomech 2005; 38: 2330-4. doi: 10.1016/j.jbiomech.2004.09.018.
- Baad-Hansen T, Kold S, Nielsen P T, Laursen M B, Christensen P H, Søballe K. Comparison of trabecular metal cups and titanium fibermesh cups in primary hip arthroplasty: a randomized RSA and bone mineral densitometry study of 50 hips. Acta Oorthop 2011; 82: 155-60. doi: 10.3109/17453674.2011.572251.
- Röhrl S M. “Great balls on fire”: known algorithm with a new instrument? Acta Orthop 2020; 91: 621-3. doi: 10.1080/17453674.2020.1840029.
- Naudie D D, Somerville L, Korczak A, Yuan X, McCalden R W, Holdsworth D, et al. A randomized trial comparing acetabular component fixation of two porous ingrowth surfaces using RSA. J Arthroplasty 2013; 28: 48-52. doi: 10.1016/j.arth.2013.06.041.
- Munzinger U, Guggi T, Kaptein B, Persoon M, Valstar E, Doets H C. A titanium plasma-sprayed cup with and without hydroxyapatite-coating: a randomised radiostereometric study of stability and osseointegration. Hip Int 2013; 23: 33-9. doi: 10.5301/hip.2013.10598.
- Zhou Z K, Li M G, Borlin N, Wood D J, Nivbrant B. No increased migration in cups with ceramic-on-ceramic bearing: an RSA study. Clin Orthop Relat Res 2006; 448: 39-45. doi: 10.1097/01.blo.0000223999.10389.c9.
Supplementary data
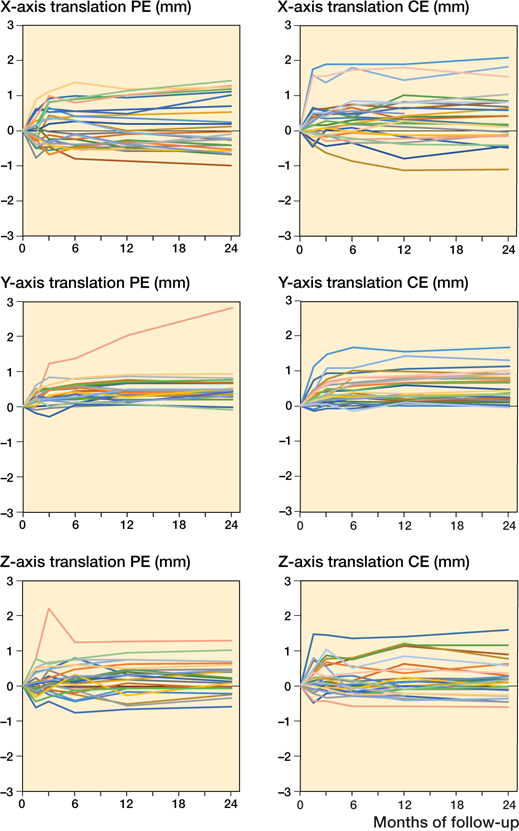
Figure 5. Individual migration patterns of the Delta-TT cup with a polyethylene liner (PE group; n = 24) on the left and with a ceramic liner (CE group; n = 26) on the right with regard to translation (mm) on the X-, Y-, and Z-axis throughout the 2-year follow-up period.
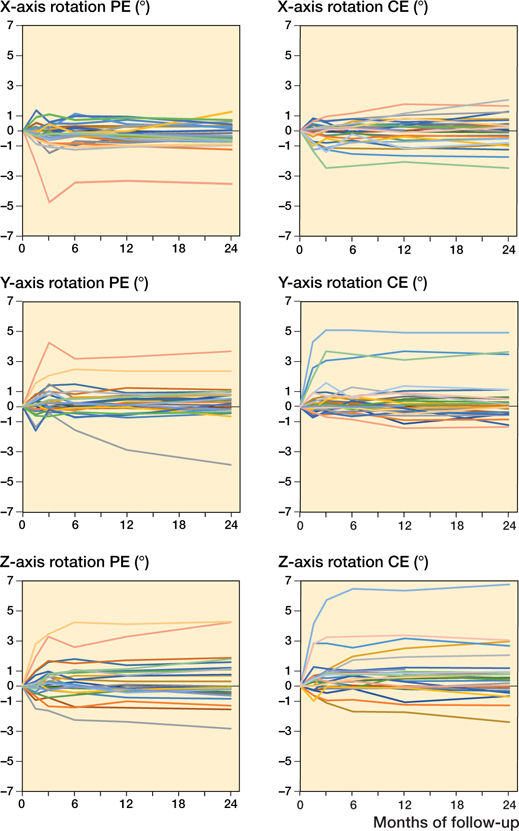
Figure 6. Individual migration patterns of the Delta-TT cup with a polyethylene liner (PE group; n = 24) on the left and with a ceramic liner (CE group; n = 26) on the right with regard to rotation (°) about the X-, Y-, and Z-axis throughout the 2-year follow-up period.