Article
A low-dose biplanar X-ray imager has RSA level precision in total knee arthroplasty
Jennifer K HURRY 1, Alan J SPURWAY 1, Elise K LAENDE 2,3, Saad REHAN 2, Janie L ASTEPHEN WILSON 4, Michael J DUNBAR 2,4, and Ron EL-HAWARY 1,4
1 Department of Orthopaedic Surgery, IWK Health Centre, Halifax, Nova Scotia; 2 Division of Orthopaedic Surgery, Nova Scotia Health Authority, Halifax, Nova Scotia; 3 Mechanical and Materials Engineering, Queen’s University, Kingston, Ontario; 4 School of Biomedical Engineering, Dalhousie University, Halifax, Nova Scotia, Canada
Background and purpose — The low radiation biplanar X-ray imager (EOS imaging, Paris, France) scans patients in a weight-bearing position, provides calibrated images, and limits radiation, an asset for serial radiostereometric analysis (RSA) studies. RSA in vivo precision values have not been published for this type of imaging system, thus the goal of this study was to assess the precision of RSA in vivo utilizing a low radiation biplanar imager.
Patients and methods — At a mean of 5 years post-surgery (range 1.4–7.5 years), 15 total knee arthroplasty (TKA) participants (mean age 67 years at the time of imaging, 12 female, 3 male) with RSA markers implanted during index surgery were scanned twice at the same visit in the EOS imager. Precision of marker-based analysis was calculated by comparing the position of the implant relative to the underlying bone between the 2 examinations.
Results — The 95% limit of precision was 0.11, 0.04, and 0.15 mm along the x, y, and z axes, respectively and 0.15°, 0.20°, and 0.14° around the same axes.
Conclusion — This precision study has shown an in vivo RSA precision of ≤ 0.15 mm and ≤ 0.20°, well within published uniplanar values for conventional arthroplasty RSA, with the added benefit of weight-bearing imaging, a lower radiation dose, and without the need for a reference object during the scan.
Citation: Acta Orthopaedica 2023; 94: 555–559. DOI: https://doi.org/10.2340/17453674.2023.19669.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-03-02. Accepted: 2023-08-17. Published: 2023-11-30.
Correspondence: jennifer.hurry@iwk.nshealth.ca
SR, JAW, JKH, AS, and REH conceived of the study, AS, JKH, MJD, EKL, and SR consulted on the settings and constructed supports, JKH conducted imaging exams, compiled and analyzed the data, and wrote the manuscript. All authors provide critical review and approved the final manuscript.
The authors wish to thank Michaela Wallace for assistance in coordination of this study and Benoit Godbout and the Laboratoire de recherche en Imagerie et Orthopédie (LIO) for their assistance with the system calibration and custom code.
Handling co-editors: Li Felländer-Tsai and Jonas Ranstam
Acta thanks Fabian van de Bunt and Rob Nelissen for help with peer review of this manuscript.
Radiostereometric analysis (RSA) is a stereo X-ray technique that has been used extensively in orthopedic research to characterize the 3-dimensional relative motion between rigid bodies [1]. It offers greater accuracy than conventional radiography in isolation and has been used to quantify fracture fixation [2], arthroplasty implant stability [3-6] and bone growth or fusion after physeal injury or physiodesis [7-11]. RSA currently uses conventional cone-beam radiography. To create registered images in such a system, 2 X-ray heads must be activated and a large reference object must be imaged simultaneously to calculate the X-ray head locations and define a coordinate system [1], or a higher dose CT-based system [12]. The requirement of a specific RSA suite with a large calibration object has limited the use of RSA. The EOS imager (EOS Imaging, Paris, France) is a low radiation dose biplanar X-ray slot scanner that has demonstrated significantly reduced radiation dose compared with computed and digital radiography [13]. This imaging modality scans patients in a weight-bearing position and provides registered and calibrated images.
The EOS imaging technology has a larger pixel size than current digital RSA systems and the vertical translation of its scanning X-ray head may impact the accuracy and precision of the RSA technique. A phantom study has demonstrated that RSA on EOS images is technically feasible [14], but to date no in vivo precision has been published.
The goal of our study was to quantify the translational and rotational precision of RSA in the EOS imager in vivo in total knee arthroplasty (TKA) patients with markers previously inserted. This study includes issues of patient motion during the scan, soft tissue scattering, and marker visibility in the precision assessment.
Patients and methods
A cohort of total knee arthroplasty (TKA) patients who had previously participated in RSA studies of their knee implants were prospectively recruited from the Halifax RSA Database [15] (Figure 1). Adult knees were used for this verification testing due to the population availability with RSA markers already implanted, and the low effective radiation dose of radiographic examinations of the knee. Additional inclusion and exclusion criteria were ability to bear weight on both legs, a BMI ≤ 40 due to limited space in the imager, and not experiencing serious postoperative pain or undergone revision surgery. All participants had received Triathlon total knee replacements (Stryker, Mahwah, NJ, USA) with 5 RSA markers inserted into the periphery of the polyethylene insert and 6–8 markers in the proximal tibia (1 mm diameter tantalum markers, Halifax Biomedical Inc, Mabou, NS, Canada). Participants were scanned twice on the same day in the EOS imager to obtain 2 sets of biplanar (anteroposterior [AP] and lateral view) examinations (Figure 2). After the first scan the participant was instructed to relax while the images were examined (a few minutes) then were set up again as described in the Image Capture Protocol below to obtain the second scan (“double examination”). The implant is assumed to have not moved relative to the bone in this short time interval, thus the comparison between examinations gives an estimate of measurement error in this system, and the method and final sample size follows the ISO standard [16] for assessing RSA precision. The study was performed in accordance with the STARD guidelines.
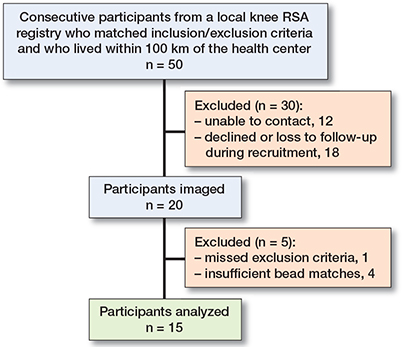
Figure 1. Recruitment diagram.
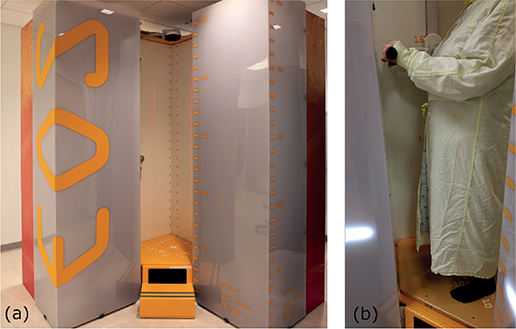
Figure 2. Radiographs of a total knee arthroplasty with RSA markers implanted in the tibia, femur, and the polyethylene of the implant: (a) anteroposterior EOS image, (b) lateral EOS image, (c) and (d) standard uniplanar images with reference box markers in a grid pattern.
Image capture protocol
The suggested protocol by EOS has the patient positioned in an AP orientation, with feet staggered to prevent overlap of the knees in the lateral view, and the scan speed set to 4 on the acquire images software (Figure 3). A previously conducted pilot study (unpublished) of 9 similar subjects using the standard knee settings for the EOS imager found that subject motion during the exam was an issue, as well as marker occlusion by the implant. The kVp energy setting and scan speed were increased to 120 kVp and 30 cm/s respectively, and additional stabilization was added for the subjects in this precision study. The increased voltage takes advantage of the absorption profile of tantalum relative to the cobalt-chrome of the implants to increase marker visibility. To provide additional stabilization and minimize patient motion during the scan, the participants were provided hand grips, were instructed to hold their breath, the ipsilateral ankle was stabilized, and the scan speed was increased 4-fold compared with standard to reduce the length of the scan from about 5 seconds to a little over 1 second. The increased voltage would normally allow for a decrease in current, but the increase in scan speed (decreasing time) necessitated keeping the current at 320 mA to maintain image quality. A set of scans of a purpose-built calibration object was done once a month to measure the geometry of the imaging system at a high enough precision for the RSA analysis [17].
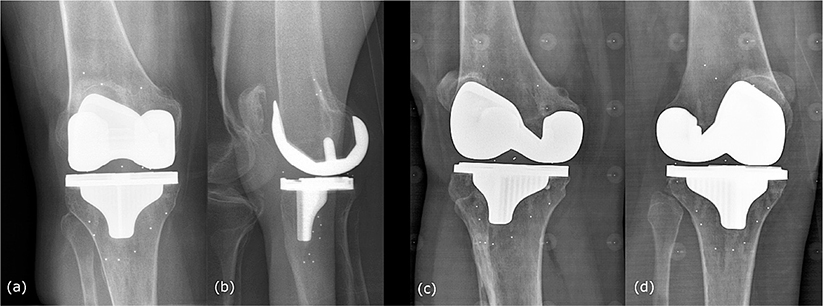
Figure 3. (a) The EOS imager, (b) an early study participant.
This protocol does not fully eliminate patient motion, especially in those populations who find it difficult to stand still for a length of time. A small reference object was created and attached to the patient in order to remove sway in the images during post-processing.
Radiographic analysis
Custom built software in MATLAB 2014a (The MathWorks Inc., Natick, MA) was used to analyze the radiographs, to determine the relative position of the implant marker cluster to the tibia bone cluster, which were compared between the 2 examinations to calculate any relative motion [15,18]. Rigid body kinematic analysis determined the relative translation in the medial, superior, and anterior directions and the rotation of the implant cluster [19,20]. Maximum total point motions (MTPM), rigid body error and condition numbers were also calculated. A custom code was also created to use the reference object to correct for patient sway during post-processing.
Statistics
The precision of the system (including hardware, software, and physical limitations) was assessed as per the ISO 16087 international standard [16]. The micromotion between the tibia and implant is calculated between 2 examinations taken one after another (double examination) for each study participant. If the mean difference in these micromotions is close to 0, the error is presented as the 95% limit around 0, or 1.96 x standard deviation (SD). As our study population was less than 30, we replaced the z score (1.96) with the appropriate t-value for our sample size.
Ethics, registration, funding, and disclosures
Ethics approval was obtained (dual-institution [Nova Scotia Health and IWK Health Centre] REB #1020211) and all participants provided written consent prior to study activities. Funding was provided by the Atlantic Canada Opportunities Agency (AIF grant #199377), with EOS imaging and Halifax Biomedical as industrial partners. The study sponsors had no role in study design, collection, analysis, or interpretation of data. EOS checked the manuscript for factual accuracy. During this research authors MJD and JLAW had consultancy agreements with Stryker, a commercial party indirectly related to this article. Previous unrestricted research grants have been received from Stryker, Zimmer, and Wright Medical Technologies Inc by the institution with which MJD and EKL are associated, and received from Depuy, Medtronic, and Zimmer by the institution with which RE and JH are associated. Complete disclosure of interest forms according to ICMJE are available on the article page, doi: 10.2340/17453674.2023.19669
Results
Of the 20 participants recruited, 1 was excluded for missed inclusion criteria (revision instead of primary implant), 2 were later excluded for unsuitable marker distribution for biplanar imaging, and 2 had insufficient bead matches for analysis. 15 participants had successfully analyzed examinations (age 67 [50–76] years, 12 female, 3 male) at a mean of 5 years post-surgery (range 1.4–7.5 years) (Figure 1). With a final sample size of 15, the 95% limit for precision was calculated as 2.1448 x SD.
Precision values of EOS were in line with standard uniplanar RSA digital X-ray suites [4,6] (Table 1). The maximum rigid body error was 0.07 mm and the highest condition number was 69. 2 examinations were post-processed with the straightening algorithm to remove sway. 1 other examination has some possible patient motion in the images, but the reference object was obscured by the implant and straightening could not be applied; the data for that exam is still included in these results. After post-processing, none of the 15 double exams had migrations > 0.2 mm in any direction, or an MTPM > 0.2 mm, which is the limit of clinical significance in both TKA stability and physiodesis [10,21]. The final 95% limit of precision determined by this study is 0.11 mm, 0.04 mm, and 0.15 mm along the x, y, and z axes respectively with a 0.15°, 0.20°, and 0.14° angular precision around those same axes. The mean MTPM of this study population is 0.11 mm. See Appendix for individual results (Table 2).
| Translation (mm) | Rotation (°) | MTPM (mm) | |||||
| Medial | Superior | Anterior | Anterior tilt | Internal rotation | Valgus rotation | ||
| Mean | 0.02 | –0.01 | –0.02 | –0.02 | –0.02 | –0.04 | 0.11 |
| SD | 0.05 | 0.02 | 0.07 | 0.07 | 0.10 | 0.07 | |
| 95% limit a | |||||||
| EOS imager | 0.11 | 0.04 | 0.15 | 0.15 | 0.20 | 0.14 | |
| Uniplanar system b | 0.10 | 0.11 | 0.17 | 0.46 | 0.27 | 0.12 | |
| a Precision given as the 95% limit around zero as per ISO 16087 standard [16], with the small sample size adjustment t-score instead of z-score. For n = 15, the 95% limit = 2.1448 x SD. b Reference uniplanar values from Dunbar et al. [6]. |
|||||||
Discussion
Our study examined whether it is feasible to conduct RSA exams in a low-dose X-ray slot scan imager considering the resolution of the detector and the motion of the source. We showed that precision comparable to standard digital radiography is possible with this system, using an in-vivo total knee replacement model.
The requirement in RSA of a specialized dual-head X-ray installation with a reference object is a hurdle to widespread use of this technique. The EOS system is an alternative radiography system with a biplanar configuration. The larger pixel size on the detector, motion of the system during the scan, image quality, and patient motion were all potential confounders to a high-precision test using this equipment. However, we have demonstrated acceptable precision with the EOS system, comparable to conventional RSA systems.
Moving the RSA technique into a low-dose slot scanner has been motivated by a desire to use RSA in orthopedic pediatrics, as the additional reduction in radiation dose is in line with as low as reasonably achievable (ALARA) principles in this vulnerable population. RSA has the potential to be a valuable tool to assess postoperative fusion (or growth) in patients treated for a number of pediatric conditions such as scoliosis fusion, physiodesis, and slipped capital femoral epiphysis. There is also increasing interest in using this scanner in adult orthopedics for applications such as joint arthroplasty as an alternative to CT or MRI imaging.
The EOS imager provides registered biplanar images and does not need a reference object in every scan [14] due to the built-in registration of the AP and lateral images. This study has demonstrated similar precision to the standard suite using only a once-a-month calibration of the system geometry.
Limitations
Among the additional participants whose examinations could not be analyzed for this study, difficulties arose due to unsuitable marker placement for biplanar imaging and due to lack of marker matching between the 2 examinations. The RSA markers in this population were placed in accordance with protocol for a uniplanar cone-beam X-ray system. The fan-beam of the EOS stays strictly horizontal, and the polyethylene component of the knee implant in the joint space is not. Depending on the height of the joint space and the distribution of the markers, the participant might be required to flex the knee or rotate their body medially or laterally to have the markers visible on the EOS radiographs. Any future studies using the EOS for RSA will need to take sight lines into account for marker placement protocols or implement model-based approaches to determine implant positioning.
Immediately following an EOS scan, a preview of the images is available at the technologist’s workstation. One of the tools available in the EOS imager control software on the workstation is a horizontal line that is displayed in both the AP and lateral image simultaneously. This allowed the research staff to verify that 3 of the same markers in each cluster were visible in both images. With the small volume in the joint space it was not always possible to verify that the same markers were again seen in the following examination, especially if the knee was rotated or tilted compared with the first. This led to some examinations being unusable as it was discovered during the later analysis that there were insufficient matching markers between examinations. This problem exists with the standard RSA technique as well, and without the preview marker matching capabilities for individual scans.
Conclusion
For an in vivo TKA population, the EOS RSA technique demonstrated a precision superior to the 0.2 mm threshold considered clinically significant for implant loosening. The 95% limit of precision of less than 0.15 mm for translations and 0.20° for rotations is within published values for this population in a standard dual-head uniplanar RSA suite.
In perspective, the use of the EOS imager to provide RSA capability is an important advancement for RSA accessibility because it requires only incremental software development with no change to the hardware.
- Selvik G. Roentgen stereophotogrammetry: a method for the study of the kinematics of the skeletal system. Acta Orthop Scand 1989; 60(Suppl 232): 1-51. Available: http://informahealthcare.com/doi/abs/10.3109/17453678909154184
- Mattsson P, Larsson S. Unstable trochanteric fractures augmented with calcium phosphate cement: a prospective randomized study using radio-stereometry to measure fracture stability. Scand J Surg 2004; 93: 223-8.
- Kärrholm J, Gill R H S, Valstar E R. The history and future of radio-stereometric analysis. Clin Orthop Relat Res 2006; 448: 10-21. doi: 10.1097/01.blo.0000224001.95141.fe.
- Dunbar M J, Wilson D A J J, Hennigar A W, Amirault J D, Gross M, Reardon G P. Fixation of a trabecular metal knee arthroplasty component: a prospective randomized study. J Bone Joint Surg Am 2009; 91: 1578-86. doi: 10.2106/JBJS.H.00282.
- Pijls B G, Valstar E R, Nouta K-A, Plevier J W, Fiocco M, Middeldorp S, et al. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop 2012; 83: 614-24. doi: 10.3109/17453674.2012.747052.
- Dunbar M J, Laende E K, Collopy D, Richardson C G. Stable migration of peri-apatite-coated uncemented tibial components in a multicentre study. Bone Joint J 2017; 99B: 1596-1602. doi: 10.1302/0301-620X.99B12.BJJ-2016-1118.R2.
- Bylander B, Aronson S, Egund N, Ingvar Hansson L, Selvik G. Growth disturbance after physeal injury of distal femur and proximal tibia studied by roentgen stereophotogrammetry. Arch Orthop Trauma Surg 1981; 98: 225-35. doi: 10.1007/BF00632981.
- Kärrholm J, Hansson L I, Selvik G. Roentgen stereophotogrammetric analysis of growth pattern after pronation ankle injuries in children. Acta Orthop Scand 1982; 53: 1001-11. PMID: 7180391.
- Kärrholm J. Roentgen stereophotogrammetry: review of orthopedic applications. Acta Orthop Scand 1989; 60: 491-503. PMID: 2683567.
- Gunderson R B, Horn J, Kibsgård T, Kristiansen L P, Pripp A H, Steen H. Negative correlation between extent of physeal ablation after percutaneous permanent physiodesis and postoperative growth: volume computer tomography and radiostereometric analysis of 37 physes in 27 patients. Acta Orthop 2013; 84: 426-30. doi: 10.3109/17453674.2013.810523.
- Holmdahl P, Backteman T, Danielsson A, Kärrholm J, Riad J. Continued growth after fixation of slipped capital femoral epiphysis. J Child Orthop 2016; 10: 643-50. doi: 10.1007/s11832-016-0793-x.
- Sandberg O H, Kärrholm J, Olivecrona H, Röhrl SM, Sköldenberg O G, Brodén C. Computed tomography-based radiostereometric analysis in orthopedic research: practical guidelines. Acta Orthop 2023; 94: 373-8. doi: 10.2340/17453674.2023.15337.
- Deschênes S, Charron G, Beaudoin G, Labelle H, Dubois J, Miron M-C, et al. Diagnostic imaging of spinal deformities: reducing patients radiation dose with a new slot-scanning X-ray imager. Spine (Phila Pa 1976) 2010; 35: 989-94. doi: 10.1097/BRS.0b013e3181bdcaa4.
- Hurry J K, Rehan S, Spurway A J, Laende E K, Astephen Wilson J L, Logan K J, et al. The reliability of radiostereometric analysis in determining physeal motion in slipped capital femoral epiphysis in standard uniplanar and low-dose EOS biplanar radiography: a phantom model study. J Pediatr Orthop B 2018; 27: 496-502. doi: 10.1097/BPB.0000000000000516.
- Laende E K, Astephen Wilson J L, Mills Flemming J, Valstar E R, Richardson C G, Dunbar M J. Equivalent 2-year stabilization of uncemented tibial component migration despite higher early migration compared with cemented fixation: an RSA study on 360 total knee arthroplasties. Acta Orthop 2019; 90: 1-11. doi: 10.1080/17453674.2018.1562633.
- ISO/TC 150 Committee. ISO 16087 Implants for surgery — Roentgen stereophotogrammetric analysis for the assessment of migration of orthopaedic implants. Switzerland; 2013.
- Allab A, Vazquez C, Cresson T, de Guise J. Calibration of stereo radiography system for radiostereometric analysis application. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS 2019; 4859-62. doi: 10.1109/EMBC.2019.8857531.
- Valstar E R, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76: 563-72. doi: 10.1080/17453670510041574.
- Challis J H. A procedure for determining rigid-body transformation parameters. J Biomech 1995; 28: 733-7. doi: 10.1016/0021-9290(94)00116-L.
- Valstar E. The use of Roentgen stereophotogrammetry to study micromotion of orthopaedic implants. ISPRS J Photogramm Remote Sens 2002; 56: 376-89. doi: 10.1016/S0924-2716(02)00064-3.
- Ryd L, Albrektsson B E B E, Carlsson L, Dansgard F, Herberts P, Lindstrand A, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br 1995; 77: 377-383. doi: 0301-620X/95/3974.
- EOS imaging Announces 350th System Worldwide Installed at University of Missouri Health Care. Press Release [Internet] 2019 [cited 6 Jan 2020]. Available from: https://www.eos-imaging.com/sites/default/files/press-release/PR_EOSI 350th Install_121119.pdf.
Appendix
| Case | x | y | z | Rx | Ry | Rz | MTPM | RBE: tibia | RBE: implant |
| 1 | –0.010 | –0.014 | 0.022 | 0.037 | 0.023 | –0.037 | 0.110 | 0.045 | 0.074 |
| 2 | 0.068 | 0.021 | –0.089 | –0.057 | 0.002 | –0.010 | 0.124 | 0.050 | 0.027 |
| 3 | –0.023 | 0.010 | –0.044 | 0.018 | –0.078 | –0.035 | 0.073 | 0.023 | 0.031 |
| 4 | 0.015 | –0.017 | –0.067 | –0.126 | –0.003 | –0.052 | 0.075 | 0.025 | 0.015 |
| 5 | 0.017 | 0.003 | 0.003 | 0.056 | –0.003 | –0.051 | 0.023 | 0.023 | 0.008 |
| 6 | 0.085 | 0.010 | 0.054 | 0.092 | –0.011 | –0.08 | 0.125 | 0.009 | 0.027 |
| 7 | Implant beads too close together | ||||||||
| 8 | 0.002 | –0.026 | 0.033 | –0.067 | 0.018 | –0.059 | 0.079 | 0.014 | 0.042 |
| 9 | Only 2 tibia beads | ||||||||
| 10 | Revision implant | ||||||||
| 11 | No bead match | ||||||||
| 12 | –0.007 | 0.013 | 0.075 | 0.006 | –0.046 | 0.041 | 0.094 | 0.051 | 0.030 |
| 13 | –0.034 | –0.005 | –0.158 | –0.069 | 0.007 | –0.014 | 0.172 | 0.023 | 0.026 |
| 14 | No bead match | ||||||||
| 15 | –0.033 | –0.040 | –0.064 | –0.037 | –0.145 | 0.039 | 0.160 a | 0.030 | 0.058 |
| 16 | 0.083 | 0.000 | –0.068 | –0.015 | –0.003 | 0.017 | 0.119 | 0.035 | 0.029 |
| 17 | –0.052 | –0.007 | 0.023 | –0.014 | 0.035 | –0.188 | 0.099 | 0.055 | 0.051 |
| 18 | –0.034 | 0.021 | –0.098 | –0.036 | 0.024 | 0.068 | 0.153 | 0.026 | 0.056 |
| 19 | 0.041 | –0.008 | 0.061 | 0.063 | 0.117 | –0.126 | 0.088 | 0.064 | 0.018 |
| 20 | 0.117 | –0.037 | –0.017 | –0.177 | –0.299 | –0.087 | 0.155 a | 0.045 | 0.054 |
| a Examinations were straightened before these final values were calculated. RBE = rigid body error. |
|||||||||