Increased mortality after total hip prosthetic joint infection is mainly caused by the comorbidities rather than the infection itself
Anders PERSSON 1, Olof SKÖLDENBERG 1, Maziar MOHADDES 2, Thomas EISLER 1, and Max GORDON 1
1 Karolinska Institutet, Department of Clinical Sciences at Danderyd Hospital, Stockholm; 2 Department of Orthopaedics, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden, and The Swedish Arthroplasty Register, Gothenburg, Sweden
Background and purpose — Periprosthetic joint infection (PJI) is a feared complication of arthroplasty surgery. There is controversy as to whether PJI also correlates with increased mortality. Our aim was to investigate in a nationwide cohort if PJI is an independent risk factor for dying.
Patients and methods — We performed a retrospective cohort study based on data from the Swedish Hip Arthroplasty Register (SHAR). All patients with a revision THA performed between 1998 and 2017 were included. The outcome is mortality; exposure is PJI according to SHAR. The control group was study participants who underwent aseptic revision. Confounders were age, sex, diagnosis, and comorbidity according to the Elixhauser index. The outcome was analyzed with a Cox proportional hazards model.
Results — 4,943 PJI revisions and 12,529 non-infected revisions were included in the analysis. The median follow-up time was 4.1 years. In the PJI group, 1,972 patients died and in the control group, 4,512. The incidence rate ratio was 1.19 (95% confidence interval [CI] 1.13–1.25), the crude hazard ratio (HR) 1.19 (CI 1.13–1.25), and the adjusted HR 1.05 (CI 0.99–1.12) for the exposed versus the unexposed group. The strongest confounder was comorbidity.
Conclusion — The increased mortality risk after revision due to PJI is mainly caused by the comorbidity of the patient, rather than by the infection itself.
Citation: Acta Orthopaedica 2023; 94: 484–489. DOI https://doi.org/10.2340/17453674.2023.18619.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-10-17. Accepted: 2023-08-07. Published: 2023-09-26.
Correspondence: anders.persson@regionstockholm.se
AP collected and analyzed the data, wrote the manuscript draft, and designed the tables and figures. OS designed the study and revised the manuscript. MM designed the study, collected the data and revised the manuscript. TE designed the study and revised the manuscript. MG designed the study, analyzed the data and revised the manuscript. All authors approved the final manuscript.
Handling co-editors: Keijo Mäkelä and Philippe Wagner
Acta thanks Mika Niemeläinen, Aleksi Reito, and Tina Strømdal Wik for help with peer review of this manuscript.
Periprosthetic joint infection (PJI) after primary total hip arthroplasty (THA) has an incidence of 0.8–1.3% [1-3]. It is associated with morbidity, mortality, long-term antibiotics, repeated surgeries, prolonged hospital stays, economic burden, and a poorer end result for individual patients [4-8]. During recent years, interest has grown in investigating the correlation between PJIs and mortality.
The national age-adjusted mortality rather than mortality after aseptic revision surgery has been used as comparator, which should better address concerns regarding the causality between PJI and death. Consequently, Shahi et al. reported that in-hospital mortality was twice as high after revision surgery related to PJI, compared with revision related to aseptic loosening [9]. Choi et al. reported higher mortality rates after revision surgery due to PJI compared with revision due to aseptic loosening, including a THA as well as TKA cohorts [10,11]. Zmistowski et al. studied almost 3,000 patients and found an increase in mortality up to 5 years postoperatively [8].
Concerns have been raised regarding whether it is the infection itself, or the surgical procedure and accompanying postoperative procedures that inflict this risk on patients [12].
The aim of our study was to investigate the risk of mortality after revision surgery due to THA PJI compared with patients revised due to any aseptic failure.
Patients and methods
Study design, setting, and participants
We performed a cohort study based on prospectively collected data from the Swedish Hip Arthroplasty Register (SHAR) [13]. All patients with a revision THA performed between 1998 and 2018 were eligible for inclusion, irrespective of cause for revision. Patients with PJI revision at any time during the study period were included at the date of their first PJI revision. Excluding PJI revision, patients revised at any time during the study period were included at the date of their first revision. Any following revisions for the same patient were excluded. Hence, every unique patient contributed only once to the study. If patients were operated on bilaterally during the study period, only the first operation was included. The study is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines regarding cohort studies.
Variables
Outcome is defined as mortality after surgery as a function of time. Exposure is defined as PJI, i.e., International Classification of Diseases (ICD) code T84.5F or free text indicating PJI, according to SHAR. The control group consists of those study participants who do not meet the criteria for exposure noted above and are therefore regarded as non-infected revision surgeries.
Data sources/measurement
Data was collected from SHAR, the Swedish National Patient Register (SNPR) [14], and the Cause of Death Register [15]. Since 1998, SHAR has collected data on all THA revisions through medical chart review. After the initial revision report on a SHAR standard form from the orthopedic department that performed the surgery has been sent in to SHAR, medical charts are collected to validate the intervention and ensure the coding is correct. The completeness of hip revisions in SHAR was 92–94% between 2013 and 2022 [16]. The completeness of revisions due to PJI in SHAR has been reported to be 67% [2].
Bias
Age, sex, revision prior to PJI revision, number of total revisions, and comorbidity according to the Elixhauser index [17] 1 year before surgery were regarded as confounders and therefore adjusted for. The elaborating of a directed acyclic graph (DAG) was used to justify the selection and definition of confounder variables (see Supplementary data). Smoking was considered as an unobserved variable and subsequently not adjusted for. Elixhauser’s index was computed by statisticians at SHAR after merging the data from SHAR and SNPR. ICD 10-codes from both in- and outpatient visits from 1 year before surgery date were used to calculate the index. Any patients without infection according to the International Statistical Classification of Diseases (ICD) or the Nordic Medico-Statistical Committee (NOMESCO) classification codes, but with SHAR codes indicating arthroplasty infections, were excluded due to the risk of misclassification bias.
Study size
Before study start, our estimation was that approximately 7,300 revisions due to PJI were performed during 1998–2016. We further assumed that 20% have died during the study period and that 10% are affected by misclassification bias. With these assumptions and this number of study participants, we can detect a risk ratio as low as 1.09 at 80% power.
Statistics
We used a Cox proportional hazards model. Testing the proportional hazards assumption of the regression model was achieved by visual inspection of the Schoenfeld residuals together with an evaluation using the Grambsch–Thernau test. Continuous variables were adjusted using restricted cubic splines if there was an indication of non-linearity and interactions among sex, age, and comorbidities. The need for this modification of the model was evaluated using likelihood ratio (LR) tests with a p-value threshold of 0.1 to include a more complex adjustment. ANOVA was used to assess model fit, interactions, importance, and non-linearity of variables. Mediation analysis was performed according to the methodology proposed by VanderWeele et al. [18], employing the difference method fitting our standard model as a parametric survival regression model and comorbidity and age as generalized linear models (see Supplementary data). The purpose of the mediation analysis was to quantify confounding effects, based on the perception that mediation and confounding are identical from a statistical and mathematical perspective [19]. Survival rates were calculated and visualized using Kaplan– Meier estimates. Data management and statistical analyses were performed in R (version 4.2.2, 2022; R Foundation for Statistical Computing, Vienna, Austria).
Ethics, funding, and disclosures
The Regional Ethics Board in Göteborg approved the study in 2014 (Dnr: 271-14). The study was performed in compliance with the Helsinki Declaration. 25,000 SEK was granted from the Tore Dalén Memorial Fund at the Swedish Hip and Knee Society. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.18619
Results
We identified 353,867 surgeries during the study period. Of those, 326,937 primary THAs were excluded. The remaining 26,930 were identified as revisions. 9,458 revisions were excluded and thus 17,472 revisions were included in the study (Figure 1).
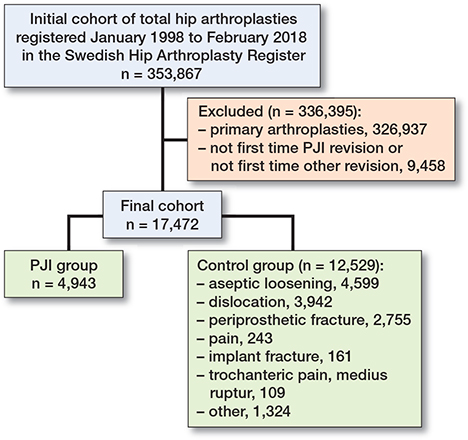
Figure 1. Flowchart of study groups. PJI = periprosthetic joint infection.
4,943 of the included revisions were defined as first time PJI revisions, and thus constituted the exposed group. The remaining 12,529 surgeries were defined as non-infected revisions, and thus constituted the control group. 9,613 (55%) of the patients were female, and the mean age was 72.8 (standard deviation [SD] 11.8) years. A majority of the patients belonged to the Elixhauser 0 group (9,932 patients) or the Elixhauser 1 group (3,597 patients). Data was missing regarding the comorbidity index in 911 patients. No data was missing regarding the other presented variables. Maximum follow-up time was 20 years. The median follow-up time was 4.1 (interquartile range [IQR] 5.6) years, and the total follow-up time of the study was 88,885 person-years. 6,484 died during the study period. All study participants were followed until death or study end. Descriptive data is presented in Table 1.
| Factor | Non-infected revisions n = 12,529 | PJI revisions n = 4,943 | Overall n = 17,472 |
| Female sex, n (%) | 7,194 (57) | 2,419 (49) | 9,613 (55) |
| Age | |||
| mean (SD) | 72.9 (11.8) | 72.5 (11.9) | 72.8 (11.8) |
| median (range) | 74 (11–104) | 74 (17–101) | 74 (11–104) |
| Comorbidity (Elixhauser) | |||
| mean (SD) | 0.6 (1.0) | 1.0 (1.3) | 0.7 (1.1) |
| median (range) | 0 (0–9) | 1 (0–9) | 0 (0–9) |
| missing, n (%) | 360 (2.9) | 551 (11) | 911 (5.2) |
| Dead, n (%) | 4,512 (36) | 1,972 (40) | 6,484 (37) |
| Follow-up, years | |||
| mean (SD) | 5.1 (4.1) | 4.8 (4.0) | 5.0 (4.1) |
| median (range) | 4.2 (0–20.0) | 3.8 (0–19.9) | 4.1 (0–20.0) |
| Revisions a | |||
| mean (SD) | 1.2 (0.7) | 2.3 (1.8) | 1.5 (1.2) |
| median (range) | 1 (1–14) | 2 (1–24) | 1 (1–24) |
| SD = standard deviation. PJI = prosthetic joint infection. | |||
| a Revisions = total number of revisions each patient has undergone during the study time. | |||
Mortality
In the PJI group, 1,972 of 4,943 patients died during the study period. The incidence rate in this group was 84 deaths per 1,000 person-years. In the non-infected revision group, 4,512 of 12,529 patients died during the same period, corresponding to 70 deaths per 1,000 person-years. The incidence rate ratio (IRR) was 1.19 (95% confidence interval [CI] 1.13–1.26) for the PJI group versus the control group. The Kaplan–Meier 6-month survival estimate was 90.6% (CI 89.8–91.4) for the PJI group and 94.5% (CI 94.1–94.9) for the control group, and at 1 year 87.9% (CI 87.0–88.9) and 91.9% (CI 91.4–92.4 95), respectively (Table 2 and Figure 2).
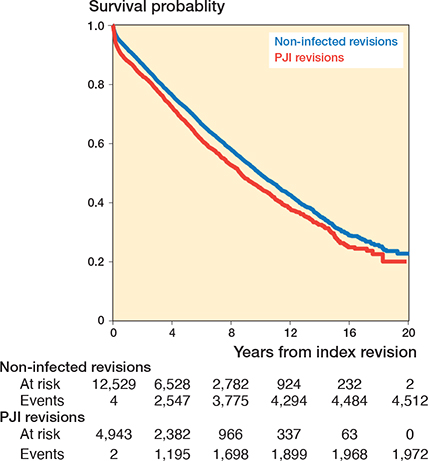
Figure 2. Kaplan–Meier survival curve based on a tabulation of the number at risk and number of events at each unique death time
Modeling mortality as a function of time resulted in a crude hazard ratio (HR) of 1.19 (CI 1.13–1.25) and an adjusted HR of 1.05 (CI 0.99–1.12, NS) (Table 3). ANOVA analysis with Wald chi-square statistics resulted in a non-significant relationship between PJI as a cause of revision and mortality risk (chi-square 0.55, P = 0.5). It further showed that all other predictor variables were significant to our model fit. The strongest predictors were age and comorbidity (chi-square 3,602, P < 0.0001 and 832, P < 0.0001, respectively). Mediation analysis quantifying confounding effects resulted in proportions of the effect caused by age at 40.4% (CI 14.4–81.0) and by comorbidity at 53.4% (CI 36.7–88.0). Supplementary data shows R code and result output regarding the mediation analysis.
ANOVA sensitivity analyses did not change the estimates when excluding sex and age from the model, both individually and together, indicating comorbidity as the key confounder of our estimates. The confounding effect ranged through all levels of the Elixhauser index. Adding an interaction term to the model showed that there was no significant interaction between PJI and comorbidity on mortality risk (P = 0.6, P = 0.9, and P = 0.2 respectively for the 3 Elixhauser groups).
The 3 major subgroups of the non-infected revisions were aseptic loosenings (4,599 patients), dislocations (3,492 patients), and periprosthetic fractures (2,755 patients) (Figure 1). Separate Cox regressions using the same model resulted in adjusted HRs of 1.84 (CI 1.67–2.02) for PJIs compared with aseptic loosenings, 0.84 (CI 0.78–0.90) compared with dislocations, and 0.84 (CI 0.77–0.90) compared with periprosthetic fractures (Table 3). The same ANOVA analyses as described above also did not change the subgroup estimates.
Using scaled Schoenfeld residuals for testing the proportional hazards assumption for our Cox model fit resulted in the plot and P values visualized in Figure 3. The minimal variation of the values over time indicates that our assumption of proportional hazards holds.
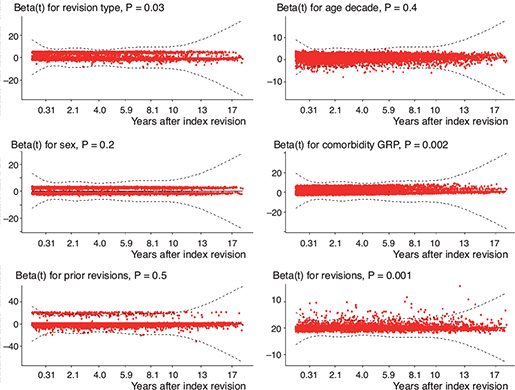
Figure 3. Plot and print of the results of testing the proportional hazards assumption for our Cox regression model fit. The plot gives an estimate of the time-dependent coefficient beta(t) with individual Schoenfeld test P values. If the proportional hazards assumption holds, then the true beta(t) function would be a horizontal line. The lines of our covariates are nearly horizontal, and therefore interpreted as indications of proportional hazards in our model fit. Global Schoenfeld test P < 0.001.
Discussion
The aim of the study was to investigate if PJI following THA is an independent risk factor for dying. We showed that there is an increased mortality risk after THA revisions due to PJI. This increase is, however, mainly caused by age and by the comorbidities of the patient rather than by the infection itself.
Our data suggests that comorbidity, alongside age, accounts for a major part of the mortality risk exerted by a PJI. Furthermore, the results from our Cox regression suggest that there probably is a minor effect of the infection itself on mortality risk (HR 1.05, CI 0.99–1.12), but from a clinical perspective this should reasonably be of limited importance.
Our results somewhat contradict earlier published reports on this subject. Gundtoft et al. [20] reported a 1-year relative mortality risk of 1.87 (CI 1.11– 3.15) after PJI revisions compared with aseptic revisions after adjusting for age, sex, comorbidity, duration of surgery, and number of secondary revisions. However, as that report focused on 1-year mortality and ours studied death up to 20 years postoperatively, the results are not entirely comparable. They also used the Charlson Comorbidity Index (CCI) when adjusting for comorbidities, compared with our usage of Elixhauser’s index. Zmistowski et al. [8] concluded that, after adjusting for CCI but also for several other comorbidities, such as diabetes, cardiac disease, and renal disease, PJI itself constitutes an independent risk factor increasing mortality after septic revision surgery. There is, however, a possibility that using the Elixhauser index is a better option when the aim is to minimize comorbidity bias in similar studies. While the CCI [21] encompasses 19 medical conditions, the Elixhauser index [17] includes 31 conditions, and has been reported to outperform the CCI in predicting inpatient mortality and morbidity after major orthopedic surgery [22].
The contradiction in our results may be explained by our elimination of more confounding factors and also by the differences between North American and Scandinavian studies in microbiological flora and resistance patterns, and various PJI treatment regimes.
However, there are also earlier reports showing similar results to ours regarding mortality rates over elapsed time [8,11,23].
The increased mortality risk in the PJI group during the first months after revision surgery is from a clinical perspective not surprising, as the comorbidity, surgical, and pharmaceutical burden connected to the surgical procedure and concomitant infectious condition reasonably should produce a heavy insult to the patient’s health status, which in our study is mainly explained by the comorbidities.
Performing subgroup analyses, our data resulted in reduced hazard ratios for PJIs comparing both with dislocations and periprosthetic fractures separately (Table 3). This is not surprising regarding the periprosthetic fractures, as several earlier studies also report increased mortality in this group compared with other subgroups of revised hip arthroplasties [24,25]. Even though not as evident as in the fracture group, revision due to dislocation has also shown trends towards increased mortality risk compared with the overall revision group [25,26]. We interpret those reports and our results as indicating a fragility of the patients in these groups, which subsequently results in an increased mortality risk. In contrast, compared with only aseptic loosenings, PJIs show an 80% increased hazard ratio. However, using only aseptic revisions as the comparison group would introduce selection bias to our study, as surgeons often choose not to revise a patient with aseptic loosening if the comorbidities are too abundant, while the same, for obvious reasons, cannot be applied to a patient with a PJI. The results of these subgroup analyses raise concerns regarding the most adequate comparison group when investigating the correlation between the infection itself and mortality. However, they further strengthen our belief that it is mainly the comorbidity that drives the mortality risk and lead us to conclude that using the entire non-infected revision group as comparison group is the most adequate choice when trying to investigate the desired correlation. The choice of which subgroups to analyze was based on the frequency table in Figure 1.
Finally, even though our results indicate that comorbidity and age are the most important contributors to the increased mortality after THA PJI revision, there remain some reasons to believe that the infection itself also contributes somewhat to this increase. Our adjusted HR was 1.05, with CI 0.99–1.12, which barely includes the 1.00 limit. With a statistical significance level of 0.05, this falls to the interpretation of non-significant. However, there is still a fair probability that the true value of the HR lies somewhat above 1.00, which would signify that there actually is an effect of the infection itself on mortality, although of clinically hesitant importance. This would also better comply with the reports mentioned earlier.
Strengths and limitations
The strengths of this study are the national coverage of the SHAR, the large number of patients included, and the completeness of data in SNPR regarding mortality.
The main limitations in our study are connected to the retrospective register design, because there is a risk of misclassification of PJI as non-infected revision due to incorrect coding in the SHAR. This will result in false low estimates on the mortality risk in the PJI group. However, Lindgren et al. [2] showed that, even if the sensitivity was as low as 60%, the specificity of PJI coding in the SHAR was 99%, but this will still contaminate our group classified as non-infected.
A further limitation is the lack of data on subgroups within the PJI group, which is rather heterogeneous regarding what specific surgical procedure has been performed. It ranges from debridement with or without modular component exchange (i.e., DAIR) to total prosthetic exchange in 1 or 2 stages. However, the total surgical insult should reasonably be the main separator between the different types of PJI surgeries, and the main purpose of this study was to determine the effect of the infection itself on mortality. By adjusting for the number of total revisions, we should at least partially compensate for the lack of data regarding PJI subgroups. We acknowledge the diversity of the PJI diagnoses and the complexity in studying the correlation between PJI and mortality.
Another limitation of the study is that we have had no possibility of separating emigrated patients from deceased ones up to December 2020. However, we now know that there is a very low actual number of emigrated patients in the entire SHAR data, comprising approximately 1,000 patients, which is why lack of emigration status in our data should reasonably not bias our results in any significant way.
Conclusion
PJI as cause of THA revision, compared with non-infected causes of revision, increases mortality postoperatively even in the long term. The most important factors contributing to this increase are comorbidity and age, even though there could be a minor effect of the infection itself on mortality. Orthopedic surgeons with an interest in hip revision surgery should be aware of the increased mortality risk after PJI revision in comparison with non-infected revisions, and that this increase is mainly attributed to the patient’s overall health status.
Supplementary data
DAG and Mediation analysis are available as Supplementary data on the article page, doi: 10.2340/17453674.2023.18619
- Gundtoft P H, Overgaard S, Schønheyder H C, Møller J K, Kjærsgaard-Andersen P, Pedersen A B. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop 2015; 86(3): 326-34. doi: 10.3109/17453674.2015.1011983.
- Lindgren J V, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord 2014; 15: 384. doi: 10.1186/1471-2474-15-384.
- Dale H, Fenstad A M, Hallan G, Overgaard S, Pedersen A B, Hailer N P, et al. Increasing risk of revision due to infection after primary total hip arthroplasty: results from the Nordic Arthroplasty Register Association. Acta Orthop 2023; 94: 307-15. doi: 10.2340/17453674.2023.13648.
- Kapadia B H, Berg R A, Daley J A, Fritz J, Bhave A, Mont M A. Periprosthetic joint infection. Lancet 2016; 387(10016): 386-94. doi: 10.1016/s0140-6736(14)61798-0.
- Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res OTSR 2010; 96(2): 124-32. doi: 10.1016/j.rcot.2010.02.005.
- Kurtz S M, Lau E, Watson H, Schmier J K, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012; 27(8 Suppl.): 61-5.e1. doi: 10.1016/j.arth.2012.02.022.
- Bozic K J, Katz P, Cisternas M, Ono L, Ries M D, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am 2005; 87(3): 570-6. doi: 10.2106/jbjs.D.02121.
- Zmistowski B, Karam J A, Durinka J B, Casper D S, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 2013; 95(24): 2177-84. doi: 10.2106/jbjs.L.00789.
- Shahi A, Tan T L, Chen A F, Maltenfort M G, Parvizi J. In-hospital mortality in patients with periprosthetic joint infection. J Arthroplasty 2017; 32(3): 948-52.e1. doi: 10.1016/j.arth.2016.09.027.
- Choi H R, Bedair H. Mortality following revision total knee arthroplasty: a matched cohort study of septic versus aseptic revisions. J Arthroplasty 2014; 29(6): 1216-18. doi: 10.1016/j.arth.2013.11.026.
- Choi H R, Beecher B, Bedair H. Mortality after septic versus aseptic revision total hip arthroplasty: a matched-cohort study. J Arthroplasty 2013; 28(8 Suppl.): 56-8. doi: 10.1016/j.arth.2013.02.041.
- Blumenfeld T J. Does the infection or the treatment kill the patient?: Commentary on an article by Zmistowski B S, et al.: :Periprosthetic joint infection increases the risk of one-year mortality:. J Bone Joint Surg Am 2013; 95(24): e200(1-2). doi: 10.2106/jbjs.M.01085.
- Garellick G K J, Lindahl H, Malchau H, Rogmark C, Rolfson O. The Swedish Hip Arthroplasty Register Annual Report 2014; 2015. Available from: http://www.shpr.se/Libraries/Documents/Arsrapport_2014_WEB.sflb.ashx.
- National Patient Register [Internet]; 2019. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register/.
- Cause of Death Register 2022; 2022. Available from: https://www.socialstyrelsen.se/statistik-och-data/register/dodsorsaksregistret/.
- Annual Report—The Swedish Arthroplasty Register; 2023.
- Elixhauser A, Steiner C, Harris D R, Coffey R M. Comorbidity measures for use with administrative data. Med Care 1998; 36(1): 8-27. doi: 10.1097/00005650-199801000-00004.
- VanderWeele T J. Mediation analysis: a practitioner’s guide. Annu Rev Public Health 2016; 37: 17-32. doi: 10.1146/annurev-publ-health-032315-021402.
- MacKinnon D P, Krull J L, Lockwood C M. Equivalence of the mediation, confounding and suppression effect. Prev Sci 2000; 1(4): 173-81. doi: 10.1023/a:1026595011371.
- Gundtoft P H, Pedersen A B, Varnum C, Overgaard S. Increased mortality after prosthetic joint infection in primary THA. Clin Orthop Relat Res 2017; 475(11): 2623-31. doi: 10.1007/s11999-017-5289-6.
- Charlson M E, Pompei P, Ales K L, MacKenzie C R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373-83. doi: 10.1016/00219681(87)90171-8.
- Menendez M E, Neuhaus V, van Dijk C N, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res 2014; 472(9): 2878-86. doi: 10.1007/s11999-014-3686-7.
- Natsuhara K M, Shelton T J, Meehan J P, Lum Z C. Mortality during total hip periprosthetic joint infection. J Arthroplasty 2019; 34(7s): S337-s42. doi: 10.1016/j.arth.2018.12.024.
- Lamb J N, Nix O, Al-Wizni A, West R, Pandit H. Mortality after postoperative periprosthetic fracture of the femur after hip arthroplasty in the last decade: meta-analysis of 35 cohort studies including 4841 patients. J Arthroplasty 2022; 37(2): 398-405.e1. doi: 10.1016/j.arth.2021.09.006.
- Khan T, Middleton R, Alvand A, Manktelow A R J, Scammell B E, Ollivere B J. High mortality following revision hip arthroplasty for periprosthetic femoral fracture. Bone Joint J 2020; 102-b(12): 1670-4. doi: 10.1302/0301-620x.102b12.Bjj-2020-0367.R1.
- Laughlin M S, Vidal E A, Drtil A A, Goytia R N, Mathews V, Patel A R. Mortality after revision total hip arthroplasty. J Arthroplasty 2021; 36(7): 2353-8. doi: 10.1016/j.arth.2021.01.022.