Major surgery for metastatic bone disease is not a risk for 30-day mortality: a population-based study from Denmark
Thea H LADEGAARD, Michala S SØRENSEN, and Michael M PETERSEN
Musculoskeletal Tumor Section of the Department of Orthopedic Surgery, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
Background and purpose — Surgery for bone metastases in the appendicular skeleton (aBM) is a trade-off between limb function and survival. A previous study from a highly specialized center found that extended surgery is not a risk for 30-day mortality and hypothesized that wide resection and reconstruction might reduce postoperative mortality. The study aimed to investigate whether parameters describing the surgical trauma (blood loss, duration of surgery, and degree of bone resection) pose a risk for 30-day mortality in patients treated with endoprostheses (EPR) or internal fixation (IF) in a population-based cohort.
Patients and methods — A population-based cohort having EPR/IF for aBM in the Capital Region of Denmark 2014–2019 was retrospectively assessed. Intraoperative variables and patient demographics were evaluated for association with 30-day mortality by logistic regression analysis. Kaplan–Meier estimate was used to evaluate survival with no loss to follow-up.
Results — 437 patients had aBM surgery with EPR/IF. No parameters describing the magnitude of the surgical trauma (blood loss/duration of surgery/degree of bone resection) were associated with mortality. Overall 30-day survival was 85% (95% confidence interval [CI] 81–88). Univariate analysis identified ASA group 3+4, Karnofsky score < 70, fast-growth primary cancer, and visceral and multiple bone metastases as risk factors for 30-day mortality. Male sex (OR 2.8, CI 1.3–6.3), Karnofsky score < 70 (OR 4.2, CI 2.1–8.6), and multiple bone metastases (OR 3.4, CI 1.2–9.9) were independent prognostic factors for 30-day-mortality in multivariate analysis.
Conclusion — The parameters describing the surgical trauma were not associated with 30-day mortality but, instead, general health status and extent of primary cancer influenced survival post-surgery.
Citation: Acta Orthopaedica 2022; 94: 447–452. DOI https://doi.org/10.2340/17453674.2023.18394.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2023-06-09. Accepted: 2023-08-02. Published: 2023-08-18.
Correspondence: Thea.hovgaard.ladegaard@regionh.dk
All authors have read and agreed to the published version of the manuscript. Conceptualization: THL, MMP, MMS; data curation: THL; formal analysis: THL; funding acquisition: MMP, MSS; investigation: THL, MMS; methodology: THL, MMP, MMS; project administration: THL; resources: THL, MMP, MMS; software: THL; supervision: MMS, MMP; validation: THL, MMP, MMS; visualization: THL; writing original draft: THL; writing, review, and editing: THL, MMP, MMS.
Handling co-editor: Ilkka Helenius
Acta thanks Elina Ekman, Michelle Ghert, and Jorrit-Jan Verlaan for help with peer review of this manuscript.
Patients with cancer are at risk of experiencing bone metastases and an impending or pathological fracture in the appendicular skeleton (aBM). Due to increasing age and improvements in diagnostics and adjuvant therapies [1] patients with aBM might live longer and therefore potentially be at prolonged risk of experiencing a pathological fracture. Treating aBM is a balance between palliative pain management and restoring extremity function and is sometimes only achievable by surgery. aBM can be managed surgically by resection and reconstruction with an endoprosthesis (EPR) or diaphyseal spacer or by internal fixation (IF). IF is in general characterized by minor surgical trauma but also by an increased risk of implant failure compared with EPR with or without resection [2,3]. EPR is in general characterized by larger surgical and anesthesiologic trauma and thus might not be suitable for every patient due to general health status and remaining life expectancy.
When choosing a surgical approach for management of aBM, it is important to investigate risk factors for mortality, ensuring that the magnitude of the surgical trauma does not pose a risk for residual life expectancy. Most studies have investigated risk factors for long-term survival [4-8], and only a few studies exist on short-term survival, and knowledge regarding surgical risk factors for this group of patients is sparse [9,10].
Earlier we have shown that extended surgery did not increase 30-day mortality, but only the general health status of the patients was essential (measured by Karnofsky performance status and American Society of Anesthesiologists [ASA] score) [11]. The study included only patients from a highly specialized tertiary referral center who had been treated with joint replacement surgery or intercalary spacing, but not IF.
The present study aimed to investigate whether parameters describing the surgical trauma (blood loss, duration of surgery, and degree of bone resection) – variables also used in other settings of orthopedic surgery for describing the surgical trauma [12-17] – pose a risk for 30-day mortality in a population-based cohort. We hypothesized that the magnitude of the surgical trauma, measured as blood loss, duration of surgery, and presence of major resection, would not influence and increase the risk of 30-day mortality in patients treated for aBM in a population-based cohort.
Patients and method
Study design and patients
We conducted a retrospective population-based cohort study on all patients from the Capital Region of Denmark (CRD) between January 1, 2014 and December 31, 2019. It is based on a previously described population-based cohort [18], receiving surgical treatment for aBM. Also, patients with hematological diseases that affected the bone, such as myeloma and lymphoma, were included as they underwent similar surgical procedures. The CRD had a mean population of 1,795 million inhabitants in the study period [19]. All orthopedic surgeries in the CRD were carried out at 6 orthopedic departments: 5 secondary surgical centers (Nordsjællands Hospital, Herlev/Gentofte Hospital, Bispebjerg/Frederiksberg Hospital, Amager/Hvidovre Hospital, and Bornholms Hospital) and the tertiary Musculoskeletal Tumor Center (Rigshospitalet). To identify eligible patients, all orthopedic procedures involving bone in the extremities (around 100,000 procedures) were examined using the regional surgical planning software EPIC or Orbit. When no biopsy was obtained during surgery or if the material was insufficient for histopathological examination, trauma mechanism (fractures sustained by no or low-energy trauma) in combination with preoperative images (radiological signs of a pathological fracture) and postoperative follow-up were assessed in the patient files. The final decision for inclusion was then made by the primary author, a senior musculoskeletal tumor surgeon, and, if required, a musculoskeletal radiologist.
In the case of multiple aBM lesions in one patient in the study period, only the first treated lesion was included in this study in order not to violate the statistical assumptions of independence. Due to the heterogeneity, and therefore indication for surgery, of the group of patients who did not receive an implant during surgery, only patients who received IF or EPR were included.
The study was reported according to the STROBE guidelines.
Data collection and variables
Patient files and operation charts were reviewed and information on sex, primary cancer diagnosis and extent of dissemination, ASA group, type of fracture, and implant were chosen. To describe the magnitude of the surgical trauma, information on surgical time (time from skin incision to wound closure), intraoperative blood loss (calculated by weighing the surgical napkins and by measuring the amount of blood removed with the surgical suction equipment), and on bone resection were used. The resection of bone was evaluated as described by Sørensen et al. [11]. In brief, major resection in the femoral bone was defined as resection through or distal to the lesser trochanter or proximal to the condyles of the knee; in the humeral bone, as resection distal to the surgical neck or proximal to the epicondyles of the elbow. Cut-off values for surgical time and blood loss could not be obtained from the previous study as this only contained patients treated with joint replacement surgery or intercalary spacing. A minor surgical trauma, such as osteosynthesis, was not reflected in the analysis by Sørensen et al. [11] regarding blood loss and surgical duration, which is why cut-off values representing this kind of procedure could not be obtained from the research group’s previous study. The cut-off was therefore done taking the medians for the entire cohort (< or ≥ 130 minutes and < or ≥ 468 mL). These values are well beyond the surgical trauma (regarding blood loss and surgical duration) for a regular nonpathological hip arthroplasty [14,15], which corresponds to an intermediate surgical trauma. Implant type was dichotomized as EPR or IF. Sex was dichotomized as male or female. ASA score was estimated before surgery by the attending anesthesiologist according to guidelines [20] and dichotomized into 1+2 or 3+4. Karnofsky performance status was dichotomized as < or ≥ 70 (equals able to care for self or Eastern Cooperative Oncology Group [ECOG] 0–1 or 2–5). Primary cancer was grouped according to the aggressiveness of the cancer into slow, moderate, and fast-growth cancer, following Katagiri et al. [21] and Sørensen et al. [11] to validate previous findings. Visceral metastases and the number of bone metastases were not included in the analysis for 30-day mortality in our previous study but were included now based on findings in the literature for overall survival [7,10,21–24]. Type of fracture (complete or impending) was included in the analysis based on findings in the literature, reporting differences in survival between these [7,18]. All the variables mentioned were used as predictive variables for the outcome, 30-day mortality, in logistic regression.
Statistics
Continuous variables were reported as mean (range) and median (IQR) and subgroups were analyzed using Student’s t-test and the Wilcoxon rank-sum test for parametric and non-parametric data, respectively. Categorical variables were reported as numbers and frequencies and subgroups were analyzed using Pearson’s chi-square test or Fisher’s exact test, subgroups being EPR versus IF.
A logistic regression model was fitted to evaluate independent risk factors for 30-day mortality. No elimination was performed in the multivariate analysis and all potential candidate variables were included regardless of significance level. As treatment center (a highly specialized referral center and a secondary surgical center) was an effect modification for bone resection and not the main scope of this paper, it was not included in the analysis. Patients with missing values were excluded from the logistic regression analysis as a pre-analysis decision.
Overall cumulated 30-day survival was calculated by Kaplan–Meier estimates. Due to the Danish Civil Registration System, which ensures accurate information on emigration and/or death, we had no loss to follow-up. All statistical analyses were performed in the statistical software R Studio (R Foundation for Statistical Computing, Vienna, Austria). Significance level was set as 0.05 and confidence intervals (CI) were reported as 95%.
Ethics, data sharing, funding, and disclosures
The Danish Patient Safety Authority (3-3013-2820/1) and the Data Protection Agency of the Capital Region of Denmark (VD-2019-132) have approved the study. The Capital Region of Denmark waived the requirement for written informed consent, as this retrospective study did not involve patient contact; therefore, no separate permission from the Danish Nation Centre for Ethics was required, according to Danish legislation. Data is not publicly available but will be sent on request. The Research Fund at Rigshospitalet, University of Copenhagen (Rigshospitalets Forskningsfond), funded the study. The funders had no role in the design of the study, collection of data, analyses, interpretation of data, writing of the manuscript, or in the decision to publish the results. The authors declare no conflict of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page. doi: 10.2340/17453674.2023.18394
Results
Patient population
515 surgical procedures for aBM were eligible for the study. Patients who received revision surgery due to previous aBM surgery but also other kinds of surgery (e.g., non-pathological fractures or osteoarthritis) were excluded (n = 22). The group with no implants consisted of patients having pelvic resections, Girdlestone procedures, and clavicular resections (n = 20). 437 patients were included in the study (Figure 1 and Table 1).
| Variable | Missing | All patients | Endo-prosthesis (n = 275) | Internal fixation (n = 162) | P value |
| Mean age at surgery | 0 | 70 | 69 | 72 | 0.01 a |
| (range) | (32–99) | (32–99) | (37–99) | ||
| Sex | 0 | 0.7 b | |||
| Female | 224 (51) | 139 (51) | 85 (52) | ||
| Male | 213 (49) | 136 (49) | 77 (48) | ||
| Cancer growth | 0 | 0.7 b | |||
| Fast | 158 (36) | 98 (36) | 60 (37) | ||
| Moderate | 132 (30) | 87 (32) | 45 (28) | ||
| Slow | 147 (34) | 90 (33) | 57 (35) | ||
| Location | 0 | 0.01 b | |||
| Lower extremity | 367 (84) | 241 (88) | 126 (78) | ||
| Upper extremity | 70 (16) | 34 (12) | 36 (22) | ||
| Fracture | 0 | < 0.001 b | |||
| Complete | 340 (78) | 199 (72) | 141 (87) | ||
| Impending | 97 (22) | 76 (28) | 21 (13) | ||
| Karnofsky score | 0 | 0.04 b | |||
| < 70 | 141 (32) | 79 (29) | 62 (38) | ||
| ≥ 70 | 296 (68) | 196 (71) | 100 (62) | ||
| ASA group | 8 | 0.4 b | |||
| 1+2 | 140 (33) | 93 (34) | 47 (30) | ||
| 3+4 | 289 (67) | 179 (66) | 110 (70) | ||
| Bone metastases | 9 | 0.03 b | |||
| Solitary lesion | 102 (24) | 74 (27) | 28 (18) | ||
| Multiple lesions | 326 (76) | 197 (73) | 129 (82) | ||
| Visceral metastases | 0 | 0.3 b | |||
| Without/unknown | 228 (52) | 149 (54) | 79 (49) | ||
| With | 209 (48) | 126 (46) | 83 (51) | ||
| Major resection | 0 | < 0.001 c | |||
| No | 278 (64) | 116 (42) | 162 (100) | ||
| Yes | 159 (36) | 159 (58) | 0 (0) | ||
| Blood loss (mL) | 45 | < 0.001 d | |||
| Median | 468 | 600 | 200 | ||
| (IQR) | (200–800) | (300–1,000) | (100–500) | ||
| Minutes of surgery | 0 | < 0.001 d | |||
| Median | 130 | 140 | 106 | ||
| (IQR) | (91–165) | (103–168) | (74–151) | ||
| a Student’s t-test. b Pearson’s chi-square test. c Fisher’s exact test. d the Wilcoxon rank-sum test. | |||||
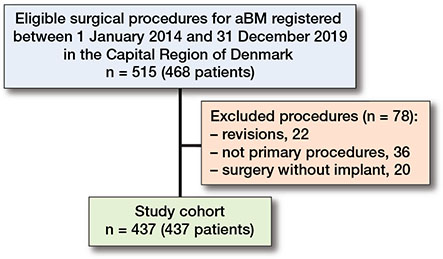
Figure 1. Flowchart illustrating patients included and excluded.
Overall survival
The cumulated probability of 30-day survival for the entire cohort was 85% (CI 81–88) (Figure 2). 30 days after surgery 67 patients were dead and 370 were still alive.
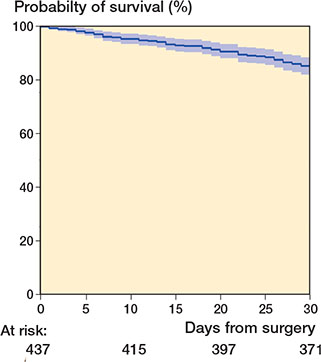
Figure 2. Kaplan–Meier analysis illustrates cumulated probability of 30-day survival for all patients at 85% (CI 81–88).
Univariate analysis
Unadjusted univariate logistic regression analysis showed that Karnofsky score < 70, ASA score 3+4, fast-growth cancer, and having visceral or multiple bone metastases were statistically significant risk factors for 30-day mortality. Complete or impending fracture, implant type, duration of surgery, intra operative blood loss, and degree of bone resection did not increase the risk of 30-day mortality in the univariate analysis.
Multivariate analysis
In the multivariate analysis containing all variables, male sex (OR 2.8, CI 1.3–6.3), Karnofsky score < 70 (OR 4.2, CI 2.1–8.6), and having multiple bone metastases (OR 3.4, CI 1.2–9.9) were independent risk factors for 30-day survival. ASA group 3+4 and fast-growth cancer were highly significant for 30-day survival in the univariate regression analysis. However, this finding did not remain statistically significant after adjusting for covariates in the multivariate analysis. No variables describing the magnitude of the surgery (blood loss, surgery time, and extent of resection) were associated with the risk of 30-day mortality in any analysis. See Table 2 for full regression analysis.
Discussion
The study aimed to investigate whether parameters describing the magnitude of the surgical trauma, measured as blood loss, surgical time, and degree of bone resection, posed a risk for 30-day mortality. We showed that none of the parameters describing the surgical trauma influenced survival. Instead, the general health status of the patients and the extent of primary cancer were found to influence survival postoperatively. The study thereby validated the research group’s previous findings of risk factors for 30-day mortality [11].
Risk factors for survival are of great importance because survival expectancies for this group of patients are low. Consequently, choosing the right surgical procedure is essential.
Few other studies have investigated perioperative variables to demonstrate an association with postoperative survival for patients with aBM. Tsuda et al. [10] investigated 1,497 patients treated for pathological fractures of the femur. In agreement with our findings on surgical time, they did not find the duration of anesthesia to be associated with postoperative mortality. Instead, they found fast-growth primary cancers, visceral metastases, no chemotherapy after surgery, and implant type to be risk factors for death. The described increased risk of 30-day mortality for patients having IF is probably a result of bias in the selection of surgical treatment for patients with a poor prognosis who tend to undergo less invasive surgery [18], as illustrated in Table 1, describing differences between patients treated with EPR and IF. Furthermore, patients undergoing IF for aBM in the femur may be limited in immediate weightbearing due to the painful lesion if not removed. Consequently, healing is sparse due to the poor quality of the bone, all contributing to an increased risk of developing thromboembolic events postoperatively [25] and hereby a possible increase in mortality. Tsuda et al. [10] did not include variables defining the general health status of the patient, such as ASA score, Karnofsky performance status, or ECOG performance score, although in the literature this is shown to have an impact on survival [21,24,26]. Hence, they were not able to detect a possible association between these variables and postoperative mortality.
A study by Dodd et al. [27] investigating non-pathological hip and pelvis fractures found ASA score and functional status to be associated with an increased risk of death 30 days post-surgery. Further, Harman et al. [28] investigated non-pathological proximal hip fractures and, among other factors, also found ASA score to be a strong predictor for 30-day survival. We consider the similarities in the surgical procedures in these studies to allow comparison with our study, hence supporting the evidence of general health status to be a strong predictor for survival.
We were able to demonstrate that patients undergoing extensive surgery (surgery time ≥ 130 minutes, blood loss ≥ 468 mL, and having major resections) were not at an increased risk of death postoperatively. Prolonged surgical time is probably a surrogate for tumor debulking and absolute stabilization of the bone, as seen in wide resection of metastases and reconstruction with tumor prostheses. The absolute stabilization of the bone enables the patient to fully bear weight with increased mobility in the early postoperative stage. A prospective study by Sørensen et al. [29] demonstrates regain of mobility 6 weeks post-surgery for aBM patients treated with EPR in the proximal and metaphyseal part of the femur, but fails to compare outcomes for patients treated with IF, due to attrition bias in this group of patients. However, studies of early mobilization and pain after surgery for aBM need to validate the hypothesis that resection of bone metastases and reconstruction with EPR results in better early mobility, and weightbearing, and protects against early postoperative mortality.
We found a 30-day survival probability of 85% (CI 81–88), slightly lower than found in our previous study [11]. This is probably explained by the present study including patients treated at secondary surgical centers, who are shown to have significantly poorer survival than patients treated at a highly specialized center [18]. Tsuda et al. [10] report a 30-day mortality after surgery for metastatic lesions in the femur of 2.6%, which is considerably lower than ours. This cannot be explained by including only proximal femur metastases or a very large patient cohort, so some selection bias or loss to follow-up must exist. As this study was not population-based but performed from an administrative database and identified only death during hospitalization, it is likely that loss to follow-up and selection resulted in an underestimation of 30-day mortality. Also, the study by Dodd et al. [27] of non-pathological hip and pelvic fractures found a 30-day mortality rate of 6.2%, indicating that the mortality rate found by Tsuda et al. is underestimated.
In the present study, dissemination of disease, described as the presence of visceral metastases and multiple bone metastases, was also found to influence risk of death in univariate analysis but in multivariate analysis only the latter was found to be an independent prognostic factor for 30-day mortality. No studies exist investigating the influence of the dissemination of disease on short-term survival for aBM patients, but several studies have shown an impact on long-term survival [21,23].
Limitations
First, the study has an inherent selection bias of both patients and the choice of treatment. As such, the bias that occurs when a certain patient is submitted to a certain treatment based on residual life expectancy and risk factors for long-term survival was not eliminated. Patients with poor performance status and extensive disease progression are often not selected for surgery and therefore were not included in the present study. Second, we acknowledge that while choosing the median as cut-off value for surgical time and blood loss, there may be a possibility of overlooking a potential increase in mortality for some procedures at the higher end of the blood loss and surgical duration scale.
Conclusion
The study found that none of the parameters describing the surgical trauma, such as blood loss, surgical time, and degree of bone resection, influenced the risk of 30-day mortality. Instead, the general health status of the patient and extent of primary cancer disease influenced survival postoperatively.
- Torre L A, Siegel R L, Ward E M, Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev 2016; 25: 16-27. doi: 10.1158/1055-9965.EPI-15-0578.
- Janssen S J, Teunis T, Hornicek F J, van Dijk C N, Bramer J A M, Schwab J H. Outcome after fixation of metastatic proximal femoral fractures: a systematic review of 40 studies. J Surg Oncol 2016; 114: 507-19. doi:10.1002/jso.24345.
- Harvey N, Ahlmann E R, Allison D C, Wang L, Menendez L R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin Orthop Relat Res 2012; 470: 684-91. doi:10.1007/s11999-011-2038-0.
- Yong M, Jensen A Ö, Jacobsen J B, Nørgaard M, Fryzek J P, Sørensen H T. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007). Breast Cancer Res Treat 2011; 129: 495-503. doi: 10.1007/s10549-011-1475-5.
- Sørensen M S, Gregersen K G, Grum-Schwensen T, Hovgaard D, Petersen M M. Patient and implant survival following joint replacement because of metastatic bone disease. Acta Orthop 2013; 84: 301-6. doi: 10.3109/17453674.2013.788437.
- Schneiderbauer M M, von Knoch M, Schleck C D, Harmsen W S, Sim F H, Scully S P. Patient survival after hip arthroplasty for metastatic disease of the hip. J Bone Joint Surg Am 2004; 86: 1684-9. doi: 10.2106/00004623-200408000-00011.
- Raschka T, Weiss S, Reiter A, Barg A, Schlickewei C, Frosch K H, et al. Outcomes and prognostic factors after surgery for bone metastases in the extremities and pelvis: a retrospective analysis of 140 patients. J Bone Oncol 2022; 34: 100427. doi: 10.1016/j.jbo.2022.100427.
- Hara H, Sakai Y, Kawamoto T, Fukase N, Kawakami Y, Takemori T, et al. Surgical outcomes of metastatic bone tumors in the extremities (surgical outcomes of bone metastases). J Bone Oncol 2021; 27: 100352. doi: 10.1016/j.jbo.2021.100352.
- Sørensen M S, Hovgaard T B, Hindsø K, Petersen M M. Prognostic value of biochemical variables for survival after surgery for metastatic bone disease of the extremities. J Surg Oncol 2017; 115: 442-8. doi: 10.1002/jso.24537.
- Tsuda Y, Yasunaga H, Horiguchi H, Fushimi K, Kawano H, Tanaka S. Complications and postoperative mortality rate after surgery for pathological femur fracture related to bone metastasis: analysis of a nationwide database. Ann Surg Oncol 2016; 23: 801-10. doi: 10.1245/s10434-015-4881-9.
- Sørensen M S, Hindsø K, Hovgaard T B, Petersen M M. Extent of surgery does not influence 30-day mortality in surgery for metastatic bone disease: an observational study of a historical cohort. Medicine (Baltimore) 2016; 95: e3354. doi: 10.1097/MD.0000000000003354.
- Boddapati V, Held M B, Levitsky M, Charette R S, Neuwirth A L, Geller J A. Risks and complications after arthroplasty for pathological or impending pathological fracture of the hip. J Arthroplasty 2021; 36: 2049-54.e5. doi: 10.1016/j.arth.2021.02.004.
- Bindels B J J, Thio Q C B S, Raskin K A, Ferrone M L, Lozano Calderón S A, Schwab J H. Thirty-day postoperative complications after surgery for metastatic long bone disease are associated with higher mortality at 1 year. Clin Orthop Relat Res 2020; 478: 306-18. doi: 10.1097/CORR.0000000000001036.
- Barrett W P, Turner S E, Leopold J P. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty 2013; 28: 1634-8. doi: 10.1016/j.arth.2013.01.034.
- Brunello M, Di Martino A, Ruta F, Ferri R, Rossomando V, Agostino C D, et al. Which patient benefit most from minimally invasive direct anterior approach total hip arthroplasty in terms of perioperative blood loss? A retrospective comparative study from a cohort of patients with primary degenerative hips. Musculoskelet Surg 2023; online ahead of print. doi: 10.1007/s12306-023-00792-z.
- Schoenfeld A J, Carey P A, Cleveland A W, Bader J O, Bono C M. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J 2013; 13: 1171-9. doi: 10.1016/j.spinee.2013.02.071.
- Pugely A J, Martin C T, Gao Y, Ilgenfritz R, Weinstein S L. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine (Phila Pa 1976) 2014; 39: 1225-34. doi: 10.1097/BRS.0000000000000365.
- Ladegaard T H, Sørensen C L, Nielsen R, Troelsen A, Al-Mousawi D A A, Bielefeldt R, et al. Surgical treatment of metastatic bone disease in the appendicular skeleton: a population-based study. Cancers (Basel) 2022; 14: 1258. doi: 10.3390/cancers14051258.
- Denmark Statistics Population—Capital Region of Denmark Available from: https://www.dst.dk/en/Statistik/emner/borgere/befolkning/befolkningstal (accessed August 7, 2023).
- ASA Physical Status Classification System. Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system.
- Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014; 3: 1359-67. doi: 10.1002/cam4.292.
- Hansen B H, Keller J, Laitinen M, Berg P, Skjeldal S, Trovik C, et al. The Scandinavian Sarcoma Group skeletal metastasis register: survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand 2004; 75: 11-15. doi: 10.1080/00016470410001708270.
- Bauer H C, Wedin R. Survival after surgery for spinal and extremity metastases: prognostication in 241 patients. Acta Orthop Scand 1995; 66: 143-6. doi: 10.3109/17453679508995508.
- Forsberg J A, Eberhardt J, Boland P J, Wedin R, Healey J H. Estimating survival in patients with operable skeletal metastases: an application of a Bayesian Belief Network. PLoS One 2011; 6: e19956. doi: 10.1371/journal.pone.0019956.
- Shallop B, Starks A, Greenbaum S, Geller D S, Lee A, Ready J, et al. Thromboembolism after intramedullary nailing for metastatic bone lesions. J Bone Joint Surg Am 2015; 97: 1503-11. doi: 10.2106/JBJS.N.01067.
- Sørensen M S, Gerds T A, Hindsø K, Petersen M M. Prediction of survival after surgery due to skeletal metastases in the extremities. Bone Joint J 2016; 98-B: 271-7. doi: 10.1302/0301-620X.98B2.36107.
- Dodd A C, Bulka C, Jahangir A, Mir H R, Obremskey W T, Sethi M K. Predictors of 30-day mortality following hip/pelvis fractures. Orthop Traumatol Surg Res 2016; 102: 707-10. doi: 10.1016/j.otsr.2016.05.016.
- Harman H, Walton T J, Chan G, Stott P, Ricketts D M, Rogers B A. Predicting 30-day mortality after hip fracture: the G4A calibrated prognostic tool. HIP Int 2022; 32: 820-5. doi: 10.1177/1120700021998959.
- Sørensen M S, Horstmann P F, Hindsø K, Petersen M M. Use of endoprostheses for proximal femur metastases results in a rapid rehabilitation and low risk of implant failure: a prospective population-based study. J Bone Oncol 2019; 19: 100264. doi: 10.1016/j.jbo.2019.100264.