No difference in risk of revision due to infection between clindamycin and cephalosporins as antibiotic prophylaxis in cemented primary total knee replacements: a report from the Norwegian Arthroplasty Register 2005–2020
Karola PAWLOY 1, Anne Marie FENSTAD 2, Tesfaye LETA 2,4, Geir HALLAN 2,3, Jan-Erik GJERTSEN 2,3, Håvard DALE 2,3, Stein Atle LIE 2, and Ove FURNES 2,3
1 Aberdeen Royal Infirmary, Aberdeen, Scotland; 2 The Norwegian Arthroplasty Register, Department of Orthopaedic Surgery, Haukeland University Hospital, Bergen, Norway; 3 Department of Clinical Medicine, University of Bergen, Bergen, Norway; 4 VID Specialized University, Bergen, Norway
Background and purpose — Systemic antibiotic prophylaxis with clindamycin, which is often used in penicillin- or cephalosporin-allergic patients’, has been associated with a higher risk of surgical revision for deep prosthetic joint infection (PJI) than cloxacillin in primary total knee replacement (TKR). We aimed to investigate whether clindamycin increases the risk of surgical revisions due to PJI compared with cephalosporins in primary cemented TKR.
Patients and methods — Data from 59,081 TKRs in the Norwegian Arthroplasty Register (NAR) 2005–2020 was included. 2,655 (5%) received clindamycin and 56,426 (95%) received cephalosporins. Cox regression analyses were performed with adjustment for sex, age groups, diagnosis, and ASA score. Survival times were calculated using Kaplan–Meier estimates and compared using Cox regression with revision for PJI as endpoint. The cephalosporins cefalotin and cefazolin were also compared.
Results — Of the TKRs included, 1.3% (n = 743) were revised for PJI. 96% (n = 713) had received cephalosporins and 4% (n = 30) clindamycin for perioperative prophylaxis. Comparing cephalosporins (reference) and clindamycin, at 3-month follow-up the adjusted hazard ratio rate (HRR) for PJI was 0.7 (95% confidence interval [CI] 0.4–1.4), at 1 year 0.9 (CI 0.6–1.5), and at 5 years 0.9 (CI 0.6–1.4). Analysis using propensity score matching showed similar results. Furthermore, comparing cefalotin (reference) and cefazolin, HRR was 1.0 (CI 0.8–1.4) at 3 months and 1.0 (CI 0.7–1.3) at 1-year follow-up.
Conclusion — We found no difference in risk of revision for PJI when using clindamycin compared with cephalosporins in primary cemented TKRs. It appears safe to continue the use of clindamycin in penicillin- or cephalosporin-allergic patients.
Citation: Acta Orthopaedica 2023; 94: 404–409. DOI: https://doi.org/10.2340/17453674.2023.16907.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-06-24. Accepted: 2023-05-13. Published: 2023-07-31.
Correspondence: karola.pawloy@nhs.scot
The authors thank the orthopedic surgeons of Norway for their conscientious reporting and the NAR for providing the first author with the necessary data for analysis.
KP: Statistical analysis, writing of the initial draft and final draft. AMF: Statistical analysis, editing of the initial and final draft. TL, GH, J-EG, and HD: Editing. SAL: Statistical advice, editing. OF: Supervision, creation of study design and protocol, editing.
Handling co-editors: Eivind Witsø and Robin Christensen
Acta thanks Johan Kärrholm, AnnetteW-Dahl, and Marianne Westberg for help with peer review of this manuscript.
Revisions for prosthetic joint infection (PJI) are on the rise [1]. This is not only a health issue with significant patient morbidity and mortality, but also has a substantial financial impact for society. The mean cost of a revision for PJI is between €11,000 and €15,000 in Europe [2].
Prophylactic systemic antibiotics are effective in reducing PJI, however, antibiotics selection remains controversial [3]. Recommendations differ from country to country, with Norway currently recommending cephalosporins (and in the case of penicillin allergy, clindamycin) whereas Sweden recommends cloxacillin preoperatively followed by 3 postoperative doses (and in the case of penicillin allergy, clindamycin) [4,5].
Robertsson et al. [5] found that systemic clindamycin as a prophylactic antibiotic was associated with a higher risk of total knee replacement (TKR) revision for PJI than cloxacillin.
We aimed to investigate whether clindamycin was associated with a higher risk of revision for PJI compared with 1st-generation cephalosporins. Additionally, 2 of the cephalosporins, cefalotin and cefazolin, were compared, to investigate their risk of revision for PJI.
Patients and methods
We identified 83,045 primary TKRs between 2005 and 2020 in the Norwegian Arthroplasty Register (NAR). Fully constrained TKRs (n = 300), unicondylar (n = 9,371), patellofemoral (n = 532), and bi-compartmental knee replacements (n = 2) as well as unspecified TKRs (n = 7) were excluded. Cases with missing information on ASA (n = 1,404) were also excluded. Cases receiving any antibiotic other than cefazolin, cefalotin, or clindamycin, or with missing data were excluded (n = 5,453). Further, uncemented TKRs were excluded (n = 6,895). This left 59,081 knees eligible for analyses (Figure 1). These were further split up into Period 1 from 2005 to 2012 (n = 22,357) and Period 2 from 2013 to 2020 (n = 36,724), as revisions due to PJI had become more frequent in later years.
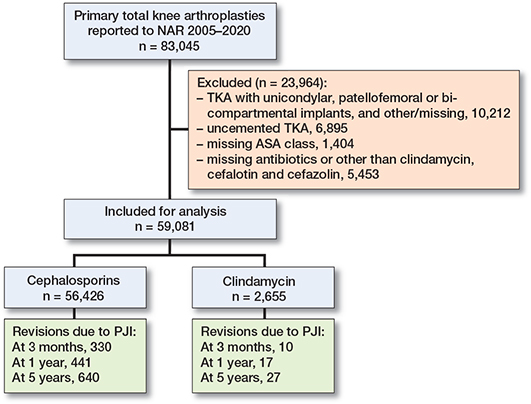
Figure 1. Demographic flowchart.
59,081 TKRs, in 48,569 patients (82% unilateral, 18% bilateral), reported to the NAR between 2005 and 2020, were included for analyses. Of these, 95% (n = 56,426) received cephalosporins (47,612 received cefalotin, 10,148 received cefazolin) and 5% (n = 2,655) received clindamycin as antibiotic prophylaxis. The majority of clindamycin patients either received 3 doses (41.5%) or 4 doses (45.3%). In the cephalosporin group 86.9% of patients received 4 doses.
The NAR has continuously monitored the quality of knee replacement surgery in Norway since 1994. Both primary and revision operations are reported by the orthopedic surgeons, who fill in a form directly after surgery [6]. Therefore, it is the surgeon who makes the diagnosis of PJI based on the clinical appearance and available evidence at the time of surgery, and the diagnosis is not always based on microbiological results. The results of perioperative cultures taken during the revision were not known at the time of diagnosis. The surgeons are educated to use the definition of PJI as described by the European Bone & Joint Infection Society [7].
The unique personal identification number of each Norwegian resident links information from any subsequent revisions to the primary operation, thus making it possible to trace them [6]. In the NAR’s most recent report, it was estimated that 97.7% of all primary TKRs and 93.2% of all revisions taking place in Norway were reported in the years 2017–2018 [6], and this rate had been equally high over the previous years [8].
Statistics
IBM SPSS 25 (IBM Corp, Armonk, NY, USA) was used for statistical analyses. Means, medians, and total numbers of TKRs were analyzed by descriptive statistics. Unadjusted time to event analysis (Kaplan–Meier) was used to calculate survival probabilities with revision due to PJI as endpoint. Comparison of revision risks (expressed as hazard rate ratios, HRR) was calculated using Cox regression, with systemic antibiotic drug as the main exposure. Sex, age groups (< 45, 45–54, 55–74, ≥ 75), diagnosis (osteoarthritis/other), and ASA class (1, 2 and ≥ 3) were used as adjustment factors, as these are known risk factors for infection [9]. BMI was not included as the registration of BMI in the NAR started as late as 2021. We reported the HRR for the clindamycin group compared with the control group (cephalosporin). HRRs and survival probabilities were presented with 95% confidence intervals (CI).
To account for the higher rate of revisions early postoperatively, 3-month, 1-year, and 5-years revision rates were analyzed. The outcome was revision due to PJI. Revision was defined as the exchange, removal, or addition of part of an implant or the whole implant. Sensitivity analysis did not show a change in statistical significance when excluding bilateral TKRs, which were therefore included in the interest of maximum numbers.
To reduce selection bias and correct for known confounding factors, propensity score matching was used for the comparison between clindamycin and cephalosporins. Age, sex, diagnosis, ASA class, and year of operation were used as predictors for calculation of the propensity of receiving treatment with clindamycin. The propensities were based on logistic regressions with the predictors as categorical variables. Cases that received clindamycin (n = 2,655) were matched 1:3, using a tolerance of 0.00001, with the cephalosporin TKRs (Table 1). Unadjusted Cox regression analyses were performed based on the propensity score matched data.
| Factor | Group 1 (cephalosporin) | Group 2 (clindamycin) | Group 1 a (PSM 1:3) |
| Procedures | 56,426 (95) | 2,655 (5) | 7,896 |
| Revisions | 2,641 (4.7) | 126 (4.7) | 369 (4.7) |
| due to PJI | 713 (1.3) | 30 (1.1) | 70 (0.9) |
| Deaths | 9,254 (16) | 301 (11) | 1,167 (15) |
| Median FU b (CI) | 6.4 (6.3–6.4) | 5.6 (5.4–5.8) | 7.0 (6.9–7.2) |
| Bilateral TKR | 10,200 (18) | 521 (19) | 1,399 (18) |
| Male sex | 22,321 (39) | 664 (24) | 1,917 (24) |
| Age, mean (SD) | 69 (9.6) | 68 (9.7) | 68 (9.6) |
| Age group | |||
| < 45 | 623 (1.2) | 41 (1.5) | 135 (1.7) |
| 45–54 | 3,397 (6.3) | 191 (7.2) | 693 (8.5) |
| 55–74 | 33,523 (63) | 1,643 (62) | 5,238 (66) |
| ≥ 75 | 15,990 (30) | 626 (24) | 1,854 (24) |
| Missing | 2,893 (5.1) | 154 (5.8) | |
| Diagnosis | |||
| Osteoarthritis | 51,897 (90) | 2,394 (90) | 7,116 (90) |
| Other | 5,863 (10) | 261 (9.8) | 780 (10) |
| ASA class | |||
| 1 | 7,020 (12) | 191 (7.2) | 567 (7.0) |
| 2 | 37,532 (67) | 1,777 (67) | 5,274 (67) |
| ≥ 3 | 11,874 (21) | 687 (26) | 2,055 (26) |
| Mean operation time, minutes | 91 | 91 | 91 |
| a PSM: propensity score matching, 69 excluded due to missing values. b Median FU: calculated by inverse Kaplan–Meier in years. |
|||
Ethics, funding, and conflicts of interest
The NAR has a license from the Norwegian Data Inspectorate based on patient consent (reference number: 16/01622-3/CDG and date of issue: latest license February 24, 2017) and collects data according to Norwegian and EU data protection rules. Data may be accessible upon application to the NAR. The study was fully financed by the NAR, and no external funding was received. The authors declare no conflict of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.16907
Results
Mean age was 68.9 years. 61.4% were female. Osteoarthritis was the most common reason for TKR (89.8%) (Table 1). Median follow-up was 6 years for the cephalosporin group and 5 years for the clindamycin group (Table 1).
73 hospitals had performed primary TKRs and 58 revision TKRs due to infection between 2005 and 2020. Some units have since stopped performing such procedures, whereas others have started. At any given year, 53–58 units were active. The completeness of the primary TKRs reported to the Norwegian register is high (> 96%).
Incidence of infection was 1.2% in unilateral TKRs (605/48,569) and 1.3% in bilateral TKRs (138/10,512) (not shown in table).
Revision rate cephalosporins vs. clindamycin
1.3% (n = 743) had a revision due to PJI; 96% (n = 713) of these had received cephalosporins and 4% (n = 30) clindamycin for perioperative antibiotic prophylaxis. 5-year mortality was 5.4% for all patients, 4.4% in the clindamycin group, 5.5% in the cephalosporin group. By the end of follow-up, 16.2% of all patients had died (Table 1).
46% of the revisions due to infection were performed within the first 3 months of follow-up and 62% within 1 year of follow-up.
At 3 months, 0.6% (n = 340) were revised due to PJI, 97% (n = 330) had received cephalosporins and 3% (n = 10) clindamycin. The unadjusted Kaplan–Meier survival probabilities free from revision due to PJI were for clindamycin 99.8% (CI 99.6–100) and for cephalosporin 99.6% (CI 99.6–99.6) (Table 2). The adjusted Cox regression analyses confirmed no statistically significant difference with cephalosporins used as the reference category (HRR 0.7, CI 0.4–1.4) (Table 2). At 3 months, there was no statistically significant difference in Period 1 (HRR 0.6, CI 0.1–2.3) or in Period 2 (HRR 0.6, CI 0.3–1.2).
| Time | Clindamycin | Cephalosporins |
| 3 months | ||
| Revised due to PJI, n | 10 | 330 |
| KM survival, % (CI) | 99.8 (99.6–100) | 99.6 (99.6-99.6) |
| Numbers at risk | 2,635 | 55,876 |
| Cox-adjusted HRR a (CI) | 0.7 (0.4–1.4) | 1 |
| 1 year | ||
| Revised due to PJI, n | 17 | 441 |
| KM survival, % (CI) | 99.4 (99.2–99.6) | 99.2 (99.2–99.2) |
| Numbers at risk | 2,609 | 55,225 |
| Cox-adjusted HRR a (CI) | 0.9 (0.6–1.5) | 1 |
| 5 years | ||
| Revised due to PJI, n | 27 | 640 |
| KM survival, % (CI) | 98.9 (98.5–99.3) | 98.8 (98.8–98.8) |
| Numbers at risk | 1,468 | 34,705 |
| Cox-adjusted HRR a (CI) | 0.9 (0.6–1.4) | 1 |
| a HRR: hazard ratio, clindamycin vs. cephalosporins (reference), adjusted for age, sex, diagnosis, ASA class. | ||
At 1 year, 0.8% (n = 458) were revised due to PJI; 96% (n = 441) had received cephalosporins and 4% (n = 17) clindamycin during primary operation (Table 2 and Figure 2). Log rank testing with unadjusted Kaplan–Meier analysis showed similar survival probabilities for clindamycin 99.4% (CI 99.2–99.6) and cephalosporin 99.2% (CI 99.2–99.2) (Table 2). Adjusted Cox regression also showed no significant difference (HRR 0.9, CI 0.6–1.5) (Table 2). At 1 year there was no significant difference in Period 1 or in Period 2 (HRR 0.9, CI 0.4–2.3 vs HRR 1.1, CI 0.6–2.0, respectively).
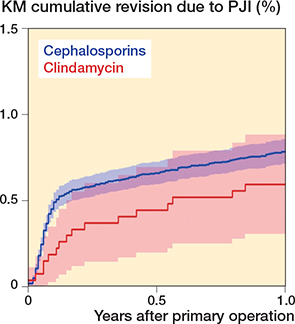
Figure 2. Kaplan–Meier cumulative revision due to PJI.
After 5 years, 1.1% (n = 667) were revised for PJI; 96% (n = 640) had received cephalosporins and 4% (n = 27) clindamycin at their primary operation (Table 2). Differences in revision rates were not statistically significant in log-rank testing in unadjusted Kaplan–Meier analysis (clindamycin 98.9%, CI 98.5–99.3 vs. cephalosporin 98.8%, CI 98.8–98.8) or adjusted Cox regression (HRR 0.9, CI 0.6–1.4) (Table 2). There was no significant difference in Period 1 or in Period 2.
Overall, male sex (HRR 2.1, CI 1.8–2.5) compared with females, ASA ≥ 3 (HRR 1.8, CI 1.8–2.5) compared with ASA 1, older age (HRR 1, CI 1–1), and osteoarthritis (HRR 0.7, CI 0.5–0.8) compared with other diagnoses were factors associated with the risk of revision due to PJI.
When comparing the risk of revision due to PJI in propensity score matched knees, the previously defined time points were analyzed with unadjusted Cox regression. At 3 months (HRR 0.9, CI 0.4–1.7), 1 year (HRR 1.0, CI 0.6–1.8), and 5 years (HRR 1.1, CI 0.7–1.8) postoperatively, similar results were found with cephalosporins used as the reference category (Table 3).
| Time | Clindamycin | Cephalosporins |
| 3 months | ||
| Revised due to PJI, n | 10 | 35 |
| Cox-unadjusted HRR a (CI) | 0.9 (0.4–1.7) | 1 |
| 1 year | ||
| Revised due to PJI, n | 17 | 46 |
| Cox-unadjusted HRR a (CI) | 1.0 (0.6–1.8) | 1 |
| 5 years | ||
| Revision due to infection, n | 27 | 71 |
| Cox-unadjusted HRR a (CI) | 1.1 (0.7–1.8) | 1 |
| a See Table 2 | ||
Our sensitivity analysis with exclusion of non-Palacos type of cements showed the same results as in the main analysis. A breakdown of types of cement used can be found in Table 4. We have previously investigated the Palacos and Palacoslike cements and have found the same results in these cement types [10].
Cefalotin vs. cefazolin
As cefazolin was introduced in 2017, only revision rates at 3 months and 1 year follow-up could be compared. Cefazolin was used in 20.8% (n = 9,905), of which 1.0% (n = 96) had a revision due to PJI and cefalotin was used in 79.2% (n = 46,521), of which 1.3% (n = 617) had a revision due to PJI. No differences in revision rate for PJI between cefazolin and cefalotin (reference) were found either at 3 months (HRR 1.0, CI 0.8–1.4) or at 1 year (HRR 1.0, CI 0.7–1.3).
Types of revisions for PJI
In the cephalosporin group (n = 713), 65% of the knees had debridement, antibiotics, and implant retention (DAIR) as a surgical procedure, 27% removal of 1 or more parts, 0.8% arthrodesis or amputation, and 0.3% did not have the type of procedure listed. In the clindamycin group (n = 30), 63% of the knees had had a DAIR, 27% removal of 1 or more parts (Table 5).
| Type of revision | Cephalo-sporin n = 713 | Clinda-mycin n = 30 | Total n = 743 |
| Change of femur, tibia or whole prosthesis | 63 (9) | 5 (17) | 68 (9) |
| DAIR a | 466 (65) | 19 (63) | 485 (65) |
| Addition of patellar component | 5 (0.7) | 1 (3) | 6 (0.8) |
| Arthrodesis or amputation | 6 (0.8) | 0 | 6 (0.8) |
| Removal of 1 or more parts | 190 (27) | 8 (27) | 198 (27) |
| Missing information | 2 (0.3) | 0 | 2 (0.3) |
| Surgeons can mark for more than 1 type of revision; hence total numbers and percentages will not add up to the numbers of revision due to PJI. a Debridement, antibiotics and implant retention with change of tibia polyethylene component only. |
|||
Discussion
We found no difference in risk of revision due to PJI with the use of clindamycin or 1st-generation cephalosporins as perioperative antibiotic prophylaxis at TKR. This finding diverges from the current view that clindamycin is inferior to cephalosporins, a notion that has only been investigated in a small number of observational studies.
Robertsson et al. [5] found an increased risk of revision in TKRs that had received clindamycin compared with cloxacillin. 79% of their revisions were done before 1 year postoperatively, compared with 62% in our study. No demographic table was provided in the paper by Robertsson et al. Hence, we were not able to determine demographic differences between their cases and ours. Prevalence of clindamycin administration was 7.2% in the Swedish study, compared with 5% in our study. The completeness of reporting of revision due to infection is lower than for other revisions in both Norway and Sweden, but there is no reason to believe that the completeness of reporting is different for the clindamycin and cephalosporin knees [9]. The power in our study was somewhat lower than in the Swedish study, but there was a trend towards a lower risk for the clindamycin group.
Dosage of clindamycin did not differ between Sweden and Norway, with the Swedish study reporting the use of 600 mg x 3 in the majority (79%) of their patient group receiving clindamycin, the same dose as in Norway [4,5].
A study from Mayo Clinic, United States, investigated the rate of PJI for both TKRs and total hip replacements (THRs) and compared cefazolin with all other antibiotics that were used at the primary operation [11]. They found that administration of cefazolin at the primary operation reduced the risk of PJI by 32% compared with the “other” group. However, it must be noted that only 28.7% of the cefazolin group and 30.9% of the “other antibiotics” group received antibioticloaded bone cement, an option that is standard in most of Europe and less so in the United States. The effectiveness of antibiotic-loaded bone cement in TKRs is disputed, and this matter is currently being studied by the NAR in a registerbased nationwide randomized control trial [12]. All TKRs in our study received antibiotic-loaded cement.
The overall number of knees strengthens this study. A total of 58,091 knee operations provides a better evaluation of the general population undergoing TKRs in various settings than randomized control trials, which usually have too few participants and a more strictly controlled environment. Moreover, propensity score matching was used, reducing bias in estimating treatment effects. The propensity score is the probability that a patient would receive the treatment based on the chosen covariates. We split our data into 2 groups, treated with clindamycin or not, and matched individuals with identical propensity scores.
There were, however, limitations that come with any register-based study. Registry data quality can vary depending on the collection method and reporting rates. The NAR has attempted to optimize this by using standardized forms and having a high reporting rate. Dale et al. [13] have reported a rate of 1% of deep surgical site infections as captured by the NOIS (Norwegian Surveillance System for Healthcare-Associated Infections), which is considered the gold standard in Norway, at 1 year after primary THR. As the definition of PJI does not differ between THRs and TKRs in the NAR, we may assume that our results are equivalent to the reporting rate, suggesting that we capture around 80% of PJIs that are reoperated. As the diagnosis of PJI is usually clinical and not always based on bacterial cultures before a revision, misclassification bias is possible.
Another limitation was a potential unrecorded variation in the timing, duration, and dosage regime of antibiotics used, as well as the type of anesthetic used. We know that over 90% of patients receive antibiotic prophylaxis as recommended by national guidelines [6]. However, timing of the preoperative dose is not recorded on the surgeon form. This is a shortcoming, as it has previously been suggested that timely administration of antibiotic prophylaxis contributes to a lower infection risk but is probably the same in both groups [14].
Finally, the relatively small number of revisions due to infection must be noted. Especially at 3 months, there were only 10 revisions in the clindamycin group and 338 revisions in the cephalosporin group. Therefore, in particular our analysis for 3 months postoperatively must be viewed critically, as we had only a small number of clindamycin cases to compare. The confidence intervals in the Cox analyses are generally quite wide, and there is a risk of a type 2 error (not being able to prove a difference that is actually there due to low power).
Conclusion
We found no difference in risk of revision surgery for PJI when using clindamycin compared with cephalosporins in primary cemented TKRs. It appears safe to continue the use of clindamycin in penicillin- or cephalosporin allergic patients.
- Dyrhovden G S, Lygre S H, Badawy M, Gøthesen Ø, Furnes O. Have the causes of revision for total and unicompartmental knee arthroplasties changed during the past two decades? Clin Orthop Relat Res 2017; 475(7): 1874-86. doi: 10.1007/s11999-017-5316-7.
- Serrier H, Julien C, Batailler C, Mabrut E, Brochier C, Thevenon S, et al. Economic study of 2-stage exchange in patients with knee or hip prosthetic joint infection managed in a referral center in France: time to use innovative(s) intervention(s) at the time of reimplantation to reduce the risk of superinfection. Front Med 2021; 8: 552669. doi: 10.3389/fmed.2021.552669.
- Allegranzi B, Bischoff P, de Jonge S, Kubilay N Z, Zayed B, Gomes S M, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016; 16(12): e276-e287. doi: 10.1016/S1473-3099(16)30398-X.
- Ortopedisk Kirurgi [Internet]. Helsedirektoratet [cited 2021 Jan 25]. Available from: https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-sykehus/antibiotikaprofylakse-ved-kirurgi/ortopedisk-kirurgi#ortopedisk-kirurgi-med-leddprotese.
- Robertsson O, Thompson O, W-Dahl A, Sundberg M, Lidgren L, Stefánsdóttir A. Higher risk of revision for infection using systemic clindamycin prophylaxis than with cloxacillin. Acta Orthop 2017; 88(5): 562-7. doi: 10.1080/17453674.2017.1324677.
- Report 2022. Bergen: Norwegian Arthroplasty Register; 2022.
- McNally M, Sousa R, Wouthuyzen-Bakker M, Chen A F, Soriano A, Vogely H C, et al. Infographic: The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021; 103-B(1): 16-17. doi: 10.1302/0301-620X.103B1.BJJ-2020-2417.
- Espehaug B, Furnes O, Havelin L I, Engesæter L B, Vollset S E, Kindseth O. Registration completeness in the Norwegian arthroplasty register. Acta Orthop 2006; 77(1): 49-56. doi: 10.1080/17453670610045696.
- Badawy M, Espehaug B, Fenstad A M, Indrekvam K, Dale H, Havelin L I, et al. Patient and surgical factors affecting procedure duration and revision risk due to deep infection in primary total knee arthroplasty. BMC Musculoskelet Disord 2017; 18(1): 544. doi: 10.1186/s12891-017-1915-4.
- Birkeland Ø, Espehaug B, Havelin L I, Furnes O. Bone cement product and failure in total knee arthroplasty. Acta Orthop 2016; 88(1): 75-81. Doi: 10.1080/17453674.2016.1256937.
- Wyles C C, Hevesi M, Osmon D R, Park M A, Habermann E B, Lewallen D G, et al. John Charnley Award: Increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin. Bone Joint J 2019; 101-B(6_Supple_B): 9-15. doi: 10.1302/0301-620X.101B6.BJJ-2018-1407.R1.
- Leta T H, Gjertsen J-E, Dale H, Hallan G, Lygre S H, Fenstad A M, et al. Antibiotic-loaded bone cement in prevention of periprosthetic joint infections in primary total knee arthroplasty: a register-based multicentre randomized controlled non-inferiority trial (Alba Trial). BMJ Open 2021; 11(1): e041096. Doi: 10.1136/bmjopen-2020-041096.
- Dale H, Skråmm I, Løwer H L, Eriksen H M, Espehaug B, Furnes O, et al. Infection after primary hip arthroplasty. Acta Orthop 2011; 82(6): 646-54. doi: 10.3109/17453674.2011.636671.
- Van Kasteren M E, Mannien J, Ott A, Kullberg B-J, de Boer A S, Gyssens I C. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin Infect Dis 2007; 44(7): 921-7. doi: 10.1086/512192.