Effects of patient-specific positioning guides (PSPGs) vs. conventional instrumentation on patient-reported outcome in total knee arthroplasty: secondary analysis of a randomized controlled trial after 5 years
Sean C S RIVRUD 1, Stephan M RÖHRL 2, and Justin A M J VAN LEEUWEN 3
1 Faculty of Medicine, University of Groningen, Groningen, The Netherlands; 2 Department of Orthopedics, Oslo University Hospital, Oslo, Norway; 3 Department of Orthopedic Surgery, Telemark Hospital, Notodden, Norway and Department of Orthopaedic Surgery, Lærdal Hospital – Helse Førde, Lærdal, Norway
Background and purpose — The use of patient-specific positioning guides (PSPGs) in total knee arthroplasty (TKA) has been advocated as a means of improving patient outcomes, but the reception of PSPGs has been mixed. The aim of our study was to compare patient-reported outcomes (KOOS, NRS-11, EQ-5D-3L, EQ-VAS) after TKA using PSPG with conventional instrumentation (CI) to determine whether there is a discernible clinical benefit to using PSPGs.
Patients and methods — This multicenter randomized controlled trial (RCT) followed 77 patients who were randomly assigned to 1 of 2 cohorts between September 2011 and January 2014—one receiving TKA with PSPGs (from Materialise NV) and one receiving TKA with CI—with each cohort followed up until 5 years after the operation. The Vanguard Cruciate Retaining Total Knee System and Refobacin Bone Cement R were used in all operations. KOOS was evaluated using confidence intervals, with differences of less than 10 KOOS units between the cohorts interpreted as indicating the absence of a clinically meaningful difference.
Results — No significant differences were found in any of the measured clinical outcomes—KOOS, NRS-11, EQ-5D-3L, EQ-VAS, range of motion, or radiolucent lines scoring—between the cohort operated on using PSPG and the cohort operated on using CI after 5 years of follow-up.
Conclusion — There was no statistically significant effect of PSPGs on patient-reported outcomes or range of motion in TKA.
Citation: Acta Orthopaedica 2023; 94: 354–359. DOI: https://doi.org/10.2340/17453674.2023.15335.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-10-16. Accepted: 2023-05-13. Published: 2023-07-20.
Correspondence: juslee@helse-forde.no
JvL and SMR designed the study. SSR and JvL gathered the data. Statistical analysis was performed by SSR. All the authors were involved in data interpretation and in the writing of the manuscript.
Handling co-editors: Keijo Mäkelä and Robin Christensen
Acta thanks Arne Lundberg, Simo Miettinen, and Christof Wagner for help with peer review of this manuscript.
The result after total knee arthroplasty (TKA) may encounter several unfavorable outcomes if the components are aligned improperly [1]. In fact, malalignment has been reported as the third most common cause of TKA revision [2]. Also, several studies have demonstrated that the proper mechanical alignment of knee prostheses is a key factor in achieving prosthesis longevity and favorable outcomes [3]. Thus, technologies have continuously been developed since the conception of TKA to improve the surgeon’s ability to achieve proper alignment consistently.
In August 1997, the first TKA using computer-assisted navigation (CAN) was performed. While early studies displayed enthusiasm towards CAN [4], current evidence of CAN as an alternative to conventional instrumentation (CI) is inconsistent [5,6]. Furthermore, CAN necessitated prolonged operative times, contributing to unclear cost efficiency in comparison with CI [7]. In 2008, the first patient-specific positioning guides (PSPGs) became available for TKA. TKA with PSPGs is a computer-assisted surgery technique that preoperatively simulates virtual surgery based on preoperative magnetic resonance imaging (MRI) to facilitate the production of PSPGs. The promise of this technology was to excel where CAN fell short by removing the computer-assisted step of CAN-TKA from the operating room and implementing it in the preoperative workflow, while not affecting operative time. The current evidence on this promise is, however, ambiguous [8].
This multicenter RCT is a continuation of a previously published study [9], and compares clinical outcomes at 3 months, 1 year, 2 years, and 5 years in 77 patients who were randomly assigned to TKA with CI technique or using PSPGs to align the components. The aim of this study is to evaluate the effects of PSGs on clinical outcomes at 5-year follow-up. Moreover, we evaluated radiolucent lines.
Patients and methods
Between September 2011 and January 2014, 109 patients were included in this RCT at 3 centers: Oslo University Hospital Ullevål, Betanien Hospital Skien, and Telemark Hospital Skien. 15 patients were excluded from the study (Figure 1). 94 patients were operated on as part of this RCT, and 77 patients were available for follow-up after 5 years (Figure 1).
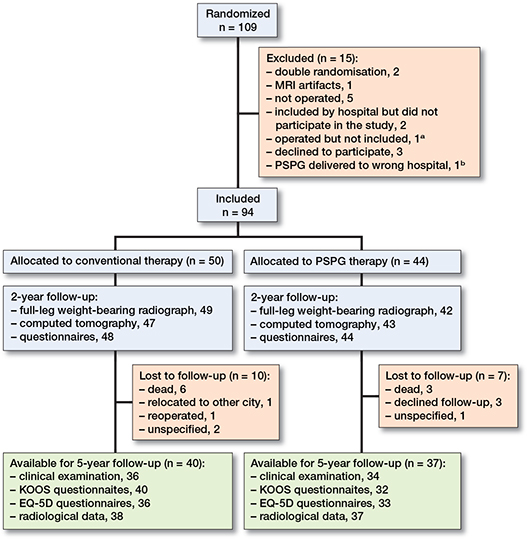
Figure 1. Flowchart of the study.
Patients with symptomatic osteoarthritis of the knee met the inclusion criteria for the study. Furthermore, only patients undergoing unilateral arthroplasty were eligible for inclusion. The following conditions were exclusion criteria: severe vascular insufficiency of the affected limb; severe instability or deformity of the ligaments and/or surrounding soft tissue, potentially precluding stability of the device; marked bone loss, potentially precluding adequate fixation of the device; non-cooperative subjects; rheumatoid arthritis and other systemic diseases; and known metal allergy [9].
A power analysis for KOOS revealed that 63 patients are required per cohort to detect a difference of 10 units in KOOS with a common standard deviation of 20, a power of 80%, and a significance level of 0.05.
This article is reported according to the CONSORT guidelines [10].
Randomization
Block randomization was carried out using varying block sizes after acquiring written consent [11]. Patients were assigned to 1 of 2 groups according to a 1:1 allocation ratio: the control group, which received TKAs with CI, or the study group, which received TKAs with PSPGs. All patients were referred for preoperative MRI, which was made according to the Signature scanning procedure (Signature MRI Inc, Monrovia, CA, USA); hence patients were not aware of what surgery they received.
Surgical technique
All patients were operated on with a standard medial parapatellar approach using the Vanguard Cruciate Retaining Total Knee system (Vanguard Complete Knee System, Zimmer Biomet, Warsaw, IN, USA) and cemented with Refobacin Bone Cement R (Zimmer Biomet, Warsaw, IN, USA). PSPGs from Materialise NV (Leuven, Belgium) were used in this study. An MRI scan of the affected limb was uploaded to the implant manufacturer for implant positioning planning. Thereafter, an engineer working for the manufacturer of the PSPGs defined relevant anatomical landmarks from the uploaded MRI. A preoperative virtual operation plan was sent to the surgeon. The surgeon was free to change and adapt the preoperative virtual operation plan according to his/her expertise by manipulating the alignment in all 3 dimensions, the depth of the bone cuts, and the size of the implant. Once the surgeon was satisfied, the approved plan was sent back to the manufacturer, and the PSPGs were manufactured and delivered to the hospital.
75 of the operations were conducted by orthopedic surgeons who specialize in TKA. The operations were carried out by a resident under the guidance of an orthopedic surgeon in 2 cases. The surgical technique has been outlined in more detail in a previous publication on this RCT [9].
Clinical assessment
All preoperative and postoperative clinical assessments were gathered by an independent physiotherapist or orthopedic surgeon who was blinded to the type of surgery that the patient had received.
Patients completed the following PROMs: Knee injury and Osteoarthritis Outcome Score (KOOS, scored 0–100, 100 indicating a healthy state) [12], Numeric (pain) Rating Scale (abbreviated NRS-11, scored 0–10, 10 being the most pain possible) [13], EuroQol Visual Analog Scale (EQ-VAS, scored 0–100, 100 being the best possible health condition) [14]. Patients also filled out a EuroQol-5-Dimensional-3-Level (EQ-5D-3L) questionnaire. The EQ-5D-3L questionnaire is a standardized assessment of health-related quality of life [14]. The 3 levels of the EQ-5D-3L questionnaire (no problems, some problems, severe problems) were dichotomized into “no problems” and “problems,” as described in a previous publication on this RCT [9]. The active range of motion (ROM) was measured by a physiotherapist or orthopedic surgeon using a universal goniometer.
Radiographic evaluation
2 observers, SMR and JvL, assessed anteroposterior and sagittal plane radiographs taken at the 5-year follow-up according to the modified Knee Society Total Knee Arthroplasty Radiographic Evaluation and Scoring System, as described by Bach et al. [15]. In this scoring system, the width (in millimeters) of radiolucent lines (RLLs) of the femoral component were recorded both anteriorly and posteriorly in the sagittal plane. The total RLL width was measured in the frontal plane for the medial and lateral part of the tibial component. RLLs of the tibial component in the sagittal plane were measured anteriorly and posteriorly. Thereafter, RLLs were categorized as none, narrow (total width ≤ 4 mm), and wide (total width RLL > 4 mm). If radiolucency was detected, the observers compared the 5-year follow-up radiographs with radiographs taken at the 2-year follow-up.
Statistics
The Shapiro–Wilk test, in conjunction with histogram visualization, was the primary tool used to determine and assess the normality of the data. When the outcome variable was continuous and non-normally distributed, the Wilcoxon rank-sum test was used for independent groups, and if it was normally distributed, then a t-test was performed. When the outcome variable for independent groups was nominal, either a chi-square test or Fisher’s exact test was used. Changes in EQ-5D-3L profiles compared preoperatively and at the 5-year follow-up were presented using the Paretian Classification of Health Change (PCHC), as described by Devlin et al. [14]. Linear mixed models were used to compare continuous variables collected at different time points between the PSPG and CI cohorts. Interaction was recorded to identify any variations in reported scores and data between the 2 groups over time. Missing data was handled by multiple imputations using the multivariate imputation by chained equations and pooled according to Rubin’s rules [16,17]. A P value of 0.05 was considered statistically significant. All analyses were performed using RStudio Team (2020) (RStudio: Integrated Development for R. Rstudio, PBC, Boston, MA, http://www.rstudio.com/).
Ethics, registration, funding, and disclosures
The Regional Committee for Medical and Health Research Ethics (REC West 2010/2056) and the institutional review board at Oslo University Hospital (2011/7613) granted this study ethical approval. All patients gave their written and oral consent prior to participation. ClinicalTrials.gov has the trial registered as NCT01696552. This research has received no financial or other forms of aid from any third parties. The authors declare no conflict of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, DOI: 10.2340/17453674.2023.15335
Results
Demographics
The baseline demographics and PROMs of CI and PSPG cohorts are given in Table 1.
Clinical outcome
KOOS
There were no statistically significant differences between the CI and PSPG cohorts in any of the dimensions of KOOS, evaluated from the preoperative score until the score at the 5-year follow-up by applying a linear mixed model as illustrated in Figure 2 and evident in Table 2. At 5-year follow-up, the difference between the CI and PSPG cohorts in the change from preoperative levels was 0.3 KOOS points (CI –11.7 to 12.3, P = 0.96) in the Pain sub-score, 0.2 KOOS points (CI –11.9 to 11.5, P = 0.98) in the Symptoms sub-score, 3.9 KOOS points (CI –17.5 to 9.8, P = 0.6) in the Sport sub-score, 1.1 KOOS points (CI –10.5 to 12.4, P = 0.8]) in the Activities of Daily Living sub-score, and 0.7 KOOS points (CI –12.7 to 14.2, P = 0.9]) in the Quality of Life sub-score.
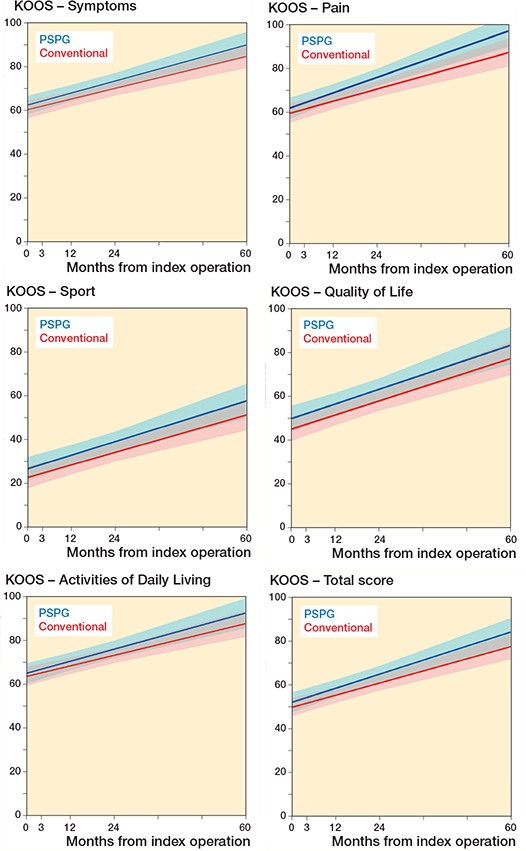
Figure 2. Linear mixed model illustrating course of KOOS dimensions per cohort
EQ-5D-3L
As presented in Table 3, we did not find any significant difference in changes in EQ-5D-3L profiles postoperatively and at the 5-year follow-up between the PSPG and CI cohorts.
| Variable | Instrumentation | P value a | |
| Conventional + / = / – | PSPG + / = / – | ||
| Mobility | 28 / 10 / 2 | 20 / 16 / 1 | 0.3 |
| Pain and distress | 18 / 22 / 0 | 20 / 17 / 0 | 0.5 |
| Usual activities | 21 / 18 / 1 | 21 / 16 / 0 | 0.9 |
| Anxiety and depression | 8 / 31 / 1 | 7 / 25 / 5 | 0.2 |
| Self-care | 10 / 28 / 2 | 6 / 28 / 3 | 0.6 |
| a Fisher’s exact test. | |||
EQ-VAS
EQ-VAS score improvement differences between the study cohorts from their respective preoperative score over time were not found to be statistically significant (P = 0.6, linear mixed model), as listed in Table 4. The difference in improvement in EQ-VAS scores measured preoperatively and at 5 years was 3.2 points (CI –10.1 to 16.5, P = 0.6).
| Variable | Instrumentation | Difference | |
| Conventional n = 40 | PSPG n = 37 | ||
| EQ-5D-VAS | |||
| Preoperative | 59 (52–65) | 64 (57–71) | –5 (–15 to 4) |
| 3 months | 70 (64–77) | 75 (70–81) | –5 (–14 to 4) |
| 1 year | 73 (67–79) | 73 (67–80) | 0 (–9 to 9) |
| 2 years | 73 (68–78) | 77 (72–83) | –4 (–12 to 3) |
| 5 years | 72 (66–79) | 74 (68–81) | –2 (–11 to 7) |
| Pain NRS-11 | |||
| Preoperative | 5.8 (5.1–6.5) | 5.7 (4.8–6.6) | 0 (–1 to 1) |
| 3 months | 2.9 (2.2–3.7) | 2.0 (1.5–2.6) | 1 (0 to 2) |
| 1 year | 2.1 (1.3–2.8) | 1.2 (0.69–1.7) | 1 (0 to 2) |
| 2 years | 2.4 (1.6–3.1) | 1.1 (0.52–1.7) | 1 (0 to 2) |
| 5 years | 1.7 (1.0–2.4) | 1.1 (0.54–1.6) | 1 (0 to 2) |
| ROM a | |||
| Preoperative | 110 (104–116) | 108 (103–113) | 1 (–6 to 9) |
| 3 months | 103 (98–108) | 110 (107–114) | –7 (–14 to –1) |
| 1 year | 113 (109–117) | 114 (109–119) | –1 (–7 to 6) |
| 2 years | 115 (111–118) | 118 (113–123) | –3 (–9 to 3) |
| 5 years | 114 (110–118) | 114 (110–119) | 1 (–6 to 5) |
| a Range of motion in degrees measured by goniometer. | |||
NRS-11
The difference in pain NRS-11 score improvement between the cohorts measured preoperatively and at 5 years was 0.6 points (CI –0.9 to 2.1, P = 0.5). Furthermore, any difference in improvement between the cohorts from their respective preoperative score over time was not found to be statically significant (P = 0.5, linear mixed model).
ROM
The difference between the CI and PSPG cohort in the change from preoperative levels was 2° (CI –11.8 to 7.8, P = 0.7) at 5-year follow-up. Furthermore, this study does not report any significant difference in ROM between the PSPG and CI cohorts over time (P = 0.1, linear mixed model) (Table 4).
Radiolucent lines
There was no significant difference between the number of narrow and wide RLLs in the femoral component (P = 0.7, Fisher’s exact test) or tibial component (P = 0.2, Fisher’s exact test) (Table 5). In all but 1 case, similar RLLs without progression were observed when compared with the 2-year follow-up radiographs. In 1 case, a minor radiolucency was observed at the anterior side of the tibial component in the sagittal plane that was not detectable in the 2-year follow-up radiograph.
| Variable | Instrumentation | P value a | |
| Conventional n = 40 | PSPG n = 37 | ||
| Femoral component | 0.7 | ||
| None | 35 | 30 | |
| Narrow (< 4 mm) | 2 | 4 | |
| Wide (> 4 mm) | 3 | 3 | |
| Tibial component | 0.2 | ||
| None | 38 | 31 | |
| Narrow (< 4 mm) | 2 | 5 | |
| Wide (> 4 mm) | 0 | 1 | |
| a Fisher’s exact test. | |||
Discussion
The aim of this secondary analysis of an RCT was to evaluate the effects of using PSPG on clinical outcomes and radiolucent lines. We found no differences after 5 years.
Patient-reported outcomes
The findings of our RCT are comparable with other studies comparing PROMs between CI and PSPG [18,19]. Furthermore, a meta-analysis performed by Huijbregts et al. found no significant difference between KOOS scores of patients undergoing TKA with CI or with PSPG [20]. Conversely, a meta-analysis conducted by Kizaki et al. found a significant improvement in all dimensions of KOOS except for “Symptoms” and “Sports” in patients undergoing TKA with PSPG compared with CI [21]. However, the mean differences in these dimensions of KOOS were below the minimum clinically important difference (MCID) for KOOS scores in the respective dimensions [22].
We found no difference in changes of EQ-5D-3L profiles and EQ-VAS postoperatively and at the 5-year follow-up between the PSPG and CI cohorts, as was the case in other RCTs that made the same comparison [19,23].
Assessment of radiolucent lines
Mainly non-progressive RLLs were observed when evaluating the 5-year follow-up radiographs. In the 1 case where progression was observed, this could potentially be explained by inappropriate cementing technique or non-optimal bone preparation. However, non-progressive RLLs, < 2 mm thick, have not been found to be correlated with an unfavorable clinical outcome [24].
Strengths and limitations
The duration of inclusion was rather long. There were 2 main reasons for this: due to renovations, one hospital had to close its operating theater for several months, and not all surgeons at participating hospitals informed patients about the study, therefore some patients were operated on but not included in this RCT.
This RCT was underpowered for KOOS. The power analysis had found that 63 patients were needed for each cohort of the RCT. As 1 center withdrew from the trial and several patients were lost to follow-up, the overall number of patients enrolled for 5-year follow-up was only 40 patients from the PSPG cohort and 37 patients from the CI cohort.
Regarding blinding in this RCT, the objective was to conceal the surgical method used in the operation record. However, in a few cases this objective was not achieved; therefore, it is feasible that some physiotherapists may have had access to the data and thereby may not have been blinded in every instance.
Concerning generalizability, PSPGs from only 1 manufacturer were utilized in this study. Therefore, the findings of this RCT might not apply to other TKA systems, particularly those that focus on a model to reestablish the lower limb’s kinematic alignment rather than the mechanical alignment, the latter being the aim of this RCT.
Our RCT assessed multiple parameters regarding clinical and functional outcomes at 5 points in time from preoperatively to 5 years postoperatively, which allowed for detailed analysis of the measured parameters, and evaluation for potential ceiling or floor effects. All surgeons were previously experienced with the PSPG procedure prior to the study’s start, thereby minimizing the possibility of bias resulting from a learning curve [9]. Another clear strength of this study is the long follow-up time, which is comparable to earlier studies [19,23].
Conclusion
Our RCT found no differences in any clinical outcome or occurrence of radiolucent lines between the PSPG and CI cohorts at 5-year follow-up.
- Longstaff L M, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty 2009; 24: 570-8. doi: 10.1016/j.arth.2008.03.002.
- Thiele K, Perka C, Matziolis G, Mayr H O, Sostheim M, Hube R. Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. J Bone Joint Surg Am 2015; 97(9): 715-20. doi: 10.2106/JBJS.M.01534.
- Mont M A, Urquhart M A, Hungerford D S, Krackow K A. Intramedullary goniometer can improve alignment in knee arthroplasty surgery. J Arthroplasty 1997; 12(3): 332-6. doi: 10.1016/S0883-5403(97)90031-0
- Krackow K A, Bayers-Thering M, Phillips M J, Bayers-Thering M, Mihalko W M. A new technique for determining proper mechanical axis alignment during total knee arthroplasty: progress toward computer-assisted TKA. Orthopedics 1999; 22(7): 698-702.
- Budhiparama N C, Lumban-Gaol I, Ifran N N, Parratte S, Nelissen R. Does accelerometer-based navigation have any clinical benefit compared with conventional TKA? A systematic review. Clin Orthop Relat Res 2019; 477(9): 2017-29. doi: 10.1097/CORR.0000000000000660.
- Jones C W, Jerabek S A. Current role of computer navigation in total knee arthroplasty. J Arthroplasty 2018; 33(7): 1989–1993. doi: 10.1016/J.ARTH.2018.01.027.
- Li J, Gao X, Li X. Comparison of iASSIST navigation system with conventional techniques in total knee arthroplasty: a systematic review and meta-analysis of radiographic and clinical outcomes. Orthop Surg 2019; 11(6): 985-93. doi: 10.1111/os.12550
- Török L, Jávor P, Hartmann P, Bánki L, Varga E. Should we abandon the patient-specific instrumentation ship in total knee arthroplasty? Not quite yet! BMC Musculoskelet Disord 2021; 22(1): 730. doi: 10.1186/S12891-021-04581-2
- van Leeuwen J A M J, Snorrason F, Röhrl S M. No radiological and clinical advantages with patient-specific positioning guides in total knee replacement: a multicenter randomized controlled trial. Acta Orthop 2018; 89(1): 89-94. doi: 10.1080/17453674.2017.139373
- Schulz K F, Altman D G, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8(1): 18. doi: 10.1186/1741-7015-8-18.
- Efird J. Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health 2011; 8:15-20. doi: 10.3390/ijerph8010015.
- Roos E M, Lohmander L S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003; 1: 64. doi: 10.1186/1477-7525-1-64.
- Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005; 14(7): 798-804. doi: 10.1111/J.1365-2702.2005.01121.X.
- Devlin N, Parkin D, Janssen B. Methods for analysing and reporting EQ-5D data. Published online 2020: 29-34. doi: 10.1007/978-3-030-47622-9.
- Bach C M, Biedermann R, Goebel G, Mayer E, Rachbauer F. Reproducible assessment of radiolucent lines in total knee arthroplasty. Clin Orthop Relat Res 2005; (434): 183-8. doi: 10.1097/01.blo.0000153077.79573.a4.
- Royston P. Division C. Multiple imputation of missing values. Stata J 2004; 4(3): 227-41.
- van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011; 45(3): 1-67. doi: 10.18637/JSS.V045.I03.
- Teeter M G, Marsh J D, Howard J L, Yuan X, Vasarhelyi E M, McCalden R W, et al. A randomized controlled trial investigating the value of patient-specific instrumentation for total knee arthroplasty in the Canadian healthcare system. Bone Joint J 2019; 101-B(5): 565-72. doi: 10.1302/0301-620X.101B5.BJJ-2018-1323.R1.
- Schotanus M G M, Boonen B, van der Weegen W, Hoekstra H, van Drumpt R, Borghans R, et al. No difference in mid-term survival and clinical outcome between patient-specific and conventional instrumented total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2019; 27(5): 1463-8. doi: 10.1007/S00167-018-4968-5
- Huijbregts H J T A M, Khan R J K, Sorensen E, Fick D P, Haebich S. Patient-specific instrumentation does not improve radiographic alignment or clinical outcomes after total knee arthroplasty: a meta-analysis. Acta Orthop 2016; 87(4): 386. doi: 10.1080/17453674.2016.1193799.
- Kizaki K, Shanmugaraj A, Yamashita F, Simunovic N, Duong A, Khanna V, et al. Total knee arthroplasty using patient-specific instrumentation for osteoarthritis of the knee: a meta-analysis. BMC Musculoskelet Disord 2019; 20(1): 561. doi: 10.1186/S12891-019-2940-2.
- Monticone M, Ferrante S, Salvaderi S, Motta L, Cerri C. Responsiveness and minimal important changes for the Knee Injury and Osteoarthritis Outcome Score in subjects undergoing rehabilitation after total knee arthroplasty. Am J Phys Med Rehabil 2013; 92(10): 864-70. doi: 10.1097/PHM.0B013E31829F19D8.
- Hampton M J, Blakey C M, Anderson A A, Tomouk W, Buckley S C, Hamer A J, et al. Minimum 5-year outcomes of a multicenter, prospective, randomized control trial assessing clinical and radiological outcomes of patient-specific instrumentation in total knee arthroplasty. J Arthroplasty 2022; 37(8): 1579-85. doi: 10.1016/j.arth.2022.01.039.
- Cawley D T, Kelly N, McGarry J P, Shannon F J. Cementing techniques for the tibial component in primary total knee replacement. Bone Joint J 2013; 95-B(3): 295-300. doi: 10.1302/0301-620X.95B3.29586.