No difference in the use of revision components and rerevision rate in conversion to total knee replacement following Oxford Partial Knee Microplasty Instrumentation: a registry study of 529 conversions
Stephan J VAN LANGEVELD 1, Stein J JANSSEN 2, Koen L M KOENRAADT 1, Joost A A M VAN DEN HOUT 1, Liza N VAN STEENBERGEN 3, and Rutger C I VAN GEENEN 1
1 Department of Orthopaedic Surgery, Foundation FORCE (Foundation for Orthopaedic Research Care and Education), Amphia Hospital, Breda; 2 Department of Orthopaedic Surgery, Academic Medical Center, Amsterdam Movement Sciences, Amsterdam; 3 Dutch Arthroplasty Register (LROI), ‘s-Hertogenbosch, The Netherlands
Background and purpose — Microplasty Instrumentation was introduced to improve Oxford Mobile Partial Knee placement and preserve tibial bone in partial knee replacement (PKR). This might therefore reduce revision complexity. We aimed to assess the difference in use of revision total knee replacement (TKR) tibial components in failed Microplasty versus non-Microplasty instrumented PKRs.
Patients and methods — Data on 529 conversions to TKR (156 Microplasty instrumented and 373 non-Microplasty instrumented PKRs) from the Dutch Arthroplasty Register (LROI) between 2007 and 2019 was used. The primary outcome was the difference in use of revision TKR tibial components during conversion to TKR, which was calculated with a univariable logistic regression analysis. The secondary outcomes were the 3-year re-revision rate and hazard ratios calculated with Kaplan–Meier and Cox regression analyses.
Results — Revision TKR tibial components were used in 29% of the conversions to TKR after failed Microplasty instrumented PKRs and in 24% after failed non-Microplasty instrumented PKRs with an odds ratio of 1.3 (CI 0.86–2.0). The 3-year re-revision rates were 8.4% (CI 4.1–17) after conversion to TKR for failed Microplasty and 11% (CI 7.8–15) for failed non-Microplasty instrumented PKRs with a hazard ratio of 0.77 (CI 0.36–1.7).
Conclusion — There was no difference in use of revision tibial components for conversion to TKR or in re-revision rate after failed Microplasty versus non-Microplasty instrumented PKRs nor in the 3-year revision rate.
Citation: Acta Orthopaedica 2023; 94: 387–392. DOI: https://doi.org/10.2340/17453674.2023.15310.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-01-11. Accepted: 2023-05-18. Published: 2023-07-31.
Correspondence: slangeveld2@amphia.nl
All authors participated in the study design. SJvL, SJJ, KLMK, LNvS, and RCIvG contributed to collection of the data. All authors participated in analyzing and interpreting the data. SJvL, SJJ, JAAMvdH, LNvS, and RCIvG participated in writing and revising the manuscript. SJvL, LNvS, and RCIvG have full access to the data and take responsibility for the integrity of the data and the accuracy of the data analyses.
Handling co-editors: Nils Hailer, Bart A Swierstra and Philippe Wagner
Acta thanks David Murray for help with peer review of this manuscript.
Medial partial knee replacement (PKR) as treatment for anteromedial osteoarthritis has several advantages over total knee replacement (TKR) with regards to functional outcome, mortality, and cost-effectiveness [1,2].
Despite these advantages, revision rates after PKR are found to be higher than after TKR [3]. When revision is imperative, failed PKR is often converted to TKR [4,5]. The tissue- and bone-sparing aspect of PKR theoretically allows for relatively simple conversion to a primary TKR [3]. Nevertheless, conversions to TKR have still been found to be technically demanding and augments, stems, and bone grafts are commonly needed, of which the majority involve the tibial side [5-7].
Microplasty instrumentation for the Oxford Mobile Partial Knee (Zimmer Biomet, Warsaw, IN, USA), was introduced as having a more consistent implant position and more accurate and bone -preserving tibial resection [8]. Recent results demonstrated improved implant position with reduced operation times using Microplasty instrumentation [9-12]. A recent group-matched registry study showed a 40% decrease in revision rate of PKRs instrumented with Microplasty (3.3% vs. 5.5%) [13]. Moreover, Microplasty instrumentation results in fewer tibial recuts and use of thinner inserts suggesting less bone removal during Microplasty surgery [14,15].
Hence, this bone-preserving technique potentially reduces the complexity of the conversion to TKR and thereby also potentially increases implant survival after conversion to TKR. Therefore, we aimed to assess the difference in use of tibial components after failed Microplasty versus non-Microplasty instrumented PKRs. Secondarily, we investigated the re-revision rates in the 2 groups.
Patients and methods
Patient population
This retrospective cohort study was conducted using the Dutch Arthroplasty Register (LROI; Landelijke Registratie Orthopedische Implantaten). The LROI contains most joint replacements implanted by orthopedic surgeons in the Netherlands and has a completeness of the registration of knee arthroplasty data of 98–100% in 2015 to 2019. All data is anonymized, and the revision procedures are linked to the primary procedures.
This study is reported according to the STROBE guidelines.
Data collection
Data of interest consisted of all patients receiving conversion to TKR for a failed medial Oxford PKR with both procedures performed between 2007 and 2019. We determined instrumentation type used (Microplasty versus non-Microplasty) for PKR placement according to the date of operation in each hospital, which was provided by the manufacturer to the LROI and blinded for the authors. PKRs performed before the first registered Microplasty instrumented PKR were defined as non-Microplasty instrumentation. PKRs performed 2 months after the last registered non-Microplasty instrumented PKR were defined as Microplasty instrumented. Tibial components used in the conversion to TKR were labeled as primary or revision components according to their surgical application as indicated by the manufacturer (Table 1, see Supplementary data). To investigate compensation for tibial bone loss by implanting a thicker insert rather than with augments or stems, we collected the insert thickness for all the conversions to primary TKR. Insert thickness was dichotomized into: < 15mm (“thin”) and ≥ 15 mm (“thick”) [16]. Additional variables used were sex, age, ASA score, insert thickness used in the PKR, type of fixation of the PKR (cementless versus cemented), reasons for the conversion to TKR, and reasons for subsequent re-revision.
PKRs were excluded if instrumentation type was unknown (PKR implanted between transition dates), PKR component fixation details were unknown, or a patellar component was implanted (as this is not possible in PKR), and if type of tibial TKR component was unknown or labeled as both a primary and revision component (Figure 1). Furthermore, we excluded conversion to TKR for periprosthetic fractures (n = 14 for Microplasty and n = 6 for non-Microplasty instrumented PKRs) due to its increased revision complexity and consequent high risk for revision component use.
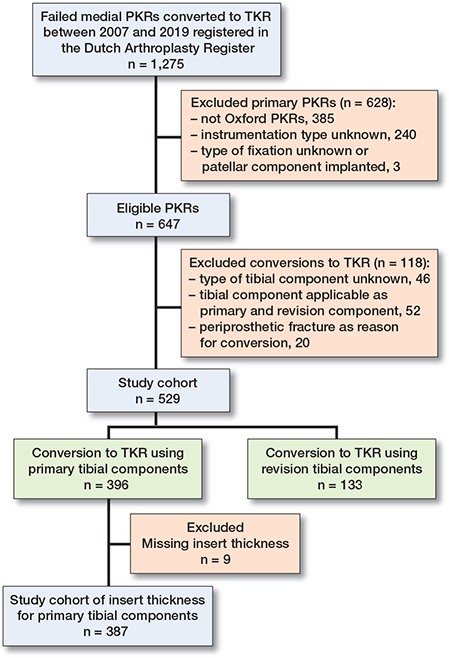
Figure 1. Flowchart. TKR = total knee replacement, PKR = partial knee replacement.
The primary outcome measure was the use of revision TKR tibial components during the conversion to TKR. The secondary outcome measure was the 3-year re-revision rate after conversion to TKR.
Statistics
None of the additional collected patient- and implant-related variables fulfilled the criteria of being a confounder, as these variables did not have a causal effect on the instrumentation type or on primary and secondary outcomes (i.e., the use of primary TKR or revision TKR tibial components in conversion to TKR and the re-revision rates) [17]. The difference in use of revision TKR tibial components between conversions to TKR for failed Microplasty and non-Microplasty instrumented PKRs (odds ratio [OR] with the 95% confidence interval [CI]) was calculated using logistic regression. The difference in insert thickness in the conversions to TKR using primary tibial components was calculated with Student’s t-test and Fisher’s exact test.
Follow-up period was defined as day of conversion to TKR until re-revision with censoring at time of death or at latest follow-up (January 1, 2020). Endpoint of survival was defined as revision with removal or replacement of any component for any reason. Kaplan–Meier analyses with a log-rank test were used to calculate and compare the 3-year re-revision rates after conversion to TKR for failed Microplasty and non-Microplasty instrumented PKRs. Moreover, we performed a Cox regression model to calculate the hazard ratio (HR) for rerevision. In addition to our secondary outcome, we assessed the association between type of tibial component (i.e., primary and revision component) used in the conversion to TKR and re-revision rates by calculating the re-revision rates and the hazard ratio for re-revision.
Proportional hazard assumptions were evaluated per variable by Schoenfeld residual analyses, and all were met.
Significance was defined as a 2-tailed p < 0.05. All data was analyzed using IBM SPSS statistics software (version 25.0, IBM Corp, Armonk, NY, USA). All figures are illustrated using Graphpad Prism (version 8.4.3, Graphpad Software, San Diego, CA, USA).
Ethics, funding, and potential conflicts of interest
Because the LROI database consisted of anonymized patient data registration, no informed consent was necessary. No International Research Board approval was needed due to the retrospective nature of the study. This study did not receive any funding. One of the authors (RCIvG) receives consultancy fees for education from Zimmer Biomet. Other authors certify that they have no conflict of interest in connection with the submitted article. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.15310
Results
Baseline characteristics
5 patients had missing variables (age 1, and ASA score 4) and were excluded from the respective analyses. Insert thickness used in conversions with primary tibial components was missing in 9 patients. Between 2007 and 2019, 529 conversions to TKR were considered eligible for further analyses (Figure 1). The numbers of conversions to TKR were 156 after Microplasty and 373 after non-Microplasty instrumented PKRs (Table 2). Cementless fixation was more frequently used in Microplasty instrumented PKRs (36% for Microplasty versus 6% for non-Microplasty). The insert thickness used in the primary PKR procedure was lower in Microplasty instrumented PKRs than in non-Microplasty instrumented PKRs (median [interquartile range, IQR] 4 mm [3–4] versus 4 mm [3–5], P = 0.03).
| Variables | Instrumentation type | |
| Microplasty (n = 156) | Non-Microplasty (n = 373) | |
| PKR characteristics | ||
| Fixation type | ||
| Cemented | 100 (64) | 349 (94) |
| Cementless | 56 (36) | 24 (6) |
| Insert thickness, mm | ||
| 3 mm | 54 (35) | 94 (25) |
| 4 | 66 (42) | 170 (46) |
| 5 | 24 (15) | 71 (19) |
| 6 | 7 (4.5) | 23 (6.2) |
| ≥ 7 | 5 (3.2) | 15 (4.0) |
| Reason for conversion | ||
| Infection | 0 (0) | 2 (0.5) |
| Malalignment | 16 (10) | 56 (15) |
| Loosening of component | 38 (24) | 117 (31) |
| Insert wear | 5 (3.2) | 16 (4.3) |
| Instability | 27 (17) | 44 (12) |
| Progression of osteoarthritis | 76 (49) | 209 (56) |
| Others | 46 (30) | 86 (23) |
| Patient characteristics a | ||
| Sex | ||
| Female | 101 (65) | 238 (64) |
| Male | 55 (35) | 135 (36) |
| Age group | ||
| < 55 | 33 (21) | 63 (17) |
| 55–64 | 51 (33) | 139 (37) |
| 65–74 | 48 (31) | 117 (32) |
| ≥ 75 | 24 (15) | 53 (14) |
| ASA score | ||
| I | 22 (14) | 60 (16) |
| II | 105 (67) | 259 (70) |
| III–IV | 29 (19) | 49 (13) |
| a Characteristics at conversion to total knee replacement. PKR = partial knee replacement. |
||
Use of revision tibial components in conversion to TKR
There was no statistically significant difference in use of revision tibial components for conversion to TKR after failed Microplasty versus non-Microplasty instrumented PKRs. Revision tibial components were used in 29% (n = 45) of the conversions to TKR for failed Microplasty instrumented PKRs and in 24% (n = 88) of those for failed non-Microplasty instrumented PKRs (OR 1.3, CI 0.86–2.0).
Insert thickness in conversion to TKR using primary tibial components
The mean insert thickness in the conversions to TKR with primary tibial components was 12.9 mm (SD 2.5) for failed Microplasty and 12.7 mm (SD 2.4) for failed non-Microplasty instrumented PKRs (P = 0.5). Use of thin inserts was similar between groups (78% in Microplasty instrumented versus 72% in non-Microplasty instrumented PKRs, P = 0.3).
Re-revision rates after TKR converted Microplasty versus non-Microplasty instrumented PKRs
The median follow-up after conversion to TKR was 2.1 years (IQR 1.0–3.5) for failed Microplasty and 5.5 years (IQR 3.2–7.8) for non-Microplasty instrumented PKRs. At 3 years after conversion to TKR, 8 re-revisions were performed in initially failed Microplasty instrumented PKRs and 35 in non-Microplasty instrumented PKRs. The 3-year re-revision rates after the conversions to TKR were 8.4% (CI 4.1–17) in failed Microplasty and 11% (CI 7.8–15) in non-Microplasty instrumented PKRs (P = 0.5) (Figure 2). The hazard ratio for rerevision was 0.77 (CI 0.36–1.7) for conversion to TKR after failed Microplasty instrumented PKRs versus non-Microplasty instrumented PKRs.
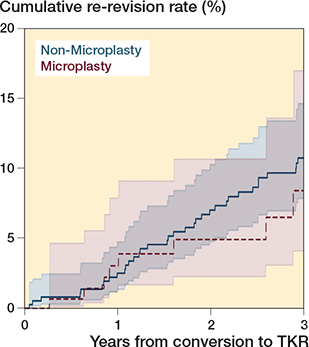
Figure 2. Re-revision rates with CI of the conversions to total knee replacement after failed Microplasty instrumented versus non-Microplasty instrumented PKRs. The 3-year re-revision rates were 8.4% (CI 4.1–17) and 11% (CI 7.8–15) respectively (P = 0.5). TKR = total knee replacement, PKR = partial knee replacement.
Re-revision rates for primary versus revision tibial components
The median follow-up after conversions to TKR using primary tibial components was 4.9 years (IQR 2.3–7.3) and 3.3 years (IQR 1.7–4.8) for conversions to TKR using revision tibial components. At 3 years after conversion to TKR, 38 re-revisions were performed after conversions to TKR with use of primary tibial components (n = 396) and 5 re-revisions after use of revision tibial components (n = 133). The 3-year re-revision rates were 12% (CI 8.6–16) after use of primary tibial components and 5.3% (CI 2.2–12) after use of revision tibial component (p = 0.06) (Figure 3). The hazard ratio for re-revision was 0.43 (CI 0.17–1.1) for use of revision tibial components compared with use of primary tibial components.
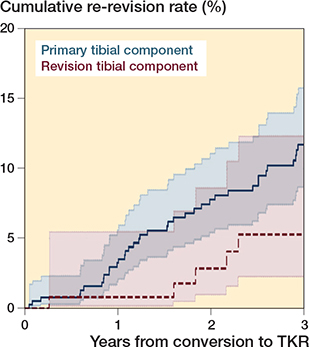
Figure 3. The 3-year re-revision rates with CI of the conversions to TKR using primary tibial components versus revision tibial components. The 3-year re-revision rates were 12% (CI 8.6–16) and 5.3% (CI 2.2–12) respectively (P = 0.06). TKR = total knee replacement.
Discussion
We assessed the use of revision tibial components in conversion to TKR for failed Microplasty versus non-Microplasty instrumented PKRs and risk of revisions. We showed no difference in use of revision tibial components for conversion to TKR or in 3-year re-revision rates. Another important finding is that our results may indicate a difference in use of revision TKR tibial component use after Microplasty compared with non-Microplasty implanted PKRs.
Conversion of a PKR to TKR can be a technically challenging procedure and is considered more complex than primary TKR. During conversion to TKR, surgeons encounter bone removal in up to 77% of procedures and revision components (i.e., stems and augments) are often needed [6,18-21]. In the case of using stems and augments, the majority involve the tibial component. Studies have shown that the tibial resection at the initial PKR is related to the bone removal encountered in the conversion to TKR. Excessive tibial resections and an increased insert thickness used in PKR are correlated with an increased use of revision tibial components in conversion to TKR [22,23]. Studies showed that using Microplasty instrumentation in PKR results in fewer tibial recuts and use of lower insert thickness compared with non-Microplasty instrumented PKR at primary implantation [14,15]. This bone-sparing aspect theoretically reduces the tibial bone loss and consequently the need for revision components at conversion to TKR. We found such a bone-sparing aspect of Microplasty instrumentation in our data, as the insert thickness at the primary procedure was statistically significantly lower in Microplasty versus non-Microplasty instrumented PKRs (median [IQR] 4 mm [3–4] versus 4 mm [3–5]; P = 0.03). This difference in thickness was mainly observed in a 10% higher use of the 3 mm inserts in Microplasty instrumented PKRs. Although expected with the decrease in insert thickness, we did not find such a decrease in use of tibial revision components.
On the contrary, we showed a tendency towards an increased use of revision tibial components in conversion to TKR after failed Microplasty compared with non-Microplasty instrumented PKRs, although with much uncertainty. Several factors might have played a role in this finding: the implant designs and techniques, together with the augments and stems, are developing rapidly, and the availability to the surgeons of revision TKR components is increasing. Surgeons are acquiring experience and are familiarizing themselves with conversion to TKR because of an increasing use of PKR overall and therefore more exposure to its conversion. This could have led to a tendency among orthopedic surgeons to use revision components in the conversion to TKR. As the Microplasty instrumentation is the successor of the non-Microplasty instrumentation, we compared groups in 2 consecutive time periods. The increase in use of revision tibial components might be the result of surgeons lowering their threshold for implanting revision components over time.
Our study results indicate that the use of tibial revision components potentially decreases the 3-year risk for re-revision by 83% or increases the risk by 10%. Similarly, recent studies showed a decrease in re-revision rates after conversion to TKR when augments and stems were used [21,24]. Augments (depending on the amount of bone loss) combined with a tibial stem are advised to improve and regain implant stability when handling tibial bone loss in conversion to TKR [25,26]. We found tibial component loosening (as sign of implant instability) in 24% (n = 9) of the re-revisions when a primary tibial component was used compared with none when a revision tibial component was used. Besides a possible benefit regarding implant stability, the decreased re-revision rates could also be attributed to a higher threshold for re-revision among surgeons as revising a revision TKR tibial component is considered more complex than revising a primary TKR tibial component.
Our study has several limitations. First, our data did not contain details regarding the conversion to TKR. A tibial component was labeled revision or primary according to their manufacturer’s indication. Whether an augment or stem was used is unknown, as well as its possible thickness or length. Moreover, we were not able to collect the PKR removal techniques, the amount of bone loss and quality encountered during the revision, or surgeon-related factors (i.e., experience and exposure). Although we did investigate the use of thicker inserts during revision of the PKR as a possible strategy to address bone loss, our study could not assess other possible differences in surgical strategy or surgeon-related rationale behind the use of the tibial revision components.
Second, we analyzed data and compared conversions to TKR with all reasons for conversion except periprosthetic fractures. Because many factors can contribute to bone loss encountered during the conversion to TKR, finding a direct causality with this type of data is not possible. We analyzed the use of tibial revision components for each reason for conversion and did not find any beneficial or harming effect of the use of Microplasty instrumentation. The cohort had a relatively short time until conversion with a median of 1.5 years for Microplasty and 3.2 years for non-Microplasty instrumented PKRs. It is possible that a longer follow-up period might demonstrate a difference. The inclusion of only specific chronic failure mechanisms and therefore selection of a more homogeneous group of patients might assess benefits of the bone-sparing effect of Microplasty instrumentation more accurately.
Third, there could be a bias within the first year after introduction of Microplasty instrumentation caused by its learning curve [13]. However, we feel that this may be limited as Microplasty only adds or changes a few steps. In addition, we had a 2-month wash-out period, which might capture this limitation. We therefore assume that the possible bias on our data resulting from a learning curve is negligible.
Finally, inadequate implantation (i.e., suboptimal alignment, positioning, or fixation) plays a role in failure mechanisms, for example bearing wear, aseptic loosening, and lateral osteoarthritis progression. It is important to note that inadequately implanted PKRs, regardless of instrumentation type, are more likely to fail, and probably fail in the same way. This might explain why we did not find a difference in use of revision TKR tibial components based on instrumentation type. However, the likelihood of inadequate implantation itself might have been affected by instrumentation type. This is the subject of further study.
Conclusion
We showed no difference in use of revision tibial components for conversion to TKR or in 3-year re-revision rates. Our findings suggest that conversion of a PKR to TKR is considered more complex than primary TKR. Based on our results, surgeons should address conversion to TKR for failed Microplasty instrumented PKRs as being similar to failed non-Microplasty instrumented PKRs.
Supplementary data
Table 1 is available on the article homepage, doi: 10.2340/17453674.2023.15310
- Beard D J, Davies L J, Cook J A, MacLennan G, Price A, Kent S, et al. The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5-year outcomes of a randomised controlled trial. Lancet (London, England) 2019; 394: 746-56. doi: 10.1016/S0140-6736(19)31281-4.
- Wilson H A, Middleton R, Abram S G F, Smith S, Alvand A, Jackson W F, et al. Patient relevant outcomes of unicompartmental versus total knee replacement: systematic review and meta-analysis. BMJ 2019; 364: l352. doi: 10.1136/bmj.l352.
- Murray D W, Liddle A D, Dodd C A, Pandit H. Unicompartmental knee arthroplasty: is the glass half full or half empty? Bone Joint J 2015; 97-B: 3-8. doi: 10.1302/0301-620X.97B10.36542.
- Kim S J, Postigo R, Koo S, Kim J H. Causes of revision following Oxford phase 3 unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2014; 22: 1895-1901. doi: 10.1007/s00167-013-2644-3.
- Vasso M, Corona K, D’Apolito R, Mazzitelli G, Panni A S. Unicompartmental knee arthroplasty: modes of failure and conversion to total knee arthroplasty. Joints 2017; 5: 44-50. doi: 10.1055/s-0037-1601414.
- Craik J D, El Shafie S A, Singh V K, Twyman R S. Revision of unicompartmental knee arthroplasty versus primary total knee arthroplasty. J Arthroplasty 2015; 30: 592-4. doi: 10.1016/j.arth.2014.10.038.
- Sun X, Su Z. A meta-analysis of unicompartmental knee arthroplasty revised to total knee arthroplasty versus primary total knee arthroplasty. J Orthop Surg Res 2018; 13: 158. doi: 10.1186/s13018-018-0859-1.
- Morris M J, Frye B M, Ekpo T E, Berend K R. Unicompartmental knee replacement with New Oxford instruments. Operative Tech Orthop 2012; 22: 189-95. doi: 10.1053/j.oto.2012.11.003.
- Berend K, Hurst J, Morris M, Adams J, Lombardi A. New instrumentation reduces operative time in medial unicompartmental knee arthroplasty using the Oxford mobile bearing design. Reconstructive Rev 2015; 5. doi: 10.15438/rr.5.4.126.
- Hurst J M, Berend K R, Adams J B, Lombardi A V Jr. Radiographic comparison of mobile-bearing partial knee single-peg versus twin-peg design. J Arthroplasty 2015; 30: 475-8. doi: 10.1016/j.arth.2014.10.015.
- Koh I J, Kim J H, Jang S W, Kim M S, Kim C, In Y. Are the Oxford((R)) medial unicompartmental knee arthroplasty new instruments reducing the bearing dislocation risk while improving components relationships? A case control study. Orthop Traumatol Surg Res 2016; 102: 183-7. doi: 10.1016/j.otsr.2015.11.015.
- Tu Y, Xue H, Ma T, Wen T, Yang T, Zhang H, et al. Superior femoral component alignment can be achieved with Oxford microplasty instrumentation after minimally invasive unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2017; 25: 729-35. doi: 10.1007/s00167-016-4173-3.
- Mohammad H R, Matharu G S, Judge A, Murray D W. New surgical instrumentation reduces the revision rate of unicompartmental knee replacement: a propensity score matched comparison of 15,906 knees from the National Joint Registry. Knee 2020; 27: 993-1002. doi: 10.1016/j.knee.2020.02.008.
- Walker T, Heinemann P, Bruckner T, Streit M R, Kinkel S, Gotterbarm T. The influence of different sets of surgical instrumentation in Oxford UKA on bearing size and component position. Arch Orthop Trauma Surg 2017; 137: 895-902. doi: 10.1007/s00402-017-2702-2.
- Gaba S, Wahal N, Gautam D, Pandit H, Kumar V, Malhotra R. Early results of Oxford mobile bearing medial unicompartmental knee replacement (UKR) with the Microplasty instrumentation: an Indian experience. Arch Bone Jt Surg 2018; 6: 301-11. https://www.ncbi.nlm.nih.gov/pubmed/30175178.
- Berend M E, Davis P J, Ritter M A, Keating E M, Faris P M, Meding J B, et al. “Thicker” polyethylene bearings are associated with higher failure rates in primary total knee arthroplasty. J Arthroplasty 2010; 25: 17-20. doi: 10.1016/j.arth.2010.04.031.
- Hernán M A, Hernández-Díaz S, Werler M M, Mitchell A A. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002; 155: 176-84. doi: 10.1093/aje/155.2.176.
- Springer B D, Scott R D, Thornhill T S. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res 2006; 446: 214-20. doi: 10.1097/01.blo.0000214431.19033.fa.
- Saragaglia D, Estour G, Nemer C, Colle P E. Revision of 33 unicompartmental knee prostheses using total knee arthroplasty: strategy and results. Int Orthop 2009; 33: 969-74. doi: 10.1007/s00264-008-0585-0.
- Khan Z, Nawaz S Z, Kahane S, Esler C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belgica 2013; 79: 699-705. https://www.ncbi.nlm.nih.gov/pubmed/24563977.
- Sierra R J, Kassel C A, Wetters N G, Berend K R, Della Valle C J, Lombardi A V. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty 2013; 28: 128-32. doi: 10.1016/j.arth.2013.02.040.
- Wynn Jones H, Chan W, Harrison T, Smith T O, Masonda P, Walton N P. Revision of medial Oxford unicompartmental knee replacement to a total knee replacement: similar to a primary? Knee 2012; 19: 339-43. doi: 10.1016/j.knee.2011.03.006.
- Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott R D. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics 2013; 36: e409-14. doi: 10.3928/01477447-20130327-14.
- Lewis P L, Davidson D C, Graves S E, de Steiger R N, Donnelly W, Cuthbert A. Unicompartmental knee arthroplasty revision to TKA: are tibial stems and augments associated with improved survivorship? Clin Orthop Relat Res 2018; 476: 854-62. doi: 10.1007/s11999.0000000000000179.
- Rawlinson J J, Closkey R F Jr, Davis N, Wright T M, Windsor R. Stemmed implants improve stability in augmented constrained condylar knees. Clin Orthop Relat Res 2008; 466: 2639-43. doi: 10.1007/s11999-008-0424-z.
- Hashemi A, Ziada S, Adili A, de Beer J. Stem requirements of tibial augmentations in total knee arthroplasty. Experimental Techniques 2014; 38: 8-17. doi: 10.1111/j.1747-1567.2012.00826.x.