Increasing risk of revision due to infection after primary total hip arthroplasty: results from the Nordic Arthroplasty Register Association
Håvard DALE 1,2, Anne Marie FENSTAD 1, Geir HALLAN 1,2, Søren OVERGAARD 3-5, Alma B PEDERSEN 5-7, Nils P HAILER 8,9, Johan KÄRRHOLM 9,10, Ola ROLFSON 9,10, Antti ESKELINEN 11,12, Keijo T MÄKELÄ 12,13, and Ove FURNES 1,2
1 The Norwegian Arthroplasty Register, Department of Orthopaedic Surgery, Haukeland University Hospital, Bergen, Norway; 2 Department of Clinical Medicine, University of Bergen, Norway; 3 Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark; 4 Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg, Denmark; 5 The Danish Hip Arthroplasty Register, Aarhus, Denmark; 6 Department of Clinical Epidemiology, Aarhus University Hospital, Denmark; 7 Department of Clinical Medicine, Aarhus University, Denmark; 8 Section of Orthopaedics, Department of Surgical Sciences, Uppsala University Hospital, Sweden; 9 The Swedish Arthroplasty Register, Gothenburg, Sweden; 10 Department of Orthopaedics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sweden; 11 Coxa Hospital for Joint Replacement, and Faculty of Medicine and Health Technologies, University of Tampere, Tampere, Finland; 12 The Finnish Arthroplasty Register, Helsinki, Finland; 13 Department of Orthopaedics and Traumatology, Turku University Hospital and University of Turku, Finland
Background and purpose — The incidence of periprosthetic joint infection after total hip arthroplasty (THA) may be increasing. We performed time-trend analyses of risk, rates, and timing of revision due to infection after primary THAs in the Nordic countries from the period 2004–2018.
Patients and methods — 569,463 primary THAs reported to the Nordic Arthroplasty Register Association from 2004 to 2018 were studied. Absolute risk estimates were calculated by Kaplan–Meier and cumulative incidence function methods, whereas adjusted hazard ratios (aHR) were assessed by Cox regression with the first revision due to infection after primary THA as primary endpoint. In addition, we explored changes in the time span from primary THA to revision due to infection.
Results — 5,653 (1.0%) primary THAs were revised due to infection during a median follow-up time of 5.4 (IQR 2.5–8.9) years after surgery. Compared with the period 2004–2008, the aHRs for revision were 1.4 (95% confidence interval [CI] 1.3–1.5) for 2009–2013, and 1.9 (CI 1.7–2.0) for 2014–2018. The absolute 5-year rates of revision due to infection were 0.7% (CI 0.7–0.7), 1.0% (CI 0.9–1.0), and 1.2% (CI 1.2–1.3) for the 3 time periods respectively. We found changes in the time span from primary THA to revision due to infection. Compared with 2004–2008, the aHR for revision within 30 days after THA was 2.5 (CI 2.1–2.9) for 2009–2013, and 3.4 (CI 3.0–3.9) for 2013–2018. The aHR for revision within 31–90 days after THA was 1.5 (CI 1.3–1.9) for 2009–2013, and 2.5 (CI 2.1–3.0) for 2013–2018, compared with 2004–2008.
Conclusion — The risk of revision due to infection after primary THA almost doubled, both in absolute cumulative incidence and in relative risk, throughout the period 2004–2018. This increase was mainly due to an increased risk of revision within 90 days of THA. This may reflect a “true” increase (i.e., frailer patients or more use of uncemented implants) and/or an “apparent” increase (i.e., improved diagnostics, changed revision strategy, or completeness of reporting) in incidence of periprosthetic joint infection. It is not possible to disclose such changes in the present study, and this warrants further research.
Citation: Acta Orthopaedica 2023; 94: 307–315. DOI: https://doi.org/10.2340/17453674.2023.13648.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-09-22. Accepted: 2023-05-03. Published: 2023-06-27.
Correspondence: havard.dale@helse-bergen.no
HD performed the analyses of the data and wrote the manuscript. All authors have contributed to the conception and design of the study, critical analyses of the data, interpretation of the findings, and critical revision of the manuscript through all stages of the study.
The authors thank the Nordic surgeons for their contribution by thoroughly reporting their THAs to the national arthroplasty registers and to the staff at these registers for their meticulous plotting and statistical support.
Handling co-editors: Eivind Witsø, Robin Christensen, and Jonas Ranstam Acta thanks Stephen Graves, Per Kjærsgaard-Andersen, and Javad Parvizi for help with peer review of this manuscript.
John Charnley stated in 1982 that “Postoperative infection is the saddest of all complications….” Periprosthetic joint infections (PJI) causes reoperations, increased morbidity and mortality, and significant cost [1,2]. Despite advances in knowledge and awareness of prophylactic perioperative routines, there are indications that the incidence of infections after total hip arthroplasty (THA) is increasing [3-6]. To disclose changes in the risk of PJI a large number of primary THAs, registered in a uniform manner, is needed. A substantial increase in demand for revisions due to PJI in the coming decades has been predicted [2]. In Norway, the risk of revision due to infection, as reported to the Norwegian Arthroplasty Register, increased through the period 2005–2019, but with a trend that the risk of revision levelled out [7].
The Nordic Arthroplasty Register Association (NARA), a cooperation between 4 Nordic national arthroplasty registers (Denmark, Norway, Sweden, and Finland), found an increasing risk of revision due to infection after primary THA during the years 1995–2010, and considered it sufficiently important to warrant an updated time trend assessment [6].
We therefore explored changes in the risk of revision due to deep infection for THAs reported to the NARA during the years 2004 to 2018, as a follow-up of our previous study [6]. In addition, we investigated changes in the time to revision.
Patients and methods
Dataset of the Nordic Arthroplasty Register Association
The NARA dataset includes individual-based data from the Danish, Norwegian, Swedish, and Finnish arthroplasty registers [8]. Within each register the selected data was transformed according to a common set of definitions, and revisions were linked to the primary procedures [8].
The dataset includes information on patient characteristics, indications for THA, and surgery-related information, including the type of implant, method of fixation, and duration of surgery. The coverage and completeness of reporting to the national registers is validated in various ways in each contributing country. The completeness of reporting of primary THAs is generally high, the reporting of revisions due to infection somewhat poorer, and the coverage of hospitals and completeness of deaths is near complete [9-12].
Reported THA revisions due to infection are not necessarily the same as rates of PJI. However, we have reason to believe that this is a close approximation, as guidelines recommend revision in the case of suspected PJI. However, there is limited data on completeness of reporting of revisions due to infection to the Nordic arthroplasty registers, as well as limited data on the accuracy of the diagnosis of infection.
The period of inclusion and observation in this study was January 1, 2004 to December 31, 2018. In this period, the NARA dataset contained data on 594,108 primary THAs. A flowchart of exclusion and inclusion is presented in Figure 1. Revisions due to infection in the case of monobloc THAs were not recorded if no implant parts were exchanged, and monobloc THAs were hence excluded. 569,463 primary THAs had complete information on all covariates and were eligible for analyses. Bilateral THAs are dependent observations, but the influence of bilaterality on the outcome has been found to be negligible; this is also true in the case of infection [13]. Hence, patients with bilateral THAs (n = 90,387) were included, and the 2 hips were evaluated independently.
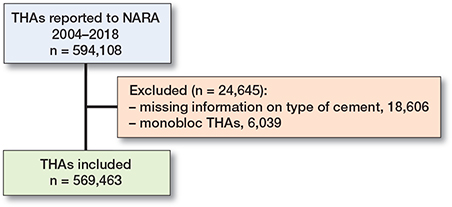
Figure 1. Flow-chart of included and excluded primary THAs.
The NARA dataset contains information on date of and reason for any first-time revision. Revision due to infection is defined as a reoperation with removal or exchange of the entire or parts of the prosthesis, where the procedure is reported as caused by infection. Isolated soft tissue debridement without the exchange of implant parts was not systematically reported and was therefore not included. The register form is completed (on paper or electronically) immediately after surgery, and the indication for the revision, infection or other, is made at the surgeon’s discretion, based on perioperative assessment and evaluation. Thus, the reported diagnosis is not dependent on bacterial cultures of tissue samples collected during the revision and, hence, the rate of revision due to infection will be only an approximation of the rate of true PJI, as defined by the European Bone and Joint Infection Society [14].
Statistics
Continuous data was described using means, medians, and interquartile ranges (IQR). Survival analyses were performed by fitting Cox regression models, with year of primary THA as the main risk factor. All THAs were followed until their first revision due to infection, revision due to other causes, death, or until December 31, 2018. Thus, follow-up varied from 0 to 15 years.
In addition to year-by-year time trend during the period 2004–2018, according to the year of primary THA, 3 time periods were compared: 2004–2008, 2009–2013, and 2014–2018, with sub-analyses for the 4 Nordic countries.
Date of revision due to deep infection was the endpoint. Adjusted hazard rate ratios (aHR) for risk of revision due to infection were calculated. We adjusted for the confounders age, sex, indication for primary THA (osteoarthritis, inflammatory disease, acute and complications after hip fracture, complications after childhood hip disease, avascular necrosis of the femoral head, other), and fixation (uncemented, cement with antibiotics, cement without antibiotics). Hybrid and reverse hybrid fixations were classified according to the cement used for fixation of the cemented component. The changes, correlation, and impact of these confounders during the study period were assessed by descriptive statistics, direct acyclic graphs, and Cox regression analyses.
We used Cox regression analyses, with time period of primary THA as the stratification factor, to construct cumulative revision curves (1 minus cumulative survival) at mean values of the covariates [15]. Analyses with follow-up restricted to 0–5 years for each period were performed, to assess for the effect of differences in follow-up. Further, we performed subgroup analyses on THAs performed due to osteoarthritis only (n = 457,949), representing a more homogeneous subgroup. In addition, we performed stratified sensitivity analyses for method of fixation (uncemented, all-cemented), hybrid (uncemented cup, cemented stem), and reverse hybrid (cemented cup, uncemented stem) to assess the impact of changes over time in type of fixation. Because we did not have data on comorbidity, we performed stratified analyses on age groups (< 55, 55–75, and >75 years), as a surrogate measure of comorbidity and frailty [16].
For absolute risk estimates of revision due to infection, both Kaplan–Meier (K–M) and competing risk (cumulative incidence function, CIF) models were used. A study from the Swedish Knee Arthroplasty Register found that potential overestimation of incidence of revision through the effect of competing risks (death and revision) is negligible, and that K–M and Cox analyses are better than competing risk analyses (Fine & Gray) in estimating revision risks in arthroplasty register data [15]. However, as we assessed only revision due to infection, revision due to causes other than infection was considered as a competing risk. Therefore, 5-year revision rates were calculated for the 3 time periods using both CIF and K–M.
Revision aHR due to infection as a function of year of the primary THA was also studied, to give a graphical display of the relationship between year of primary THA and risk of revision due to infection, based on a generalized additive model for survival data [17]. The analyses were performed in accordance with the guidelines for statistical analyses of arthroplasty register data [18]. The proportional hazard assumptions of the Cox survival analyses were not completely fulfilled among the 3 time periods when visually tested by smoothed Schoenfeld residuals. We therefore assessed the risk of revision due to infection of 0–30 days, 31–90 days, 91 days–1 year, and 1–5 years postoperatively.
We calculated 95% confidence intervals (CIs) for revision rates and relative risks. We used IBM SPSS 26.0 (IBM Corp, Armonk, NY, USA) and the R statistical software package (R Foundation for Statistical Computing, Vienna, Austria) for analyses, and the study was performed in accordance with the RECORD statements.
Ethics, data sharing plan, funding, and disclosures
The registration of data and the study were performed confidentially according to national legislation. Formal approval for the study was granted by the ethical approval process of each national register. Permission numbers from each country: the Danish Data protection agency (1-16-02-54-17), Denmark; the National Institute of Health and Welfare (Dnro THL/1743/.5.05.00/2014), Finland; the Norwegian Data Inspectorate (ref 24.1.2017: 16/01622-3/CDG), Norway; and the Swedish Ethical Review Authority (1184-18/2019-00812), Sweden. The data was de-identified nationally before the anonymous data was merged into the NARA dataset and treated in full confidentiality and in compliance with Nordic and EU data protection rules. The study was fully financed by the NARA and the Norwegian Arthroplasty Register, and no conflict of interest is declared. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi:10.2340/17453674.2023.13648
Results
569,463 primary THAs in 479,076 patients met the inclusion criteria. 5,653 (1.0%) first revisions due to infection after primary THA were reported. Median follow-up for the primary THAs was 5.4 (IQR 2.5–9.0) years, and median age was 69 (IQR 62–76) years.
Time trends of revision due to infection
There was an annual increase in risk of revision due to infection (1.07, CI 1.06–1.08) throughout the study period (Table 1, Figure 2). This increased risk was also found when comparing 2009–2013 and 2014–2018 with 2004–2008 (Table 2 and Figure 3). The absolute risk estimates showed a corresponding increase over time (Table 3).
| Year of primary THA | THAs included | Number of THAs revised due to infection | Annual aHR a of revision (CI) | Adjusted 5-year revision rate (CI) | |||||
| Total n (%) | 0–30 days | 31–90 days | 91 days–1 year | 1–5 years | 0–5 years | ||||
| 2004 | 28,850 | 235 (0.8) | 23 | 18 | 43 | 79 | 163 | 1 | 0.5 (0.5–0.6) |
| 2005 | 31,769 | 228 (0.7) | 21 | 21 | 31 | 92 | 165 | 0.9 (0.7–1.1) | 0.5 (0.4–0.6) |
| 2006 | 32,405 | 274 (0.8) | 45 | 27 | 47 | 88 | 207 | 1.2 (0.9–1.4) | 0.6 (0.5–0.7) |
| 2007 | 33,299 | 317 (1.0) | 75 | 48 | 42 | 87 | 252 | 1.4 (1.1–1.7) | 0.7 (0.7–0.8) |
| 2008 | 34,057 | 344 (1.0) | 102 | 37 | 59 | 89 | 287 | 1.6 (1.3–1.9) | 0.8 (0.7–0.9) |
| 2009 | 37,138 | 353 (1.0) | 109 | 44 | 49 | 86 | 288 | 1.4 (1.2–1.7) | 0.8 (0.7–0.9) |
| 2010 | 37,650 | 354 (0.9) | 139 | 38 | 51 | 92 | 320 | 1.6 (1.3–1.9) | 0.8 (0.7–0.9) |
| 2011 | 38,313 | 403 (1.1) | 165 | 65 | 53 | 93 | 376 | 1.8 (1.5–2.2) | 1.0 (0.9–1.1) |
| 2012 | 39,086 | 425 (1.1) | 181 | 51 | 61 | 109 | 402 | 1.9 (1.6–2.3) | 1.0 (0.9–1.1) |
| 2013 | 39,784 | 443 (1.1) | 198 | 73 | 71 | 97 | 439 | 2.0 (1.7–2.4) | 1.1 (1.0–1.2) |
| 2014 | 40,514 | 437 (1.1) | 201 | 74 | 64 | 98 | 437 | 2.0 (1.7–2.4) | 1.1 (1.0–1.1) |
| 2015 | 42,266 | 448 (1.1) | 221 | 98 | 57 | (72) b | (448) b | 2.1 (1.7–2.5) | 1.0 (0.9–1.1) c |
| 2016 | 44,292 | 532 (1.2) | 275 | 101 | 90 | (66) b | (532) b | 2.5 (2.1–3.0) | 1.2 (1.1–1.3) c |
| 2017 | 45,041 | 481 (1.1) | 279 | 111 | 74 | (17) b | (481) b | 2.5 (2.1–3.0) | 1.1 (1.0–1.2) c |
| 2018 | 44,999 | 379 (0.8) | (247) b | (101) b | (31) b | (0) b | (379) b | 2.6 (2.2–3.2) | 0.9 (0.8–1.1) c |
| 569,463 | 5,653 (1.0) | 2,281 | 907 | 823 | 1,165 | 5,176 | |||
| a aHR is adjusted for age, sex, indication for primary THA, and type of fixation b Not applicable due to incomplete follow-up, number of THAs revised due to infection so far in parenthesis. c Underestimation due to incomplete follow-up. |
|||||||||
| Year | THAs included | Revised due to infection | aHR a for revision (CI) |
| 2004–2008 | 160,380 | 1,398 | 1 |
| 2009–2013 | 191,971 | 1,978 | 1.4 (1.3–1.5) |
| 2014–2018 | 217,112 | 2,277 | 1.9 (1.7–2.0) |
| a Adjusted for sex, age, indication for primary THA, and fixation. | |||
| Year | 5-year revision rates due to infection | |||
| Crude | K–M | CIF | Cox adjusted | |
| 2004–2008 | 0.7 | 0.7 (0.7–0.7) | 0.7 (0.7–0.7) | 0.7 (0.6–0.7) |
| 2009–2013 | 1.0 | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) | 0.9 (0.9–1.0) |
| 2014–2018 a | 1.0 | 1.2 (1.2–1.3) | 1.2 (1.2–1.3) | 1.1 (1.1–1.2) |
| a The rates are underestimate due to incomplete 5-year follow-up. | ||||
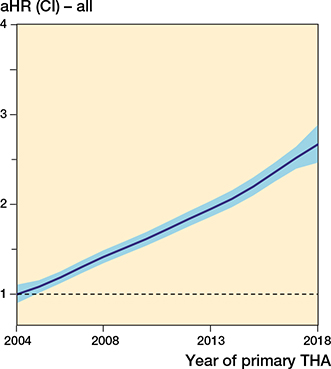
Figure 2. The relationship between year of primary surgery and risk of revision due to deep infection (with 95% CI) for all THAs, adjusted for sex, age, indication for primary THA, and fixation. The broken line represents the aHR in 2004 (aHR = 1).
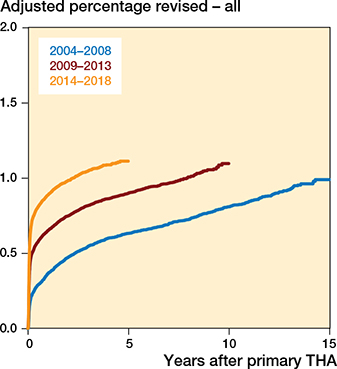
Figure 3. Adjusted revision percentage due to deep infection, for all THAs for 3 periods of primary surgery, adjusted for sex, age, indication for primary THA, and fixation.
The increased risk of revision due to infection was observed only during the first 90 days after index surgery (Table 4).
| Years | THAs included | 0–30 days | Timespan of revisions due to infection | 1–5 years | |||||
| 31–90 days | 91 days–1 year | ||||||||
| Revised | aHR a (CI) | Revised | aHR a (CI) | Revised | aHR a (CI) | Revised | aHR a (CI) | ||
| 2004–2008 | 160,380 | 266 | 1 | 151 | 1 | 222 | 1 | 435 | 1 |
| 2009–2013 | 191,971 | 792 | 2.5 (2.1–2.9) | 271 | 1.5 (1.3–1.9) | 285 | 1.1 (0.9–1.3) | 477 | 0.9 (0.8–1.1) |
| 2014–2018 b | 217,112 | 1,223 | 3.4 (3.0–3.9) | 485 | 2.5 (2.1–3.0) | 316 | 1.1 (1.0–1.4) | 253 | 0.9 (0.8–1.0) |
| a Adjusted for sex, age, indication for primary THA, and fixation. b The number revised due to infection will be an underestimate due to incomplete follow-up. |
|||||||||
Excluding THAs performed for reasons other than primary osteoarthritis did not alter our findings (data not shown). In addition, restricting follow-up for each period to 0–5 years also showed similar results (data not shown).
There were differences in risk of revision due to infection between the countries (Tables 5 and 6, Figures 4 and 5, see Appendix).
Among the 4 countries, Norway had the highest risk of revision due to infection (1.3, CI 1.2–1.4), compared with Sweden. However, in Norway, we found no increase in the risk of revision between the latter 2 time periods. In Norway, there was a subsequent decreased risk of revision due to infection after the first postoperative year during the period 2013–2018 when compared with 2004–2008.
Finland had a lower risk of revision (0.7, CI 0.7–0.8), compared with Sweden. In contrast to Norway, Finland had no risk increase during 2009–2013 when compared with 2004–2008, but during the subsequent period (2014–2018) the risk doubled. Finland had a 10-fold increase in revisions within 30 days of THA in the last period studied, but at the same time a risk reduction of 50% beyond 1 year postoperatively, compared with 2004–2008.
Denmark and Sweden had a similar risk of revision due to infection. Both countries also had an increased risk of revision due to infection for both consecutive time periods after 2004–2008, mainly due to an increased risk of revision within 90 days of THA. In addition, in Sweden, the risk of revision remained increased during the first postoperative year for 2014–2018, compared with 2004–2008, as opposed to the other countries.
The distribution of factors potentially associated with year of primary THA and revision due to infection, and used as adjustment covariates in the analyses, is presented in Table 7 (see Appendix). Patient-related factors such as age, sex, and indication for primary THA were stable throughout the period studied. There was a shift towards more uncemented fixation, also in patients older than 75 years in the latter 2 time periods, at 22% and 21%, compared with 9% in 2004–2008.
Discussion
Our main finding was a nearly doubled relative risk and absolute rate of revision due to infection after primary THA through the period 2004–2018. The rate of revision due to infection was 0.7% in 2004–2008 but increased to 1.2% in 2014–2018. The risk increased especially within 90 days after primary THA. This observation confirms the findings in an earlier study from NARA [6].
There are also other studies reporting an increased risk of PJI over time [3-5]. The finding that the increase in risk of infection is flattening out after 2010, as found in New York State, and in Norway in the present study, is not supported by the compiled NARA data [7,19].
Several infection surveillance registries report a trend for decreasing rates of surgical site infection (SSI) after THA, including both superficial and deep infections [20,21]. The European Centre for Disease Prevention and Control (ECDC) reports a stable in-hospital incidence of SSI after THA since 2011 [22]. This variety in trends might be explained by the differences in definitions and duration of observation between SSIs reported in regional or national surveillance systems, and revision due to infection, as reported to the arthroplasty registers. SSI is observed on discharge from hospital or at post-discharge surveillance by a general physician (30 days, 90 days, or 1 year postoperatively), in accordance with a specific set of diagnostic criteria and strict definition, and may be superficial or deep [22]. In the NARA, however, the surgeon reports revision due to infection to the register at any time after THA, and not during a limited period as in the surveillance systems.
There may be several changes that result in an apparent increase in risk of revision due to infection, without an actual increased incidence of PJI.
There has been an increase in the reporting of minor revision procedures, such as soft tissue debridement with exchange of removable parts of modular implants, and retention of the femoral stem and acetabular cup, the so called DAIR procedures (debridement, antibiotics, and implant retention) [23]. Such revisions were typically performed 3–12 weeks after primary THA or onset of symptoms of PJI, to avoid a later revision of the whole prosthesis. The threshold to perform these minor procedures may be lower than for full revisions with removal or exchange of the whole prosthesis. These factors may explain the increase in reported revisions due to infection during the first 3 months postoperatively as found for the latter 2 time periods in our study. For these time periods we also found a trend for reduced risk of revision due to infection later than 1 year after primary THA. This may indicate that these early DAIRs result in fewer later major revisions due to infection. It should be mentioned that DAIRs also are performed beyond 3 months of primary THA in the case of PJI due to acute hematogenous spread.
Surgeons may also perform and report DAIR due to prolonged wound drainage to “save” the implant, and report this to the register as revision due to infection. This revision strategy is well motivated due to the strong association between prolonged wound drainage, superficial SSIs, and PJI [24,25]. As mentioned earlier, the reported diagnosis is not dependent on bacterial cultures of tissue samples collected during the revision and, hence, there may be a certain level of misdiagnosis.
There have been improvements in the diagnostics of PJI, and more standardized sampling, culturing, and analyzing techniques have led to fewer samples being false negative [26,27]. In addition, bacteria like Staphylococcus epidermidis and Cutibacterium acnes have emerged as important agents of implant infection [28]. These changes may have resulted in more low-grade infections being correctly diagnosed, revised, and reported, whereas such infections may previously have been overlooked or misdiagnosed as aseptic loosening.
Probably a major contributor to the increased risk of revision due to infection is improved completeness and coverage of reporting. DAIRs may have been underreported as reported by Kamp et al. [29]. This may also have changed during the study period. Compared with several other arthroplasty registers, the Nordic registers have higher rates of revision due to infection [5,29,30]. This may reflect a “true” higher risk of PJI in the Nordic countries, but more likely is due to improved reporting as this has been subject to attention and validation in recent years [9-11]. Compared with validation studies from Sweden and Denmark, our reporting of revision due to infection at 1% resemble the “true” incidence of PJI reported from these countries [11,31]. Focus on the importance of thorough reporting has probably improved the reporting of revisions due to infection in all the Nordic countries, and not only Finland. However, a time-trend evaluation of this has not been performed.
There is also the possibility that the increased risk of revision due to infection reflects a true increase in PJI after THA. A possible explanation may be that THA is now being performed in frailer patients. Some comorbidities, e.g., obesity and diabetes with hyperglycemia, are found to be potent risk factors for postoperative infection and have an increasing incidence in the population [32,33]. These confounders could contribute to an increased risk of infection, but comorbidity covariates are unfortunately not included in the NARA dataset.
Also, increased use of uncemented fixation in patients over 75 years of age may partly explain the increased risk [7]. Both uncemented fixation and advanced age were found to have slightly higher risk of revision due to infection, but the impact on our findings is probably minor.
Strengths and limitations
Registers can provide a useful source of information on incidences and trends because of large numbers and long duration of observation. The NARA dataset contains information on THA patients and primary and revision surgery, gathered uniformly over a long period. Our data are prospective and with information on possible risk factors for primary and revision THA [8]. We therefore have a base for a trend study on a relatively rare complication like PJI. Since a large number of THAs from nationwide populations were included, external validity is expected to be good.
Register studies, however, have inherent limitations [34]. Even if we adjusted for several important factors that could be associated with revision due to infection, there will be residual time-dependent confounding that may only partly be elucidated in an arthroplasty register (i.e., lower threshold of DAIR procedures, increasingly obese population, more diabetes etc.).
Reported THA revisions due to infection are not necessarily the same as rates of PJI. However, we have reason to believe that it is a close approximation, as guidelines recommend revision in the case of suspected PJI, and reporting of revisions to the Nordic arthroplasty registers appears to be acceptable [7].
Interpretation
Our finding of an increased risk of revision due to infection is probably multifactorial. It might not represent a true increase in PJI if it could be explained by changes over time in completeness of reporting, lower revision threshold, improved diagnostics, and/or increased surgeon awareness. On the other hand, factors such as increasing number of operations on frail patients with more comorbidity and higher age at primary THA and increasing use of uncemented implants may contribute to increased incidence of PJI.
Conclusion
The relative risk and absolute rate of revision due to infection after THA increased throughout the period 2004–2018 in the Nordic countries. The increase was mainly caused by more revisions being performed during the first 90 days postoperatively. This may reflect a “true” increase (i.e., frailer patients or more use of uncemented implants) and/or an “apparent” increase (i.e., improved diagnostics, changed revision strategy, or completeness of reporting) in incidence of periprosthetic joint infection. It is not possible to disclose such changes in the present study and this warrants further research.
- Li K, Cuadra M, Scarola G, Odum S, Otero J, Griffin W, et al. Complications in the treatment of periprosthetic joint infection of the hip: when do they occur? J Bone Jt Infect 2021; 6(7): 295-303. doi: 10.5194/jbji-6-295-2021.
- Premkumar A, Kolin D A, Farley K X, Wilson J M, McLawhorn A S, Cross M B, et al. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J Arthroplasty 2021; 36(5): 1484-9.e3. doi: 10.1016/j.arth.2020.12.005.
- Brochin R L, Phan K, Poeran J, Zubizarreta N, Galatz L M, Moucha C S. Trends in periprosthetic hip infection and associated costs: a population-based study assessing the impact of hospital factors using national data. J Arthroplasty 2018; 33(7s): S233-s8. doi: 10.1016/j.arth.2018.02.062.
- Kurtz S M, Lau E C, Son M S, Chang E T, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty 2018; 33(10): 3238-45. doi: 10.1016/j.arth.2018.05.042.
- Lenguerrand E, Whitehouse M R, Beswick A D, Jones S A, Porter M L, Blom A W. Revision for prosthetic joint infection following hip arthroplasty: evidence from the National Joint Registry. Bone J Res 2017; 6(6): 391-8. doi: 10.1302/2046-3758.66.Bjr-2017-0003.R1.
- Dale H, Fenstad A M, Hallan G, Havelin L I, Furnes O, Overgaard S, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 2012; 83(5): 449-58. doi: 10.3109/17453674.2012.733918.
- Dale H, Høvding P, Tveit S M, Graff J B, Lutro O, Schrama J C, et al. Increasing but levelling out risk of revision due to infection after total hip arthroplasty: a study on 108,854 primary THAs in the Norwegian Arthroplasty Register from 2005 to 2019. Acta Orthop 2021; 92(2): 208-14. doi: 10.1080/17453674.2020.1851533.
- Mäkelä K T, Furnes O, Hallan G, Fenstad A M, Rolfson O, Kärrholm J, et al. The benefits of collaboration: the Nordic Arthroplasty Register Association. EFORT Open Rev 2019; 4(6): 391-400. doi: 10.1302/2058-5241.4.180058.
- The Norwegian Arthroplasty Register Annual Report 2019. Available from: http://nrlweb.ihelse.net/eng/Rapporter/Report2019_english.pdf.
- Gundtoft P H, Pedersen A B, Schonheyder H C, Overgaard S. Validation of the diagnosis “prosthetic joint infection” in the Danish Hip Arthroplasty Register. Bone Joint J 2016; 98-b(3): 320-5. doi: 10.1302/0301-620x.98b3.36705.
- Lindgren J V, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord 2014; 15: 384. doi: 10.1186/1471-2474-15-384.
- The Swedish Arthroplasty Register’s Annual Report 2019. Available from: https://registercentrum.blob.core.windows.net/shpr/r/VGR_Annual-report_SHAR_2019_EN_Digital-pages_FINAL-ryxaM-BUWZ_.pdf.
- Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen A B, et al. Statistical analysis of arthroplasty data, II: Guidelines. Acta Orthop 2011; 82(3): 258-67. doi: 10.3109/17453674.2011.588863.
- McNally M, Sousa R, Wouthuyzen-Bakker M, Chen A F, Soriano A, Vogely H C, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J 2021; 103-b(1): 18-25. doi: 10.1302/0301-620x.103b1.Bjj-2020-1381.R1.
- Lie S A, Fenstad A M, Lygre S H L, Kroken G, Dybvik E, Gjertsen J E, et al. Kaplan–Meier and Cox regression are preferable for the analysis of time to revision of joint arthroplasty: thirty-one years of follow-up for cemented and uncemented THAs inserted from 1987 to 2000 in the Norwegian Arthroplasty Register. JBJS Open Access 2022; 7(1): e21.00108. doi: 10.2106/jbjs.Oa.21.00108.
- Cnudde P H J, Nemes S, Bülow E, Timperley A J, Whitehouse S L, Kärrholm J, et al. Risk of further surgery on the same or opposite side and mortality after primary total hip arthroplasty: a multi-state analysis of 133,654 patients from the Swedish Hip Arthroplasty Register. Acta Orthop 2018; 89(4): 386-93. doi: 10.1080/17453674.2018.1475179.
- Hastie T J, Tibshirani R J. Generalized additive models. London: Chapman & Hall; 1990.
- Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen A B, et al. Statistical analysis of arthroplasty data, I: Introduction and background. Acta Orthop 2011; 82(3): 253-7. doi: 10.3109/17453674.2011.588862.
- Perfetti D C, Boylan M R, Naziri Q, Paulino C B, Kurtz S M, Mont M A. Have periprosthetic hip infection rates plateaued? J Arthroplasty 2017; 32(7): 2244-7. doi: 10.1016/j.arth.2017.02.027.
- Choi H J, Adiyani L, Sung J, Choi J Y, Kim H B, Kim Y K, et al. Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J Hosp Infect 2016; 93(4): 339-46. doi: 10.1016/j.jhin.2015.12.021.
- Sodhi N, Anis H K, Garbarino L J, Gold P A, Kurtz S M, Higuera C A, et al. Have we actually reduced our 30-day short-term surgical site infection rates in primary total hip arthroplasty in the United States? J Arthroplasty 2019; 34(9): 2102-6. doi: 10.1016/j.arth.2019.04.045.
- ECDC Healthcare-associated infections: surgical site infections Annual Epidemiological Report 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-SSI.pdf.
- Engesæter L B, Dale H, Schrama J C, Hallan G, Lie S A. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop 2011; 82(5): 530-7. doi: 10.3109/17453674.2011.623572.
- Scheper H, Mahdad R, Elzer B, Löwik C, Zijlstra W, Gosens T, et al. Wound drainage after arthroplasty and prediction of acute prosthetic joint infection: prospective data from a multicentre cohort study using a telemonitoring app. J Bone Jt Infect 2023; 8(1): 59-70. doi: 10.5194/jbji-8-59-2023.
- Zhu Y, Zhang F, Chen W, Liu S, Zhang Q, Zhang Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. J Hosp Infect 2015; 89(2): 82-9. doi: 10.1016/j.jhin.2014.10.008.
- Dudareva M, Barrett L, Figtree M, Scarborough M, Watanabe M, Newnham R, et al. Sonication versus tissue sampling for diagnosis of prosthetic joint and other orthopedic device-related infections. J Clin Microbiol 2018; 56(12). doi: 10.1128/jcm.00688-18.
- Sigmund I K, Yeghiazaryan L, Luger M, Windhager R, Sulzbacher I, McNally M A. Three to six tissue specimens for histopathological analysis are most accurate for diagnosing periprosthetic joint infection. Bone Joint J 2023; 105-b(2): 158-65. doi: 10.1302/0301-620x.105b2.Bjj-2022-0859.R1.
- Patel R. Periprosthetic joint infection. N Engl J Med 2023; 388(3): 251-62. doi: 10.1056/NEJMra2203477.
- Kamp M C, Liu W Y, Goosen J H M, Rijnen W H C, van Steenbergen L N, van der Weegen W. Mismatch in capture of periprosthetic joint infections between the Dutch Arthroplasty Register (LROI) and a detailed regional periprosthetic joint infection registry. J Arthroplasty 2022; 37(1): 126-31. doi: 10.1016/j.arth.2021.09.001.
- Springer B D, Cahue S, Etkin C D, Lewallen D G, McGrory B J. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today 2017; 3(2): 137-40. doi: 10.1016/j.artd.2017.05.003.
- Gundtoft P H, Overgaard S, Schonheyder H C, Moller J K, Kjaersgaard-Andersen P, Pedersen A B. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties. Acta Orthop 2015: 1-9. doi: 10.3109/17453674.2015.1011983.
- Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am 2012; 94(14): e1011-e9. doi: 10.2106/JBJS.J.01935.
- Pedersen A B, Mehnert F, Johnsen S P, Sørensen H T. Risk of revision of a total hip replacement in patients with diabetes mellitus: a population-based follow up study. J Bone Joint Surg Br 2010; 92(7): 929-34. doi: 10.1302/0301-620X.92B7.24461.
- Varnum C, Pedersen A B, Gundtoft P H, Overgaard S. The what, when and how of orthopaedic registers: an introduction into register-based research. EFORT Open Rev 2019; 4(6): 337-43. doi: 10.1302/2058-5241.4.180097.
Appendix
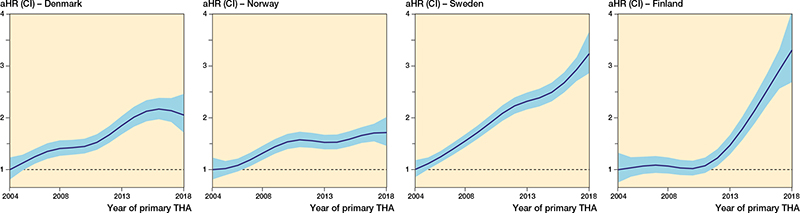
Figure 4. The relationship between year of primary surgery and risk of revision due to deep infection (with 95% CI) for all THAs, adjusted for sex, age, indication for primary THA, and fixation. The broken line represents the aHR in 2004 (aHR = 1).
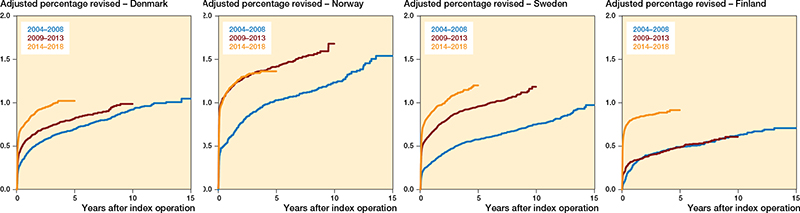
Figure 5. Adjusted revision percentage due to deep infection, for each Nordic country, for all THAs, for 3 periods of primary surgery, adjusted for sex, age, indication for primary THA, and fixation.
| Country Year | THAs included | Revised due to infection | aHR a for revision (CI) | Adjusted 5-year revision rate (CI) |
| Denmark | ||||
| 2004–2008 | 34,812 | 350 | 1 | 0.7 (0.6–0.7) |
| 2009–2013 | 43,119 | 431 | 1.2 (1.0–1.4) | 0.9 (0.8–1.0) |
| 2014–2018 b | 47,727 | 488 | 1.7 (1.4–1.9) | 1.2 (1.0–1.3) |
| Norway | ||||
| 2004–2008 | 26,548 | 304 | 1 | 0.9 (0.8–1.0) |
| 2009–2013 | 35,882 | 497 | 1.4 (1.2–1.6) | 1.2 (1.1–1.3) |
| 2014–2018 b | 43,806 | 508 | 1.4 (1.2–1.7) | 1.1 (1.0–1.2) |
| Sweden | ||||
| 2004–2008 | 70,113 | 550 | 1 | 0.6 (0.5–0.6) |
| 2009–2013 | 79,996 | 856 | 1.6 (1.4–1.8) | 0.9 (0.9–1.0) |
| 2014–2018 b | 86,927 | 920 | 2.1 (1.8–2.3) | 1.2 (1.1–1.2) |
| Finland | ||||
| 2004–2008 | 28,713 | 194 | 1 | 0.5 (0.4–0.6) |
| 2009–2013 | 32,780 | 194 | 1.0 (0.8–1.2) | 0.5 (0.4–0.6) |
| 2014–2018 b | 38,652 | 361 | 2.1 (1.8–2.6) | 1.0 (0.9–1.1) |
| a Adjusted for sex, age, indication for primary THA, and fixation. b The number revised due to infection will be an underestimate due to incomplete follow-up. |
||||
| Years | THAs included | 0–30 days | Timespan of revisions due to infection | 1–5 years | |||||
| 31–90 days | 91 days–1 year | ||||||||
| Revised | aHR a (CI) | Revised | aHR a (CI) | Revised | aHR a (CI) | Revised | aHR a (CI) | ||
| Denmark | |||||||||
| 2004–2008 | 34,812 | 72 | 1 | 39 | 1 | 61 | 1 | 97 | 1 |
| 2009–2013 | 43,119 | 135 | 1.6 (1.2–2.1) | 79 | 1.8 (1.2–2.7) | 78 | 1.1 (0.8–1.5) | 103 | 0.9 (0.7–1.2) |
| 2014–2018 b | 47,727 | 247 | 2.6 (2.0–3.4) | 111 | 2.4 (1.7–3.5) | 63 | 0.8 (0.6–1.2) | 67 | 1.1 (0.8–1.5) |
| Norway | |||||||||
| 2004–2008 | 26,548 | 82 | 1 | 35 | 1 | 29 | 1 | 98 | 1 |
| 2009–2013 | 35,882 | 277 | 2.5 (2.0–3.3) | 54 | 1.2 (0.8–1.8) | 53 | 1.3 (0.8–2.0) | 82 | 0.6 (0.4–0.8) |
| 2014–2018 b | 43,806 | 309 | 2.3 (1.8–3.0) | 83 | 1.5 (1.0–2.2) | 72 | 1.5 (0.9–2.3) | 44 | 0.5 (0.4–0.8) |
| Sweden | |||||||||
| 2004–2008 | 70,113 | 98 | 1 | 61 | 1 | 73 | 1 | 178 | 1 |
| 2009–2013 | 79,996 | 321 | 2.8 (2.3–3.6) | 122 | 1.7 (1.2–2.3) | 109 | 1.3 (1.0–1.8) | 239 | 1.1 (0.9–1.4) |
| 2014–2018 b | 86,927 | 441 | 3.5 (2.8–4.4) | 221 | 2.8 (2.1–3.7) | 135 | 1.6 (1.2–2.1) | 123 | 1.1 (0.8–1.4) |
| Finland | |||||||||
| 2004–2008 | 28,907 | 14 | 1 | 16 | 1 | 59 | 1 | 62 | 1 |
| 2009–2013 | 32,974 | 59 | 3.4 (1.9–6.1) | 16 | 0.8 (0.4–1.5) | 45 | 0.7 (0.5–1.0) | 53 | 0.8 (0.5–1.1) |
| 2014–2018 b | 38,652 | 226 | 10.8 (6.3–18.7) | 70 | 3.0 (1.7–5.2) | 46 | 0.6 (0.4–0.9) | 19 | 0.5 (0.3–0.9) |
| a Adjusted for sex, age, indication for primary THA, and fixation. b The number revised due to infection will be an underestimate due to incomplete follow-up |
|||||||||
| Variable | THAs included | Revised due to infection | 2004–2008 (%) | 2009–2013 (%) | 2014–2018 (%) | aHR a for revision (CI) |
| Age | ||||||
| < 45 | 15,565 | 169 | 3 | 3 | 3 | 0.9 (0.8–1.1) |
| 45–54 | 46,327 | 458 | 7 | 8 | 9 | 0.9 (0.8–1.0) |
| 55–64 | 130,774 | 1,259 | 24 | 23 | 22 | 1.0 (0.9–1.0) |
| 65–74 | 207,619 | 2,019 | 35 | 37 | 38 | 1 |
| 75–84 | 142,478 | 1,434 | 26 | 24 | 25 | 1.1 (1.0–1.2) |
| ≥ 85 | 26,700 | 314 | 5 | 5 | 5 | 1.3 (1.2–1.5) |
| Sex | ||||||
| Female | 336,383 | 2,550 | 60 | 59 | 59 | 1 |
| Male | 233,080 | 3,103 | 40 | 41 | 41 | 1.9 (1.8–2.0) |
| Indication for primary THA | ||||||
| Osteoarthritis | 457,949 | 4,226 | 79 | 81 | 81 | 1 |
| Inflammatory disease | 10,073 | 109 | 3 | 2 | 1 | 1.4 (1.1–1.7) |
| Acute and complications after hip fracture | 52,354 | 744 | 10 | 9 | 9 | 1.7 (1.6–1.9) |
| Complications after childhood hip disease | 19,499 | 149 | 3 | 3 | 4 | 0.8 (0.7–0.9) |
| Necrosis of the femoral head | 11,586 | 184 | 2 | 2 | 2 | 1.7 (1.5–2.0) |
| Other diagnoses | 18,002 | 241 | 4 | 3 | 3 | 1.6 (1.4–1.8) |
| Cement | ||||||
| No | 225,250 | 2,219 | 28 | 42 | 46 | 1.1 (1.0–1.2) |
| With antibiotics | 317,006 | 3,119 | 63 | 55 | 51 | 1 |
| Without antibiotics | 27,207 | 315 | 9 | 3 | 3 | 1.4 (1.3–1.7) |
| Country | ||||||
| Denmark | 125,658 | 1,269 (1.0%) | 22 | 22 | 22 | 0.9 (0.8–1.0) |
| Norway | 106,236 | 1,309 (1.2%) | 17 | 19 | 20 | 1.3 (1.2–1.4) |
| Sweden | 237,036 | 2,326 (1.0%) | 44 | 42 | 40 | 1 |
| Finland | 100,533 | 749 (0.7%) | 18 | 17 | 18 | 0.7 (0.7–0.8) |
| a Adjusted for age, sex, indication for primary THA, fixation, and year of primary THA. | ||||||