The incidence of physeal fractures in the lower limb and the frequency of premature physeal closure: a cohort study of 236 patients
David A L W Cant 1,2 and Christian Faergemann 1,2
1 Section for Pediatric Orthopaedics, Department of Orthopaedics and Traumatology, Odense University Hospital, Odense; 2 Orthopaedic Research Unit, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense C, Denmark
Background and purpose — Physeal fractures represent 15–20% of all pediatric fractures and may lead to premature physeal closure (PPC). The aim of our study was to determine the incidence rates of physeal fractures in the lower limb and the proportion of PPC that lead to limb length discrepancy (LLD), and/or angular deformity (AD).
Patients and methods — This retrospective study included 236 consecutive children with physeal fracture in the tibia, distal femur, or distal fibula. We estimated incidence rates and reviewed medical records and radiographs to obtain information regarding the development of PPC leading to LLD and AD. Of the 236 children, 100 had planned growth control or were referred for growth control due to symptoms of PPC.
Results — The total incidence rate was 35 (95% CI 30–39) per 100,000 person-years, with 1.2 (CI 0.5–23) for distal femur, 5.7 (CI 3.1–7.8) for proximal tibia, 14 (CI 11–17) for distal tibia, and 14 (CI 11–17) for distal fibula. The overall prevalence of PPC was 9.7% (CI 6.3–14), while the prevalence was 38% (CI 8.5–76) for distal femur, 15% (CI 5.9–31) for proximal tibia, 14% (CI 7.4-–22) for distal tibia, and 1.1% (CI 0.3-–59) for distal fibula. We found a significant higher hazard of PPC in fractures with ≥ 3 mm displacement (hazard ratio: 12, CI 1.5–97).
Conclusion — 10% of children with physeal fractures developed PPC that led to LLD or AD. The highest hazard ratio was in children who had an initial fracture displacement. This study highlights the importance of routine and uniform growth evaluation after a physeal fracture.
Citation: Acta Orthopaedica 2023; 94: 289–294. DOI: https://doi.org/10.2340/17453674.2023.13429.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-01-31. Accepted: 2023-05-01. Published: 2023-06-16.
Correspondence: Christian.faergemann@rsyd.dk
DC reviewed the medical records and analyzed the radiographs and CT scans assisted by CF. DC wrote the first draft of the article. Final editing and corrections were made in collaboration. CF designed the study and acquired the necessary data and approvals.
Handling co-editors: Ivan Hvid and Philippe Wagner
Acta thanks Julio de Pablos and Klaus Parsch for help with peer review of this manuscript.
Fractures constitute 10–25% of all pediatric injuries, and the risk of sustaining a fracture during childhood is 27% in girls and 42% in boys [1,2]. In a previous Danish study of children aged 0–15 years, the annual fracture incidence rates (IR) per 10,000 population/years were 215 for girls and 393 for boys [3]. Between 15% and 20% of all pediatric fractures involve the physeal growth plates [4,5]. Physeal fractures may be associated with complications such as premature physeal closure (PPC) that can lead to limb-length discrepancy (LLD), and/or angular deformity (AD) [6-11].
PPC occurs most often after physeal fractures in the lower limbs, most frequently in the distal tibia, distal femur, and proximal tibia [3,12]. Studies have reported that PPC occurs in 12–15% of all physeal fractures in the distal tibia, 26–64% of physeal fractures in the distal femur, and 10–45% of the physeal fractures in the proximal tibia [6,7,9-11,13-16]. However, most studies have had small patient numbers or were case studies. No previous studies have estimated the IR of physeal fractures in the lower limbs.
The aim of our study was to determine the IRs of physeal fractures in the lower limb and the proportion of PPC that leads to LLD and AD.
Patients and methods
The present study was reported according to the STROBE guidelines. We conducted a retrospective cohort study of children aged 0–15 years treated for a physeal fracture in the proximal or distal tibia, distal femur, or distal fibula 2013–2020 at the emergency department (ED) of Odense University Hospital or Svendborg Hospital in Denmark. The population base for this study was the island of Funen in the Region of Southern Denmark, which is a well-defined geographical area with a population of 498,506 [17]. During the study period, the population of children aged 0–15 years decreased from 88,000 in 2013 to 83,269 in 2020 [17]. The 2 EDs had open access 24 hours a day and were the only EDs on Funen during the study period.
We reviewed all radiographs and CT scans of children aged 0–15 years with relevant ICD-10 diagnoses (DS724, DS821, or DS823–DS829) in the study period. 831 radiographs were reviewed (Figure 1). We excluded fractures without involvement of the growth plates. Furthermore, we excluded children with radiographic signs of physiological closure of the growth plates due to maturity, including Tillaux fractures. Each fracture was analyzed by both authors and compared with the diagnosis from the radiology department. The final diagnosis was obtained by consensus. 236 children with fractures involving the growth plate were identified and included in the study. The physeal fractures were subsequently evaluated according to the Salter–Harris classification. At both hospitals all children with fracture displacement on the plain radiograph undergo CT scan to evaluate the fracture displacement. The initial displacement on the CT scan was measured as the greatest distance (in millimeters) in diastasis or cortex displacement.
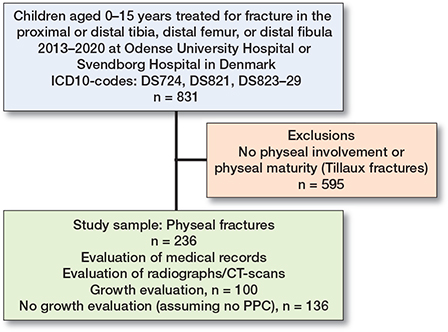
Figure 1. Flowchart of inclusions and exclusions.
The medical records of all cases were systematically reviewed to obtain information on age, sex, date of injury, cause of injury, initial treatment, and the presence of growth disturbance including the date of verification and corrective treatment. The review of medical records ended on July 1, 2022 with an observation period of at least 18 months. We registered the occurrence of PPC with or without LLD and/ or AD. PPC was defined as a radiologically verified physeal bridge formation that suggested either partial or total PPC. CT scans or additional radiographs of the contralateral limb were undertaken to distinguish cases with physiological closure and PPC. The children were examined clinically for limping and signs of asymmetry/angulation in the distal femur, proximal tibia, and distal tibia. LLD was assessed by evaluating the height of the iliac crest in standing position. In the case of clinical and/or radiological signs of LLD and/or AD a low-dose 2D radiograph (EOS imaging system) of the lower limbs was undertaken. Based on this 2D radiograph LLD was defined as a radiographically verified discrepancy of ≥ 5 mm [18,19]. The length of each bone was compared with the contralateral side. AD was defined as ≥ 5° angulation compared with the reference value [18,19].
Growth disturbances were registered in children who participated in planned growth evaluations or were referred for growth evaluation due to symptoms of PPC (e.g., pain, limping, deformity). Only growth evaluations consisting of a combined clinical and radiographic (conventional radiographs and/ or CT scans) examination performed a minimum of 6 months after treatment were considered adequate. Children without a planned growth evaluation or not referred for a growth evaluation were assumed to have no PPC. Of 236 children, 100 had an adequate growth evaluation.
The children were divided into 2 age groups, those < 8 years old and those ≥ 8 years old. Cause of injury was classified as transport-related injuries, sports injury, fall from ground level, or fall from above ground level.
Patients were treated in 1 of 4 ways: conservative treatment with immobilization in a cast or brace, closed reduction without fixation (CRNF), internal fixation with K-wires, or internal fixation with cannulated screws. CRNF was considered as operative treatment. Physeal fractures were treated uniformly at the 2 hospitals, and the recommended treatment is specified in the regional instruction for the 2 EDs and the orthopedic departments. In general, children had surgical intervention if the physeal fracture had > 2 mm displacement, was intra-articular, or was angulated or rotated. After surgery, the fractures were immobilized in a cast or brace without weight-bearing for 4–6 weeks depending on age and fracture site. Conservatively treated fractures were immobilized in a cast or brace for 4–6 weeks, also without weight-bearing.
Statistics
We calculated the annual fracture IR in the different anatomical subgroups including 95% confidence intervals (CI). The IRs were estimated based on mid-year (July 1) population counts of children aged 0–15 years living on the Island of Funen each year extracted as population at risk from Statistics Denmark [17]. The IRs were estimated as incidence densities in a dynamic cohort, allowing subjects to enter and leave the cohort by migration. Children with fractures were not excluded from the population at risk. Mann–Whitney statistics was used to compare age in boys and girls.
Survival statistics were used to analyze age, sex, treatment, and fracture displacement as risk factors of development of growth disturbances. Time of observation was defined as time from date of fracture to the first date of radiologically (radiography or CT) confirmed PPC or censored at end of observation (July 1, 2022). Failure was defined as radiological signs of PPC. Hazard ratios were estimated in a crude and an adjusted model. According to the Akaike and Bayesian information criterion, we built Cox regression models including sex, age group, treatment, and fracture displacement as risk factors. We examined residuals using the Schoenfeld test for proportionalhazard assumption. The Schoenfeld residuals were calculated for each variable to see if each variable independently satisfies the assumptions of the final adjusted Cox model. Hazard ratios were estimated in both a crude model and in a final adjusted model with CIs and p-values. Additionally, we estimated a Kaplan–Meier survival curve of the timespan from physeal fracture to the time of diagnosed PPC, including CI and the number of children at risk at different time points. Statistical analyses were undertaken using Stata 15 (StataCorp LLC, College Station, TX, USA). P < 0.05 was considered significant.
Ethics, funding, and disclosures
The study received approval from the Region of Southern Denmark (Journal number: 21/3509) and informed content was obtained from the Danish Patient Safety Authority (Journal number: 21/23581). The study received no funding or benefits of any kind, and no direct or indirect commercial interests are attached to the findings. The authors have no conflicts of interest to declare. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.13429
Results
We identified 236 (28%) physeal fractures in 831 children with fracture of either the proximal or distal tibia, distal femur, or distal fibula during the study period. Overall, 41% of the physeal fractures involved the distal tibia, 39% the distal fibula, 17% the proximal tibia, and 3.4% the distal femur (Table 1). The total IR of physeal fractures was 35 (CI 30–39) per 100,000 persons/year. The respective IRs were 1.2 (CI 0.50–23) in the distal femur, 5.7 (CI 3.1–7.8) in the proximal tibia, 14 (CI 11–17) in the distal tibia, and 14 (CI 11–17) in the distal fibula.
126 (53%) of the children were boys and the median age was 12 (range 2–15) years for boys and 10.5 (2–15) years for girls (Mann–Whitney test, p = 0.1) (Table 1). The median age was 11 (4–14) for physeal fractures in the distal femur, 9 (2–15) years for the proximal tibia, 12 (3–15) years for the distal tibia, and 10.5 (2–15) years for the distal fibula. Falls from above ground level were the most frequent cause of injury (32%) followed by sports (31%), transport-related injuries (19%), and falls from ground level (18%).
Salter–Harris type 2 was the most frequent type of physeal fracture, accounting for 51% (Table 1). All physeal fractures in the distal femur were classified as type 2, which also accounted for 69% of the proximal tibia fractures, 34% of the distal tibia fractures, and 57% of the distal fibula fractures. Most children (n = 165, 70%) were treated conservatively, while 71 (30%) had surgery; of these, 44 (19%) were treated with screw fixation, 25 (11%) with K-wires, and 2 with CRNF (Table 1).
100 (42%) children participated in a growth evaluation within a mean of 48 (SD 3.0) weeks after the initial injury (Table 2). At the time of growth evaluation, 23% of these children presented with either intermittent or consistent pain in relation to the injury site and 8% had detectable joint stiffness (Table 2).
23 (9.7%, CI 6.3–14) children had growth disturbances verified on radiographs and/or CT scan and diagnosed within a mean of 72 (SD 59) weeks after the initial injury. The proportion of growth disturbances within each fracture site was 38% (CI 8.5–76) for the distal femur, 14% (CI 7.4–22) for the distal tibia, 15% (CI 5.9–31) for the proximal tibia, and 1.1% (CI 0.3–59%) for the distal fibula. Among the children treated surgically, 24% (CI 15–36) developed growth disturbance compared with the 3.6% (CI 1.3–7.7) seen in conservatively treated children (Table 2). After excluding children who did not receive growth evaluation, the total prevalence of growth disturbances was 23% (CI 15–33), where 13% (CI 4.7–22) were treated conservatively and 33% (CI 20–47) were treated operatively. Similarly adjusted, the frequencies of growth disturbance within each anatomical subgroup were 60% (CI 15–95) in the distal femur, 23% (CI 9.0–44) in the proximal tibia, 26% (CI 14–40) in the distal tibia, and 5.6% (CI 0.1–27) in the distal fibula.
In children with growth disturbances, the most common disturbance was PPC leading to AD (39%), followed by equal proportions of PPC leading to LLD (26%) and PPC leading to both LLD and AD (26%). Only 8.7% developed isolated PPC without LLD or AD yet (Table 3). The proportion of growth disturbances was highest in children with Salter–Harris type 2 fractures (61%), followed by type 4 fractures (35%) (Table 3).
| Factor | Distal femur | Proximal tibia | Distal tibia | Distal fibula | Total |
| Children | 3 | 6 | 13 | 1 | 23 |
| Age (median) | 12 | 7 | 11 | 8 | 11 |
| Sex | |||||
| Boys | 2 | 4 | 6 | 0 | 12 |
| Girls | 1 | 2 | 7 | 1 | 11 |
| Treatment | |||||
| Conservative | 1 | 3 | 1 | 1 | 6 (26) |
| Surgical | 2 | 3 | 12 | 0 | 17 (74) |
| Salter–Harris type | |||||
| 1 | 0 | 0 | 0 | 0 | 0 (0) |
| 2 | 3 | 5 | 5 | 1 | 14 (61) |
| 3 | 0 | 0 | 1 | 0 | 1 (4.3) |
| 4 | 0 | 1 | 7 | 0 | 8 (35) |
| Accompanying fracture | 0 | 1 | 7 | 0 | 8 (35) |
| Growth disturbance | |||||
| PPC only | 0 | 0 | 2 | 0 | 2 (8.7) |
| PPC + LLD | 1 | 2 | 3 | 0 | 6 (26) |
| PPC + AD | 1 | 3 | 4 | 1 | 9 (39) |
| PPC + LLD + AD | 1 | 1 | 4 | 0 | 6 (26) |
| Corrective surgery | 3 | 2 | 11 | 1 | 17 (74) |
| For abbreviations, see Table 2. | |||||
Of the 23 children with growth disturbance, 17 had corrective surgery: 16 had physiodeses, 3 had Langenskjöld’s procedure, and 4 had angular corrections. A further patient was treated with an internal bone-lengthening nail. We found that 20% of the transport-related injuries were complicated by growth disturbances, compared with 9.3% of falls above ground level, 8.1% of sports, and 2.4% of falls from ground level.
The adjusted Cox regression analyses showed that children with an initial fracture displacement ≥ 3 mm had a significantly higher hazard for developing growth disturbances (hazard ratio 12, CI 1.5–97) than children with < 3 mm displacement (Table 4). We found no significant difference regarding age, sex, or treatment in the adjusted analyses. The vast majority of growth disturbances were diagnosed before 2 years of follow-up (Figure 2). The latest time of diagnosed growth disturbance was after 3.5 years.
| Factor | Growth disturbance a | Normal growth | Hazard ratio (CI) | |
| crudeb | adjustedc | |||
| Age groups | ||||
| 0–7 | 3 | 40 | 1.0 (reference) | 1.0 (reference) |
| 8–15 | 20 | 173 | 1.4 (0.41–4.7) | 0.98 (0.25–3.9) |
| Sex | ||||
| Girls | 11 | 99 | 1.0 (reference) | 1.0 (reference) |
| Boys | 12 | 114 | 0.88 (0.39–2.0) | 0.99 (0.27–3.6) |
| Treatment | ||||
| Conservative | 6 | 159 | 1.0 (reference) | 1.0 (reference) |
| Surgical | 17 | 54 | 9.9 (3.9–25) | 1.0 (0.21–4.8) |
| Fracture displacement | ||||
| < 3 mm | 1 | 21 | 1.0 (reference) | 1.0 (reference) |
| ≥ 3 mm | 10 | 18 | 10 (4.4–24) | 12 (1.5–97) |
| a Comparison includes only patients who had a CT scan done before the initial treatment. b Crude model analyzing age group, sex, treatment, and fracture displacement separately. c Adjusted model including age group, sex, treatment, and fracture displacement. |
||||
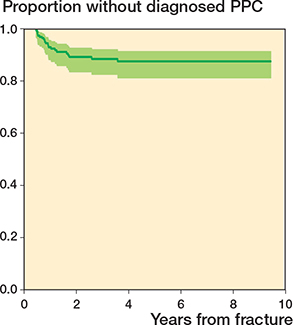
Figure 2. Kaplan–Meier survival curve of the timespan from physeal fracture to the time of diagnosed growth disturbance including 95% CI.
Discussion
The aim of the study was to determine the IRs of physeal fractures in the lower limb and the proportion of PPC that led to LLD and AD. We showed the IR of physeal fractures to be 1.2 (CI 0.5–23) per 100,000 person-years in the distal tibia, 5.7 (CI 3.1–7.8) in the proximal tibia, 14 (CI 11–17) in the distal tibia, and 14 (CI 11–17) in the distal fibula. The IRs in our study are consistent with a systematic review (not including avulsion of the tibial tubercle), except that we found a higher rate of physeal fractures in the proximal tibia compared with the distal femur [12]. In addition, we found that Salter–Harris type 2 fractures were the most frequent type, followed by type 3, type 4, and type 1. This reflects the general understanding of the proportion of each fracture type [5].
We found that 14% of children with physeal fractures in the distal tibia developed PPC, which is consistent with previous studies showing that PPC occurs in 12–15% of cases [7,9-11]. In a previous study, 12.8% developed growth arrest defined by a LLD > 1 cm or a LDTA > 5° [11]. We found that 11 (12%) children developed either LLD, AD, or a combination of both. In our study we defined LLD as ≥ 5 mm.
In children with physeal fractures of the proximal tibia, we found that 15% developed PPC and all had either LLD, AD, or a combination of the two. It is reported that growth disturbance occurs in 10% of all physeal fractures in the proximal tibia [16], which is lower than earlier reported with up to 45% developing growth disturbances and 25.5% who had major deformities in terms of LLD ≥ 25 mm and/or AD ≥ 5° [15]. Our inclusion of all physeal fractures involving the growth plate, including avulsions of the tibial tubercle, may have contributed to an overall higher frequency and a lower proportion of growth disturbances.
Our study included only 8 children with distal femur physeal fractures, of whom 3 developed PPC, all with AD and/ or LLD. Eid and Hafez [14] studied 151 injuries of the distal femoral physis and found LLD in 38.4% and AD in 51%. They reported a range of LLD between 0.5 cm and 11 cm (mean = 1.7), indicating the severity of LLD after physeal injuries in the distal femur. In our study, both patients with LLD after distal femur fractures developed a minimum of 20 mm of LLD.
We included 93 children with physeal fracture of the distal fibula, of whom only 1 developed PPC with AD. Almost all (97%) children were treated conservatively. These findings support the suggestion that fractures treated conservatively have a low risk of developing growth disturbances [4].
Previous studies have reported several risk factors of PPC, such as initial displacement, high-energy trauma, accompanying fractures, preoperative delay, age at the time of trauma, fracture classification, multiple attempts at reduction, and residual displacement after intervention [6-8,11,20-23]. Our study showed an increased risk of developing PPC in surgically treated physeal fractures and in fractures with an initial displacement ≥ 3 mm. However, in the adjusted analysis only fracture displacement was associated with an increased hazard of developing PPC. A meta-analysis of 970 distal tibia physeal fractures found no significant association between different treatment methods and the development of growth disturbances [9]. Previous studies have shown a similar association between fracture displacement and growth disturbances [6,7,11]. In our analyses, we included only children with a preoperative CT scan. We are aware that some children with undisplaced fracture according to the plain radiographs may have had a displaced fracture on a CT scan. We have no reliable information on the size of this bias.
This study has some limitations, primarily related to its retrospective design. First, only 100 out of 236 children had a planned growth evaluation or were referred for growth evaluation due to symptoms of PPC. Only 73% of the surgically treated and 29% of the conservatively treated children were systematically examined for growth disturbances. We have assumed that the majority of children without growth evaluation have not developed PPC, but we are aware that some children may have had undiagnosed PPC or will develop PPC. We have no reliable information on the importance of this bias. The prevalence of PPC in our study is comparable with other studies. Second, some children may have had growth evaluations and treatment at other hospitals due to migration during the study period. This bias is considered of minor importance, due to the structure of the Danish healthcare system with free and equal access to the healthcare system for all and the geographic conditions of Funen as an island. Third, our study included children in the period 2013–2020, with follow-up until July 1, 2022. This resulted in different lengths of follow-up and thus an unequal range of time to develop PPC. All children had follow-up of at least 18 months. However, it is likely that some children, especially in the youngest group, may develop PPC later in childhood. We have limited information on the importance of this bias. Additionally, the Kaplan–Meier survival curve showed that the vast majority of growth disturbances were diagnosed within 2 years of follow-up.
We defined LLD as at least 5 mm. Some studies have found that discrepancy of less than 1.25 cm does not cause significant compensatory mechanisms [24,25]. However, a recent study suggests that discrepancies of 5 mm can lead to long-term pathology [18]. Our choice of 5 mm was primarily based on the clinical relevance of early diagnosis preventing further aggravation. We defined PPC as a radiologically verified physeal bridge formation suggesting either partial or total premature closure of the growth plate that could lead to LLD and/ or AD. Other studies have used the terms “growth arrest” or “physeal bone bridge.” We have chosen to distinguish PPC from growth arrest measured as LLD or AD to avoid misinterpretations, as PPC or physeal bone bridges do not necessarily lead to further manifestations such as LLD and AD.
Conclusion
Our study showed incidence rates of physeal fractures in the lower limb between 1.2 and 14.1 per 100,000 population/ years and that approximately 10% of children with physeal fractures developed PPC that led to LLD or AD. We emphasize the importance of routine and uniform growth evaluations of children with physeal fractures.
- Landin L A. Epidemiology of children’s fractures. J Pediatr Orthop Part B 1997; 6(2): 79-83.
- Landin L A. Fracture patterns in children: analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979. Acta Orthop Scand 1983; Suppl. 202: 1-109. PubMed PMID: 6574687.
- Larsen A V, Mundbjerg E, Lauritsen J M, Faergemann C. Development of the annual incidence rate of fracture in children 1980–2018: a population-based study of 32,375 fractures. Acta Orthop 2020; 91(5): 593-7. doi: 10.1080/17453674.2020.1772555.
- Peterson H A. Epiphyseal growth plate fractures. Berlin/Heidelberg: Springer Science & Business Media; 2007.
- Levine R H, Foris L A, Nezwek T A, Waseem M. Salter Harris fractures. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021. Tampa, FL: StatPearls Publishing LLC; 2021.
- Arkader A, Warner W C Jr, Horn B D, Shaw R N, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop 2007; 27(6): 703-8. doi: 10.1097/BPO.0b013e3180dca0e5.
- Leary J, Handling M, Talerico M, Yong L, Bowe J A. Physeal fractures of the distal tibia: predictive factors of premature physeal closure and growth arrest. J Pediatr Orthop 2009; 29(4): 356-61. doi: 10.1097/BPO.0b013e3181a6bfe8.
- Petratos D V, Kokkinakis M, Ballas E G, Anastasopoulos J N. Prognostic factors for premature growth plate arrest as a complication of the surgical treatment of fractures of the medial malleolus in children. Bone Joint J 2013; 95-b(3): 419-23. doi: 10.1302/0301-620x.95b3.29410.
- Asad W A, Younis M H S, Ahmed A F, Ibrahim T. Open versus closed treatment of distal tibia physeal fractures: a systematic review and meta-analysis. Eur J Orthop Surg Traumatol 2018; 28(3): 503-9. doi: 10.1007/s00590-017-2062-1.
- Stenroos A, Kosola J, Puhakka J, Laaksonen T, Ahonen M, Nietosvaara Y. Routine radiographic follow-up is not necessary after physeal fractures of the distal tibia in children. Acta Orthop 2019; 90(6): 610-3. doi: 10.1080/17453674.2019.1643632.
- Jung H S, Park M S, Lee K M, Choi K J, Choi W Y, Sung K H. Growth arrest and its risk factors after physeal fracture of the distal tibia in children and adolescents. Injury 2021; 52(4): 844-8. doi: 10.1016/j.injury.2021.01.014.
- Peterson C A, Peterson H A. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma 1972; 12(4): 275-81. doi: 10.1097/00005373-197204000-00002.
- Young E Y, Stans A A. Distal femoral physeal fractures. J Knee Surg 2018; 31(6): 486-9. doi: 10.1055/s-0038-1627465.
- Eid A M, Hafez M A. Traumatic injuries of the distal femoral physis: retrospective study on 151 cases. Injury 2002; 33(3): 251-5. doi: 10.1016/s0020-1383(01)00109-7.
- Gautier E, Ziran B H, Egger B, Slongo T, Jakob R P. Growth disturbances after injuries of the proximal tibial epiphysis. Arch Orthop Trauma Surg 1998; 118(1-2): 37-41. doi: 10.1007/s004020050307.
- Flynn J M, Skaggs D, Waters P M. Rockwood and Wilkins’ fractures in children. 9th ed. Philadelphia, PA: Wolters Kluwer; 2019.
- Statistics D. FOLK1A: Population at the first day of the quarter by region, sex, age and marital status 2021 [cited 2021, Dec 19]. Available from: https://www.statbank.dk/FOLK1A.
- Gordon J E, Davis L E. Leg length discrepancy: the natural history (and what do we really know). J Pediatr Orthop 2019; 39(6, Suppl 1.): S10-13. doi: 10.1097/bpo.0000000000001396.
- Paley D. Principles of deformity correction: Berlin/Heidelberg: Springer Science & Business Media; 2002.
- D’Angelo F, Solarino G, Tanas D, Zani A, Cherubino P, Moretti B. Outcome of distal tibia physeal fractures: a review of cases as related to risk factors. Injury 2017; 48(Suppl. 3): S7-11. doi: 10.1016/s0020-1383(17)30650-2.
- Park H, Lee D H, Han S H, Kim S, Eom N K, Kim H W. What is the best treatment for displaced Salter–Harris II physeal fractures of the distal tibia? Acta Orthop 2018; 89(1): 108-12. doi: 10.1080/17453674.2017.1373496.
- Wuerz T H, Gurd D P. Pediatric physeal ankle fracture. J Am Acad Orthop Surg 2013; 21(4): 234-44. doi: 10.5435/jaaos-21-04-234.
- Barmada A, Gaynor T, Mubarak S J. Premature physeal closure following distal tibia physeal fractures: a new radiographic predictor. J Pediatr Orthop 2003; 23(6): 733-9. doi: 10.1097/00004694-200311000-00010.
- Goel A, Loudon J, Nazare A, Rondinelli R, Hassanein K. Joint moments in minor limb length discrepancy: a pilot study. Am J Orthop (Belle Mead NJ) 1997; 26(12): 852-6. PMID: 9413588.
- Song K M, Halliday S E, Little D G. The effect of limb-length discrepancy on gait. J Bone Joint Surg Am 1997; 79(11): 1690-8. doi: 10.2106/00004623-199711000-00011.