Intra-articular injection of secretome, derived from umbilical cord mesenchymal stem cell, enhances the regeneration process of cartilage in early-stage osteo-arthritis: an animal study
Andri Maruli Tua LUBIS 1,2, Petrus APRIANTO 1,2, Jeanne Adiwinata PAWITAN 3, Bambang Pontjo PRIOSOERYANTO 4, Tri Isyani Tungga DEWI 4, and Achmad Fauzi KAMAL 1,2
1 Department of Orthopaedics and Traumatology, Cipto Mangunkusumo General Hospital, Jakarta; 2 Department of Orthopaedics and Traumatology, Faculty of Medicine Universitas Indonesia, Jakarta; 3 Department of Histology, Cipto Mangunkusumo General Hospital – Faculty of Medicine Universitas Indonesia, Jakarta; 4 Department of Veterinary Pathology, Faculty of Agriculture, IBP University, Bogor, Indonesia
Background and purpose — Mesenchymal stem cells (MSCs), both endogenous and exogenous, enhance chondrocyte proliferation by stimulating collagen type II. Secretome, an MSC derivate, has shown to also provide this mechanism through a paracrine effect. We aimed to evaluate the use of secretome and MSC in the management of early osteoarthritis (OA).
Animals and methods — 19 (1 control) male sheep (Ovies aries), which were operated on with total lateral meniscectomy to induce knee OA, were divided into 3 groups: the secretome group, hyaluronic acid group, and MSC group. Each group was injected with the respective substances and was evaluated macroscopically and microscopically. The Osteoarthritis Research Society International (OARSI) score was calculated for all subjects and a descriptive and comparative statistical analysis was undertaken.
Results — The macroscopic analysis of the treated groups revealed better OARSI score in the secretome group compared with the other 2 groups. The secretome group showed a significantly better microscopic score compared with the hyaluronic acid group (mean difference [MD] 6.0, 95% confidence interval [CI] 0.15–12), but no significant difference compared with the MSC group (MD 1.0, CI –4.8 to 6.8).
Conclusion — Intra-articular injection of secretome is effective in managing early-stage osteoarthritis in the animal model compared with hyaluronic acid and has similar efficacy to MSC injection.
Citation: Acta Orthopaedica 2023; 94: 300–306. DOI: https://doi.org/10.2340/17453674.2023.12359.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-11-10. Accepted: 2023-03-20. Published: 2023-06-27.
Correspondence: fauzikamal@yahoo.com
AMTL conceived, designed, and supervised this research. PA conceived, designed, collected data, and analyzed data. JAP conceived, designed, and supervised this research. BPP prepared the animal, collected data, and analyzed data. TITD prepared the animal, collected data, and analyzed data. AFK conceived, directed, analyzed, and supervised the overall process of this research.
The authors would like to thank Dr Gunanti for her continuous support in this research. The authors would also like to thank Ragil Parasmadan, Riky Setyawan, Anita Heppy Rahayu, Nuzli Farida, Aufa Kunti, and Tri Kuniawati for their contributions and support in this research.
Handling co-editors: Ivan Hvid and Robin Christensen
Acta thanks Mats Brittberg and Asbjørn Årøen for help with peer review of this study.
Surgical and non-surgical treatment options for osteoarthritis (OA) have been confined to alleviation of symptoms or correction of isolated lesions. However, OA is progressive, causing the necessity for total knee replacement at a later date. Therapies that can alter the disease’s progression are being researched and have the potential to become the key OA management. Articular injections of hyaluronic acid have been used as a therapy for OA in the past. Even though there is still debate over its efficacy and adverse effects, hyaluronic acid has been authorized for intraarticular injection in knee OA across the world (1,2). Mesenchymal stem cells (MSCs) have the capacity to proliferate into chondrocytes and create secretome, which influences the cellular environment. As a result, MSCs offer potential as a treatment in knee cartilage diseases (3–5). Secretomes are potentially safer than stem cell injection since they have fewer immunogenic effects, are easier to store, and have a lower risk of producing malignancies, but no studies have compared the treatments (5,6).
Therefore, we aimed to analyze the efficacy of hyaluronic acid, MSC, and secretome intraarticular injections on macroscopic and microscopic cartilage change in an animal model.
Animals and methods
We conducted an animal experimental study in Veterinary Medicine, PPB University and Stem Cell and Tissue Engineering Research Cluster Indonesian Medical Education and Research Institute (SCTE IMERI). We compared the cartilage regeneration in early OA between the intra-articular injection of hyaluronic acid, MSC, and secretome in an animal model. We used male local sheep (Ovies aries) aged 3 years old or more, with weight of 23–30 kg, and which were healthy and acclimatized. The sheep should have skeletal maturity and no history of trauma or congenital disorder that caused impairment or abnormalities. The animal was excluded if no OA developed or if infection in the targeted knee occurred.
Mesenchymal stem cell culture, isolation, and expansion
Fifth passage HUC-MSCs (human umbilical cord mesenchymal stem cells) were taken from a human umbilical cord source. The complete medium consisted of 1% penicillin-streptomycin, 1% amphotericin, 1% heparin, 1% GlutaMax (L-alanyl-L-glutamine dipeptide), 10% serum platelet-rich plasma (PRP), and medium basal MEM α (Minimum Essential Medium α). The MSCs were cultured after the cell confluence reached 80–90%. The cultured cells were later divided into containers consisting of 2x106 cells for each container and were kept in coolboxes and transported to the animal laboratory to be intra-articularly injected on the same day.
The secretome
We used laboratory standard procedure in extracting secretome from MSCs. The 6th stage of HUC-MSCs was cultured in 6 flasks of 25 cm3. Each flask was filled with 5 mL of complete medium. The medium was replaced every 2–3 days. Centrifugation was done on the suspense and medium at 1,200 RPM. Cell culture was grown in an incubator at 37°C with 5% level of CO2. When the cell confluence achieved 80–90%, the conditioned medium was used to process secretome. Total conditioned medium collected from the 6 flasks was 30 mL. The conditioned medium was centrifuged at 3,500 RPM in 30 minutes. Supernatant was then filtered with filters sized 0.45 μm and 0.22 μm and collected as secretome.
Secretome contains paracrine protein secreted by cells and exosome with its microvesicle, where exosome in particular isolates proteins that are used in intercellular communication. In this study, we separated the cell and its fluid containing secretome as a whole and did not isolate specific proteins.
Meniscectomy-induced osteoarthritis in sheep model
Subjects and surgical approach
19 sheep included in this experiment were acclimatized in the laboratory for 2 weeks with a controlled temperature of 28–30°C and humidity of 55%. 1 sheep was killed as control and the other sheep were divided into 3 equal groups.
Sample size calculation
Sample size was estimated using Mead’s formula (7). Based on theis formula, the sample size required for each group was 5. This number was later increased by 1 to anticipate the possibility of dropout. Therefore, 6 sheep were required for each treatment group.
Randomization and blinding
All 18 sheep were randomized into 3 equal groups using simple randomization. Authors and the technician were blinded to the treatment given to the animal. Killing and meniscectomy procedures were performed by a veterinarian. Macroscopic and microscopic examination were performed by a certified animal pathologist.
The scoring was not done by the author, but by the histology team of IPB University, which consists of 2 people who did the scoring for all samples in the 3 groups
Meniscectomy
Meniscectomy was performed on all animal subjects on the right hind leg knee. Prior to the surgery, prophylactic antibiotics were administered using intramuscular amoxicillin 5 mg/kg and premedication of atropine sulfate 0.15 mg/kg subcutaneously. The anesthesia was induced using ketamine 22 mg/kg and xylazine 0.20 mg/kg intramuscularly. A lateral meniscectomy was then conducted with a lateral parapatellar approach.
Rehabilitation protocol
The sheep were then kept in the cage for 10 days before the rehabilitation procedure. The sheep were hand-walked daily on a hard surface (asphalt) for 360 meters for 3 weeks. After 3 weeks, the sheep’s knees were examined radiologically to make sure that early OA had occurred. One sheep was killed and the right knee, as osteoarthritis defect control, and the left knee, as normal control, were harvested.
Treatment protocol
Intra-articular secretome, hyaluronic acid injection, and mesenchymal stem cell in hyaluronic acid implantation
4 weeks after meniscectomy, injection was carried out on the right posterior knee joint after aseptic and antiseptic procedure with 70% alcohol and povidone iodine was performed. Injection of 2 mL of secretome, 2 mL of hyaluronic acid, or 2x106 cells of HUC-MSCs was conducted accordingly. Intra-articular injection was performed using fluoroscopic guidance.
Evaluation of the treatment outcomes
Macroscopic scoring confirmation
After an observation period of 1 month, all sheep were killed using exsanguination. The joint cartilage with its subchondral bone was retrieved and evaluated macroscopically and microscopically. On macroscopic examination, the cartilage surface, osteophyte formation on the joint, and synovium characteristics of the joint were scored based on the OARSI scoring system (Tables 1 and 2, see Appendix).
Modified OARSI score has been used for several studies, for example Liu et al. (8), Kraus et al. (9), and Waldstein et al. (10). The modifications made in this study were done to increase the homogenization of score calculation and do not change the core components of the original OARSI score.
Histopathological evaluation
Cartilage tissue and subchondral bone samples were retrieved from central coronal cutting of the lateral femoral condyle and lateral proximal tibia cartilage, which were then submersed in nitric acid 20% solution for 3 weeks. The histopathological examination was then conducted with the measurement of 5 parameters. The microscopic structure, chondrocyte density, and cell multiplication were stained using hematoxylin-eosin (HE) (Figure 1) and the interterritorial and tidemark area were stained using toluidine blue (Figure 2). The score was then measured based on the OARSI scoring system.
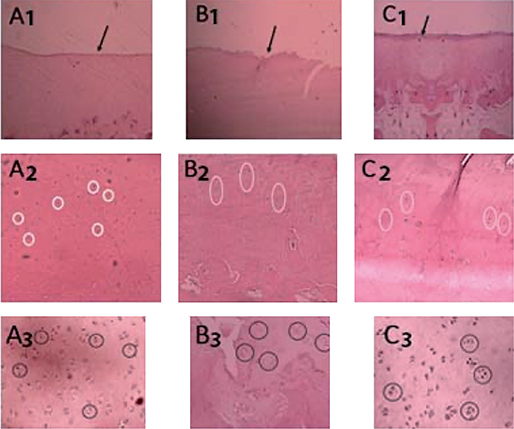
Figure 1. Structure, density, and cell cloning evaluation. A1: secretome group, normal structure; B1: HA group, defect to 1/3 of depth (transitional zone); C1: MSC group, moderate surface irregularity; A2: secretome group, density decreases slightly; B2: HA group, density decreases slightly; C2: MSC, density decreases slightly; A3: secretome group, some double cells and some cell cages; B3: HA group, double cell and cell cage; C3: MSC group, multiple double cell and cell cage.
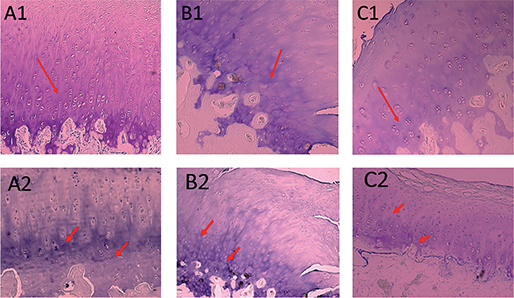
Figure 2. Interterritorial and tidemark evaluation. A1: the secretome group, normal interterritorial; B1: HA group, less colored by coloring; C1: MSC group, coloring is reduced to middle zone 1/3 of depth; A2: secretome group, tidemark duplication; B2: HA group, tidemark duplication; C2: MSC group, tidemark duplication.
Statistics
The retrieved data is presented with mean and standard deviation (SD) and mean difference (MD) with 95% confidence interval (CI). The data was analyzed using the Statistical Program for the Social Sciences (SPSS) version 25.0 (IBM Corp, Armonk, NY, USA). All data is presented with descriptive analysis to show the samples’ characteristics. The comparative analysis of each variable was analyzed using a one-way ANOVA test with post-hoc Bonferroni analysis to obtain the significance of each comparative parameter. The results were interpreted accordingly with p-value < 0.05 and effect size as considerations.
Ethics, data sharing, funding, and disclosures
The authors declare that all the procedures and experiments in this study respect the ethical standards in the Helsinki Declaration of 1975, as revised in 2008 (5), as well as national law. This research received ethical approval from the Animal Ethics Committee Faculty of Veterinary Medicine Bogor Agricultural University (No 013/KEH/SKE/X/2020). The datasets generated and/or analyzed during the current study are not publicly available due to regulation of the institutional review board but are available from the corresponding author on reasonable request.
This research is funded by the Ministry of Education, Culture, Research, and Technology, Republic of Indonesia (contract number 008/E4.1/AK.04.PRN/2021). The authors declare that they have no competing interests. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.12359
Results
The secretome group showed better total macroscopic OARSI score (6.8, SD 1.5) compared with the hyaluronic acid group (15, SD 3.1) and MSC group (13, SD 3.3). The one-way ANOVA parametric test showed significant differences between groups (p = 0.001). The test was continued with post-hoc Bonferroni test and showed significantly better macroscopic score in the secretome group compared with the hyaluronic acid group (p = 0.01, MD 6.3, CI 3.7–9.0) and the MSC group with the hyaluronic acid group (p = 0.02, MD 4.0, CI 1.4–6.6), while there was no significant difference between the secretome and MSC groups (p = 0.3, MD 2.3, CI –0.20 to 5.0). Similar results were found on each component of scoring except for the synovium score, which showed similar results in all groups (Table 3).
| Parameter Samples’ group | Macroscopic score | p-value | Post-hoc analysis | Mean difference (CI) | ||
| vs group | p-value | |||||
| Total macroscopic score | ||||||
| Secretome | 6.8 (1.5) e | 0.001 a | HA | 0.001 c | 6.3 (3.7 to 9.0) | |
| Hyaluronic acid | 15 (3.1) e | MSC | 0.02 c | 4.0 (1.4 to 6.6) | ||
| MSC | 13 (3.3) e | Secretome | 0.3 c | 2.3 (–0.2 to 5.0) | ||
| Cartilage score | ||||||
| Secretome | 4.2 (0.8) e | < 0.001 a | HA | < 0.001 c | 4.0 (2.7 to 5.3) | |
| Hyaluronic acid | 9.3 (1.2) e | MSC | 0.08 c | 2.7 (1.3 to 4.0) | ||
| MSC | 6.3 (2.1) e | Secretome | 0.05 c | 1.3 (0.10 to 2.7) | ||
| Osteophyte score | ||||||
| Secretome | 2 (2–3) f | 0.003 b | HA | 0.002 d | 2.0 (0.27 to 3.7) | |
| Hyaluronic acid | 5 (3–7) f | MSC | 0.8 d | 1.3 (–0.30 to 3.1) | ||
| MSC | 4.5 (3–6) f | Secretome | 0.006 d | 0.66 (–1.0 to 2.4) | ||
| Synovial score | ||||||
| Secretome | 0.5 (0–1) f | 0.4 b | HA | 0.2 d | 0.33 (–0.30 to 1.0) | |
| Hyaluronic acid | 1 (0–1) f | MSC | 1.0 d | 0.0 (–0.70 to 0.71) | ||
| MSC | 1 (0–1) f | Secretome | 0.4 d | 0.33 (–0.30 to 1.0) | ||
| a One-way ANOVA test. b Kruskal–Wallis test. c Bonferroni post-hoc analysis. d Mann–Whitney U-test. e Mean (SD). f Median (min–max). |
||||||
On the scoring evaluation on various surfaces of the knee, the Kruskal–Wallis test showed significant differences between groups on the surfaces of distal femur inferior side (contact with proximal tibial cartilage) (p = 0.003) and tibia (p = 0.001) (Table 4).
| Parameter Samples’ group | Macroscopic score a | p-value b | Post-hoc analysis | Mean difference (CI) | |
| vs group | p-value c | ||||
| Total distal femur inferior side (contact with proximal tibial cartilage) | |||||
| Secretome | 3 (3–3) | 0.003 | HA | 0.001 | 1.8 (0.55 to 3.1) |
| Hyaluronic acid | 4.5 (4–6) | MSC | 0.4 | 1.5 (0.21 to 2.8) | |
| MSC | 4 (3–5) | Secretome | 0.02 | 0.33 (–0.90 to 1.6) | |
| Total distal femur anterior side (contact with patellar cartilage) | |||||
| Secretome | 1 (0–3) | 0.3 | HA | 0.4 | 0.33 (–0.8 to 1.5) |
| Hyaluronic acid | 1.5 (1–2) | MSC | 0.5 | –0.30 (–1.5 to 0.88) | |
| MSC | 2 (1–3) | Secretome | (0.2) | 0.66 (–0.50 to 1.9) | |
| Total tibial cartilage | |||||
| Secretome | 2 (2–2) | 0.001 | HA | < 0.001 | 2.8 (1.3 to 4.3) |
| Hyaluronic | 5 (3–7) | MSC | 0.2 | 2.0 (0.51 to 3.5) | |
| MSC | 3.5 (3–4) | Secretome | 0.09 | 0.83 (–0.60 to 2.3) | |
| Total patella cartilage | |||||
| Secretome | 0 (0–1) | 0.1 | HA | 0.007 | 1.2 (–0.01 to 2.4) |
| Hyaluronic acid | 1.5 (0–3) | MSC | 0.2 | 0.83 (–0.40 to 2.1) | |
| MSC | 1 (0–3) | Secretome | 0.4 | 0.33 (–0.90 to 1.6) | |
| a Median (min–max). b Kruskal–Wallis test. c Mann–Whitney U-test. |
|||||
On the microscopic scoring analysis, the total microscopic OARSI scores of the secretome group showed a lower score (26, SD 4.2) compared with the hyaluronic acid group (37, SD 6.4) and the MCS group (28, SD 4.6). There was a significant difference in the total microscopic score of the secretome group (p = 0.007) and MSC group (p = 0.02) when compared with the hyaluronic acid group as gold standard. However, secretome and MSC showed a non-significant difference (p = 1). As for the comparison of each component, the structure score (p = 0.03) and the interterritorial score (p = 0.007) showed significant differences among the 3 groups, while on the microscopic surface scoring, significant differences were found in the distal femur inferior side (contact with proximal tibial cartilage) (p = 0.009) and tibial surfaces (p = 0.04), similar to the macroscopic score (Tables 5 and 6).
| Parameter Samples’ group | Microscopic score a | p-value b | Post-hoc analysis | Mean difference (CI) | |
| vs group | p-value c | ||||
| Total microscopic score | |||||
| Secretome | 26 (4.2) | 0.005 | HA | 0.007 | 6.0 (0.15 to 12) |
| Hyaluronic acid | 37 (6.4) | MSC | 0.02 | 5.0 (–0.80 to 11) | |
| MSC | 28 (4.6) | Secretome | 1.0 | 1.0 (–4.8 to 6.8) | |
| Structural score | |||||
| Secretome | 8.6 (3.5) | 0.03 | HA | 0.03 | 6.0 (0.15 to 12) |
| Hyaluronic acid | 16 (4.6) | MSC | 0.2 | 5.0 (–0.80 to 11) | |
| MSC | 11 (3.6) | Secretome | 1.0 | 1.0 (–4.8 to 6.8) | |
| Density score | |||||
| Secretome | 3.7 (1.3) | 0.6 | HA | 1.0 | 0.33 (–1.3 to 2.0) |
| Hyaluronic acid | 4.0 (0.60) | MSC | 1.0 | 0.0 (–1.6 to 1.7) | |
| MSC | 4.3 (1.2) | Secretome | 1.0 | 0.33 (–1.3 to 2.0) | |
| Cloning cell score | |||||
| Secretome | 6.1 (1.9) | 0.1 | HA | 0.3 | 1.7 (–0.80 to 4.2) |
| Hyaluronic acid | 7.8 (1.1) | MSC | 0.3 | 1.7 (–0.80 to 4.2) | |
| MSC | 6.1 (1.9) | Secretome | 0.3 | 0.0 (–2.4 to 2.5) | |
| Interterritorial score | |||||
| Secretome | 1.6 (1.6) | 0.007 | HA | 0.006 | 3.3 (1.4 to 5.4) |
| Hyaluronic acid | 4.6 (1.6) | MSC | 0.2 | 1.7 (–0.20 to 3.6) | |
| MSC | 3.0 (0.60) | Secretome | 0.4 | 1.7 (–0.20 to 3.6) | |
| Tidemark score | |||||
| Secretome | 4.7 (1.0) | 0.5 | HA | 1.0 | 0.16 (–2.5 to 2.9) |
| Hyaluronic acid | 4.8 (1.8) | MSC | 0.8 | 1.2 (–1.5 to 3.9) | |
| MSC | 3.7 (2.1) | Secretome | 0.8 | –1.0 (–3.7 to 1.7) | |
| a Mean (SD) b One-way ANOVA test. c Kruskal–Wallis test. |
|||||
| Parameter Samples’ group | Microscopic score a | p-value b | Post-hoc analysis | Mean difference (CI) | |
| vs group | p-value c | ||||
| Total distal femur inferior side (contact with proximal tibial cartilage) | |||||
| Secretome | 8.8 (3.5) | 0.009 | HA | 0.02 | –5.0 (–9.2 to –0.7) |
| Hyaluronic acid | 14 (2.6) | MSC | 0.02 | 4.8 (0.60 to 9.1) | |
| MSC | 9.2 (2.7) | Secretome | 1.0 | 0.16 (–4.0 to 4.4) | |
| Total distal femur anterior side (contact with patellar cartilage) | |||||
| Secretome | 4.7 (2.1) | 0.8 | HA | 1.0 | –0.80 (–4.1 to 2.5) |
| Hyaluronic acid | 4.5 (1.0) | MSC | 1.0 | 0.16 (–3.1 to 3.5) | |
| MSC | 4.3 (3.2) | Secretome | 1.0 | 0.66 (–2.6 to 4.0) | |
| Total tibial cartilage | |||||
| Secretome | 8.5 (1.6) | 0.04 | HA | 0.05 | –5.1 (–10 to –0.0) |
| Hyaluronic acid | 14 (4.0) | MSC | 0.2 | 3.7 (–1.4 to 8.8) | |
| MSC | 9.8 (3.3) | Secretome | 1.0 | 1.5 (–3.6 to 6.6) | |
| Total patellar cartilage | |||||
| Secretome | 4.0 (1.4) | 0.6 | HA | 1.0 | –0.50 (–2.8 to 1.8) |
| Hyaluronic acid | 4.8 (0.5) | MSC | 1.0 | 0.83 (–1.5 to 3.2) | |
| MSC | 4.0 (1.4) | Secretome | 1.0 | –0.30 (–2.6 to 2.0) | |
| a Mean (SD) b One-way ANOVA test. c Kruskal–Wallis test. |
|||||
Meanwhile on microscopic surface scoring, there was no significant difference among the 3 groups in the distal femur anterior side (contact with patellar cartilage) (p = 0.8) and patellar score (p = 0.6) (Table 6).
Discussion
OARSI macroscopic scoring analysis
Our study showed there was a significant difference among the 3 groups in total macroscopic score of OARSI, score components, and each surface of the knee joint. The secretome group showed a better result, followed by the MSC group and the hyaluronic acid group. In research conducted by Colombini et al. (11) and Mancuso et al. (12) the secretome works on cartilage defects and promotes chondrocyte regeneration to replace damaged cells. According to Schneider et al. (13), the cellular matrix secretes proteins that act as regulators which will bind to the extracellular matrix and act as receptors for cell surface molecules, growth factors, and matrix metalloproteinases (MMP). Administration of secretomes containing many growth factors can bind to these receptors and stimulate cartilage regeneration. Waldstein et al. (10) reported that thrombospondin (TSP2) was known to be a cartilage regulator and bone differentiator secreted by MSC to induce proliferation through autocrine mechanisms.
Secretome and MSC have a positive effect on the cartilage structure, which may be explained by various growth hormones that can directly promote chondrocytes’ growth to regenerate cartilage, which is absent in the hyaluronic acid group. This finding is in accordance with Jeong et al. (6), who reported that TSP2 induces differentiation from chondroprogenitor cells that trigger cartilage regeneration and prevent chondrocyte hypertrophy. Injection of hyaluronic acid in OA patients does not produce a direct effect on cartilage regeneration as it produces reactive oxygen species (ROS) products, leading to cartilage degeneration and increased chondrocyte apoptosis (14). The growth of osteophytes, found to differ in all 3 groups, resulted from differences in inhibition mechanisms of osteophytic formation that occurred indirectly in all 3 groups.
OARSI microscopic scoring analysis
There was a significantly lower total microscopic score of OARSI in secretome and MSC treated groups compared with the hyaluronic acid group but no significant difference between the secretome group and the MSC group. This result is similar to the study of Khatab et al. (15), who found that secretome and MSC administration is better compared with hyaluronic acid in maintaining the structure of cartilage. The other factors which contribute to the better result of the secretome and MSC groups are that they have IL-6, prostaglandin E2 (PGE2), TNF stimulated gene 6 (TSG-6), and hepatocyte growth factor (HGF), which stimulates chondrocyte proliferation. This mechanism helps to maintain the integrity of the cartilage structure.
In our study, significant matrix differences in secretome and MSC when compared with hyaluronic acid may be caused by differences in mechanisms of chondrocyte regeneration. Studies by Iannone et al. (16) and Loeser (17) found that regeneration and degeneration of cartilage are influenced by chondrocyte cells and their extracellular matrix (18,19). Integrin is a protein in the extracellular matrix of cartilage that can regulate cartilage synthesis and degradation to modulate cells or extracellular matrix signaling (18).
In the evaluation of microscopic scores, the components of the density score, the cell multiplication score, and the tidemark score were obtained and showed no significant difference among the 3 groups. Our result supports the research by Waldstein et al. (10), who found that secretome, hyaluronic acid, and MSC had a positive effect in regenerating chondrocyte in knee OA subjects.
Our study also used tidemark condition assessment as a microscopic parameter to assess the effects of secretome, MSC, and hyaluronic acid on the tidemark layer of cartilage. In this study, there was no significant difference in tidemark among the 3 groups. In research conducted by Bail et al. (20), it was found that the growth factors present in the secretome and MSC help in maintaining the cellularity of cartilage cells (regeneration) in OA. With the reduced damage done to the cartilage, tidemark was found not to be suitable because there was no need for more effort to maintain the cartilage structure. In the study by Altman et al. (21), hyaluronic acid caused an effect on subchondral bones by suppressing MMP-13 and IL-6 mechanisms through CD44 bonds preventing abnormal metabolism of subchondral bone tissue. These mechanisms explain the absence of difference in tidemark condition among the 3 groups.
Host rejection process causing higher OARSI score in MSC than secretome
Despite no significant difference between the secretome group and MSC group, secretome showed better OARSI score compared with the MSC group, theoretically caused by host rejection process on the latter group. The study by Le Blanc et al. showed the existence of some immunogenicity of MSC as it expressed low class I MHC (major histocompatibility complex) (18). This is also in accordance with research conducted by Giannasi et al., who found that when stem cells are in the joint they secrete autoimmune mediators which cause an autoimmune reaction that could damage chondrocytes if the number increased (19).
Our study has several limitations. We did not determine the effective dose of secretome and MSC and therefore it is difficult with the expected results to compare with treatments in other studies (22). This study also did not determine the optimal frequency of intraarticular injection in knee OA therapy. Further research is necessary to investigate and study the appropriate therapeutic dosage for knee OA.
Our study is still in the animal trial phase, analyzing the histological images assessed by OARSI. The histological images are basic data used as reference for cartilage condition in OA and tissue engineering therapy effect compared with hyaluronic acid as the gold standard. The effect of histological changes on clinical outcome cannot be seen in this study as histological assessment is done at a certain time interval and requires that experimental animals are killed. Therefore, further and more complex studied are needed for clinical application on humans.
Conclusions
The application of secretome showed significantly higher cartilage regeneration in a sheep model based on OARSI scoring compared with hyaluronic acid, both macroscopically and microscopically. There is no significant difference between the secretome and MSC groups.
- Bowman S, Awad M E, Hamrick M W, Hunter M, Fulzele S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med 2018; 7(1): 6. doi: 10.1186/s40169-017-0180-3.
- Johansen M, Bahrt H, Altman R, Bartels E, Juhl C, Bliddal H, et al. Addressing controversies around intra-articular injections with hyaluronic acid in the treatment of osteoarthritis: meta-regression analyses of randomized trials. Osteoarthritis Cartilage 2015; 23: A47. doi: 10.1016/j.joca.2015.02.103.
- Lubis A M, Lubis V K. Adult bone marrow stem cells in cartilage therapy. Acta Med Indones 2012; 44(1): 62-8. PMID: 22451188.
- Newman B, Wallis G A. Is osteoarthritis a genetic disease? Clin Invest Med 2002; 25(4): 139-49. PMID: 12220042.
- Rani S, Ryan A E, Griffin M D, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 2015; 23(5): 812-23. doi: 10.1038/mt.2015.44/
- Jeong S Y, Kim D H, Ha J, Jin H J, Kwon S-J, Chang J W, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells 2013; 31(10): 2136-48. doi: 10.1002/stem.1471/
- Festing M F W. Randomized block experimental designs can increase the power and reproducibility of laboratory animal experiments. ILAR J 2014; 55(3): 472-6. doi: 10.1093/ilar/ilu045/
- Liu H, Ding J, Wang J, Wang Y, Yang M, Zhang Y, et al. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. Shi X-M, editor. PLoS One 2015; 10(3): e0120596. doi: 10.1371/journal.pone.0120596.
- Kraus V B, Huebner J L, DeGroot J, Bendele A. The OARSI histopathology initiative: recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage 2010; 18: S35-52. doi: 10.1016/j.joca.2010.04.015.
- Waldstein W, Perino G, Gilbert S L, Maher S A, Windhager R, Boettner F. OARSI osteoarthritis cartilage histopathology assessment system: a biomechanical evaluation in the human knee. J Orthop Res 2016; 34(1): 135-40. doi: 10.1002/jor.23010.
- Colombini A, Perucca Orfei C, Kouroupis D, Ragni E, De Luca P, Viganò M, et al. Mesenchymal stem cells in the treatment of articular cartilage degeneration: new biological insights for an old-timer cell. Cytotherapy 2019; 21(12): 1179-97. doi: 10.1016/j.jcyt.2019.10.004.
- Mancuso P, Raman S, Glynn A, Barry F, Murphy J M. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol 2019; 7: 9. doi: 10.3389/fbioe.2019.00009.
- Schneider M C, Barnes C A, Bryant S J. Characterization of the chondrocyte secretome in photoclickable poly(ethylene glycol) hydrogels. Biotechnol Bioeng 2017; 114(9): 2096-108. doi: 10.1002/bit.26320.
- Kim H J, Park J-S. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages. Dev Reprod 2017; 21(1): 1-10. doi: 10.12717/DR.2017.21.1.001.
- Khatab S, van Osch G, Kops N, Bastiaansen-Jenniskens Y, Bos P, Verhaar J, et al. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur Cells Mater 2018; 36: 218-30. doi: 10.22203/eCM.v036a16.
- Iannone M, Ventre M, Formisano L, Casalino L, Patriarca E J, Netti P A. Nanoengineered surfaces for focal adhesion guidance trigger mesenchymal stem cell self-organization and tenogenesis. Nano Lett 2015; 15(3): 1517-25. doi: 10.1021/nl503737k.
- Loeser R F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol 2014; 39: 11-16. doi: 10.1016/j.matbio.2014.08.007.
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003; 31(10): 890-6. doi: 10.1016/s0301-472x(03)00110-3.
- Giannasi C, Niada S, Della Morte E, Casati S, Orioli M, Gualerzi A, et al. Towards secretome standardization: identifying key ingredients of MSC-derived therapeutic cocktail. Paspaliaris V, editor. Stem Cells Int 2021; 2021: 1-13. doi: 10.1155/2021/3086122.
- Bail H, Klein P, Kolbeck S, Krummrey G, Weiler A, Schmidmaier G, et al. Systemic application of growth hormone enhances the early healing phase of osteochondral defects: a preliminary study in micropigs. Bone 2003; 32(5): 457-67. doi: 10.1016/s875-63282(03)00051-6.
- Altman R, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 2015; 16(1): 321. doi: 10.1186/s12891-015-0775-z.
- Maheshwer B, Polce E M, Paul K, Williams B T, Wolfson T S, Yanke A, et al. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: a systematic review and meta-analysis. Arthroscopy 2021; 37(1): 362-78. doi: 10.1016/j.arthro.2020.05.037.
Appendix