In old men Scheuermann’s disease is not associated with neck or back pain: a Swedish cohort study
Anette JÖNSSON 1, Henrik DAMM 1, Mehrsa HOFVANDER 1, Björn E ROSENGREN 1, Inga REDLUND-JOHNELL 1,a, Claes OHLSSON 2, Dan MELLSTRÖM 3, and Magnus K KARLSSON 1
1 Clinical and Molecular Osteoporosis Research Unit, Departments of Orthopedics and Clinical Sciences, Lund University, Skåne University Hospital, Malmö; 2 Sahlgrenska Osteoporosis Centre, Center for Bone Research, Departments of Internal Medicine and Geriatrics, Gothenburg University, Sahlgrenska University Hospital, Göteborg; 3 Departments of Internal Medicine and Geriatrics, Gothenburg University, Sahlgrenska University Hospital, Göteborg, Sweden
a Diseased 2022-09-01
Background and purpose — Scheuermann’s disease is characterized by kyphosis and frequently mild back pain. As the level of kyphosis may progress over time, also the level of pain may increase. We evaluated the prevalence of Scheuermann’s disease, and their pain, in Swedish elderly men.
Patients and methods — The Osteoporotic Fractures in Men (MrOS) Study Sweden (n = 3,014) is a population-based prospective observational study of community-living men aged 69–81 years. At baseline, participants answered a questionnaire including history of neck/back pain during the preceding year and characteristics of any pain (severity, sciatica, and neurological deficits). Lateral thoracic/lumbar spine radiographs were taken of 1,453 men. We included the 1,417 men with readable radiographs. Scheuermann’s disease was defined as 3 or more consecutive vertebrae with > 5° wedging with no other explanation for the deformity.
Results — 92 of the 1,417 men (6.5%, 95% confidence interval 5.3–7.9) had Scheuermann’s disease. 31% of men with and 31% without Scheuermann’s disease reported neck pain (P = 0.90) and 51% with and 55% without the disease reported back pain (P = 0.4). Among men with Scheuermann’s disease and back pain, none reported severe pain, 57% moderate, and 43% mild, compared with 7%, 50%, and 44% in those without Scheuermann’s disease (P = 0.2). In those with Scheuermann’s disease 63% reported no sciatica, 15% sciatica without neurological deficits, and 22% sciatica with neurological deficits, compared with 56%, 16%, and 28% in those without the disease (P = 0.6).
Conclusion — The prevalence of Scheuermann’s disease in elderly Swedish men is between 5.3% and 7.9%. The condition seems at this age not to be associated with neck or back pain.
Citation: Acta Orthopaedica 2023; 94: 236–242. DOI: https://doi.org/10.2340/17453674.2023.12358.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-05-25. Accepted: 2023-03-16. Published: 2023-05-10.
Correspondence: anette.r.jonsson@gmail.com
CO, DM, and MK collected the data and financed the study; IRJ read all radiographs; AJ, HD, MK, and BJ validated the data; AJ, BJ, and MK designed the study; AJ, HD, MH, and MK conducted all calculations and created all tables and figures; AJ, HD, MH, and MK wrote the first draft of the manuscript; all authors reviewed the manuscript and accepted it in the final form.
Handling co-editors: Ivan Hvid and Robin Christensen
Acta thanks Jens Ivar Brox and Federico Canavese for help with peer review of this study.
Scheuermann’s disease is reported with a prevalence between 0.4% and 25.0% (1-3). The disease becomes usually apparent in puberty (4), when a hyperkyphosis develops in the thoracic spine, with or without a compensatory hyperlordosis in the lumbar spine (5). The disease is linked to a dominant autosomal genetic component, with a heritability of 74% (6), and is reported with no sex difference (2), and up to double male prevalence (7). Increased release of growth hormone, defective formation of collagen fibrils with subsequent weakening of the vertebral endplate, juvenile trauma, vitamin A deficiency, and epiphysitis, may all be involved (8) in the defective growth of the cartilage endplate due to disorganized enchondral ossification (5). The defect results in a wedging of ≥ 3 adjacent vertebrae, but other radiographic findings such as Schmorl’s nodes, vertebral endplate irregularities, and disc space narrowing are also common (9).
Scheuermann’s disease is at younger ages often associated with mild neck and/or back pain (10), in most patients addressed by no therapy, analgesics, or physiotherapy (10). Corrective surgery is an option only in those with extensive deformity and/or severe pain (11). The prognosis regarding neck and/or back pain and quality of life is usually reported to be benign (4), but the kyphosis may progress (12), then being compensated by hyperlordosis in the lumbar and/or cervical spine. These changes may lead to an increased risk of neck and/or back pain (13), while other studies refute this (14).
The aim of our study was to (i) identify prevalence of the disease, and (ii) evaluate if men with the disease have more often or more severe neck/back pain than men without the disease.
Patients and methods
Data source
The data in our study was retrieved from the Osteoporotic Fractures in Men (MrOS) Sweden Study (15) which is a multicenter study that includes 3,014 men aged 69-–81 years, evaluated in medical centers in Gothenburg (n = 1,010), Malmö (n = 1,005), and Uppsala (n = 999) (Figure 1). The primary aim of this prospective observational study is to evaluate risk factors for osteoporosis and fractures, where details of the study have been published earlier (16). The men were randomly selected from the national population register. To be included, the men had to have a contact address, be able to walk without assistance, and be without bilateral hip replacements. The attendance rate was 45% (16). The study is reported according to the STROBE guidelines.
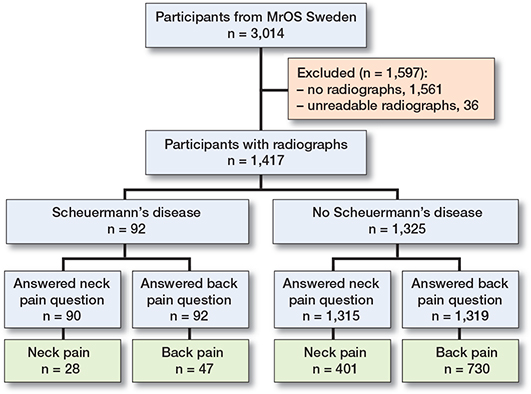
Figure 1. Flow-chart of study participants.
Outcomes
At baseline, the 3,014 participants answered a questionnaire that included questions on lifestyle, medical conditions, and if, during the preceding 12 months, the individuals had experienced any period with neck and/or back pain. Back pain was defined as pain in the thoracic or lumbar region and neck pain in the neck region. Those who had experienced pain also answered questions regarding pain severity (mild, moderate, or severe), and if the pain was associated with radiating pain and/or neurological deficits, defined as sensory or motor function deficits in the extremities. Men with back pain also registered the frequency of the pain (rarely, some of the time, most of the time, constantly) and if the pain was associated with any restriction in activities of daily living (ADL) (i) in bending down, (ii) in lifting a 5 kg object, (iii) in reaching objects above their heads, (iv) in putting on socks, (v) in getting in and out of a car, (vi) in sitting in a chair for ≥ 30 minutes, and (vii) in any ADL restriction for > 2 hours/day.
Height and weight were measured using standard equipment (16). We performed 2 consecutive measurements of height in the same session and used the average of these. In the case of a discrepancy of ≥ 5 mm between the measurements, we performed a third measurement, and used the average of the 2 nearest values. Body mass index (BMI) was calculated.
Radiological evaluation
A senior radiologist (IR-J), blinded to the clinical presentation of the individuals, evaluated the radiographs according to the semi-quantitative method proposed by Genant et al. (17). This method involved assessing the visual shape of the vertebra, i.e., if there is height reduction in the anterior, middle, and/or posterior part of the vertebral body. The radiographs in which Scheuermann’s disease was possible, i.e., with height reduction in the anterior part in at least 3 vertebrae, were identified. Cases where other reasons for the deformity were apparent, such as congenital abnormality, hemi-vertebrae, degenerative abnormalities, and fractures were then discarded, after which the remaining cases were objectively measured with a protractor. Only those where the objective protractor measurement verified that the wedge shape of at least 3 consecutive vertebrae exceeded 5° were classified as Scheuermann’s disease (Figure 1). The number of affected vertebrae, localization of affected vertebrae, kyphosis angle of the most wedge-shaped vertebra, kyphosis angle over all affected vertebrae, kyphosis from upper endplate thoracic vertebra 4 to lower endplate thoracic vertebra 12 (T4 to T12), and lordosis from upper endplate lumbar vertebra 1 to lower endplate lumbar vertebra 5 (L1 to L5) in those with diagnosed Scheuermann’s disease were registered by 1 of the authors (AJ).
The diagnosis of Scheuermann’s disease was made only with help of the radiographs. We have no information on whether at the time of this examination the participants had already been diagnosed with the disease, and if so, at what age the diagnosis was stated, and whether any of them had been exposed to brace treatment. None of the participants had been exposed to corrective surgery for their deformity.
Statistics
We used the Statistical Package for the Social Sciences (SPSS version 27, IBM, Armonk, NY, USA) for statistical analyses. Data is presented as numbers (n), proportions (%), means with standard deviations (SD), or mean with 95% confidence intervals (CI). For group comparisons we used the chi-square test (categorical data) and Student’s t-test between means (continuous data). We considered P < 0.05 as a statistically significant difference.
Ethics, data sharing, funding, and disclosures
The study was approved by the ethics committees in Lund (Registration number: LU 693-00) and Gothenburg and the radiological committee at each of the hospitals. All participants gave written consent before study start. Data can be obtained on request from the corresponding author. Financial support was received from ALF, Region Skåne (FoU), Skåne University Hospital, Herman Järnhardt, and Österlund Foundations. None of the authors has any conflict of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.12358
Results
At study start, all participants in Malmö and the second half of those evfaluated in Gothenburg were invited to have a standard radiological lateral examination of the thoracic and lumbar spine. 1,453 men (988 in Malmö and 465 in Gothenburg) accepted. 36 of the radiographs were not readable from the fourth thoracic (T4) to the fifth lumbar (L5) vertebrae. These patients were excluded (Figure 1).
92 of the 1,417 men (6.5%, CI 5.3–7.9) had Scheuermann’s disease. Background data on men with and without Scheuermann’s disease is presented in Table 1, and data in men with neck pain, or, respectively, back pain in Table 2. We found similar ADL restrictions in men with and without Scheuermann’s disease (Table 2). Radiographic characteristics in the men with Scheuermann’s disease are depicted in Figures 2–5.
| Factor | Men with Scheuermann’s disease | Men without Scheuermann’s disease | ||
| Missing data | (n = 92) | Missing data | (n = 1,325) | |
| Anthropometry | ||||
| Age a | 0 | 75 (3.3) | 0 | 75 (3.0) |
| Height a | 0 | 1.75 (0.7) | 0 | 1.75 (0.6) |
| Weight a | 0 | 83 (12) | 0 | 81 (12) |
| Body mass index a | 0 | 26.9 (3.0) | 0 | 26.4 (3.6) |
| Lifestyle factors | ||||
| Daily normal routine walking distance (km) a | 2 | 1.3 (1.5) | 26 | 1.2 (1.5) |
| Drinking > 12 units of alcohol the last year | 0 | 87 | 0 | 1,135 (86) |
| Current smoker | 26 | 6 | 413 | 143 (16) |
| Diagnosis | ||||
| Diabetes | 0 | 4 | 2 | 127 (10) |
| Hyperthyreosis | 0 | 3 | 8 | 13 (1) |
| Hypothyreosis | 2 | 2 | 10 | 39 (3) |
| Osteoporosis | 0 | 0 | 4 | 31 (2) |
| Stroke | 3 | 3 | 5 | 90 (7 ) |
| Parkinson’s disease | 1 | 1 | 4 | 12 (1) |
| High blood pressure | 0 | 36 | 8 | 473 (36) |
| Myocardial infarction | 2 | 11 | 10 | 179 (12) |
| Angina | 2 | 13 | 12 | 199 (15) |
| Congestive heart failure | 2 | 12 | 24 | 113 (9) |
| COPD, asthma or emphysema | 1 | 5 | 11 | 105 (8) |
| Prostatitis | 3 | 9 | 20 | 168 (13) |
| Arthritis or gout | 1 | 19 | 19 | 246 (19) |
| Ever had cancer | 0 | 14 | 3 | 211 (16) |
| Medication | ||||
| Use of ibuprofen 3 or more times a week | 1 | 1 | 9 | 8 (1) |
| Use of naproxen/diklofenak 3 or more times a week | 1 | 4 | 11 | 27 (2) |
| Currently using steroids | 0 | 3 | 5 | 72 (5) |
| Ever used medication for osteoporosis | 0 | 1 | 1 | 20 (2) |
| a mean (SD) | ||||
| Men with Scheuermann’s disease | Men without Scheuermann’s disease | Percentage point difference (CI) | P value a | |||
| Missing data | (n = 92) | Missing data | (n = 1,325) | |||
| Factor | ||||||
| Among all men | ||||||
| Neck pain | 2 | 28 (31) | 10 | 401 (30) | 1 (–9 to 11) | 0.9 |
| Back pain | 0 | 47 (51) | 6 | 730 (55) | –4 (–15 to 6) | 0.4 |
| Among men with neck pain | ||||||
| Severity of neck pain | 0 | 0 | 0.02 | |||
| Mild | 7 (25) | 144 (36) | –11 (–28 to 5) | |||
| Moderate | 18 (64) | 153 (38) | 26 (8 to 45) | |||
| Severe | 3 (11) | 104 (26) | –15 (–27 to –3) | |||
| Neck pain characteristics | 0 | 0 | 0.4 | |||
| Neck pain, no radiating pain, neurological deficits | 14 (50) | 239 (60) | –10 (–29 to 10) | |||
| Neck pain, radiating pain, no neurological deficits | 7 (25) | 64 (16) | 9 (–7 to 25) | |||
| Neck pain, radiating pain, no neurological deficits | 7 (25) | 98 (24) | 1 (–16 to 17) | |||
| Among men with back pain | ||||||
| Severity of back pain | 0 | 6 | 0.2 | |||
| Mild | 20 (43) | 315 (44) | –1 (–16 to 14) | |||
| Moderate | 27 (57) | 359 (50) | 8 (–7 to 22) | |||
| Severe | 0 (0) | 50 (7) | –7 (–9 to -5) | |||
| Back pain characteristics | 1 | 7 | 0.6 | |||
| Back pain, no radiating pain, no neurological deficits | 29 (63) | 403 (56) | 7 (–7 to 22) | |||
| Back pain, radiating pain, no neurological deficits | 7 (15) | 116 (16) | –1 (–12 to 10) | |||
| Back pain, radiating pain, neurological deficits | 10 (22) | 204 (28) | –6 (–19 to 6) | |||
| Frequency of the back pain | 0 | 2 | 0.04 | |||
| Rarely | 7 (15) | 136 (19) | –4 (–14 to 7) | |||
| Some of the time | 36 (77) | 413 (57) | 20 (7 to 32) | |||
| Most of the time | 3 (6) | 119 (16) | –10 (–17 to –2) | |||
| Constantly | 1 (2) | 60 (8) | –6 (–11 to –2) | |||
| Restrictions in activities of daily living (ADL) due to the back pain | ||||||
| Any limitation >2 hours/day | 1 | 13 (28) | 6 | 218 (30) | –2 (–15 to 12) | 0.8 |
| Difficulty bending down to pick up light objects | 1 | 11 (24) | 2 | 271 (37) | –13 (–26 to 0) | 0.07 |
| Difficulty lifting a 5 kg object from the floor | 1 | 6 (13) | 3 | 120 (17) | –3 (–14 to 7) | 0.5 |
| Reaching above head to grab an item | 1 | 7 (15) | 2 | 58 (8) | 7 (–3 to 18) | 0.09 |
| Difficulty putting socks on either foot | 0 | 12 (26) | 1 | 252 (35) | –9 (–22 to 4) | 0.2 |
| Difficulty getting in or out of front seat of a car | 0 | 14 (30) | 1 | 249 (34) | –4 (–18 to 9) | 0.5 |
| Difficulty sitting in a chair for 30 minutes | 1 | 6 (13) | 1 | 125 (17) | –4 (–14 to 6) | 0.5 |
| a Chi-square test | ||||||
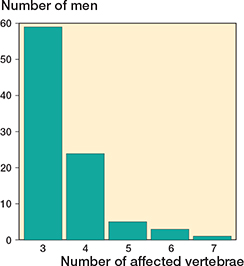
Figure 2. Number of individuals with different numbers of vertebrae affected in the 92 men with Scheuermann’s disease.
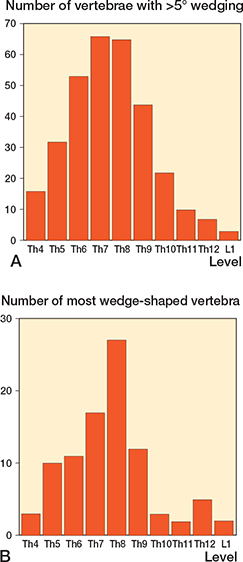
Figure 3. Distribution of the 323 vertebrae with >5° wedge angle (A) and distribution of the most wedge-shaped vertebra (B) in the 92 men with Scheuermann’s disease in the thoracic (Th) and lumbar (L) spine.
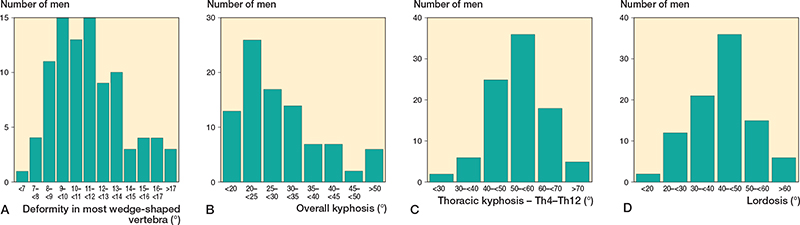
Figure 4. Number of men with different severity of the most deformed vertebra (A), number of men with different s kyphosis over all affected vertebrae (B), number of men with different thoracic kyphosis (Th4 to Th12) (C) and number of men with different lordosis (L1 to L5) (D) in the 92 men with Scheuermann’s disease.
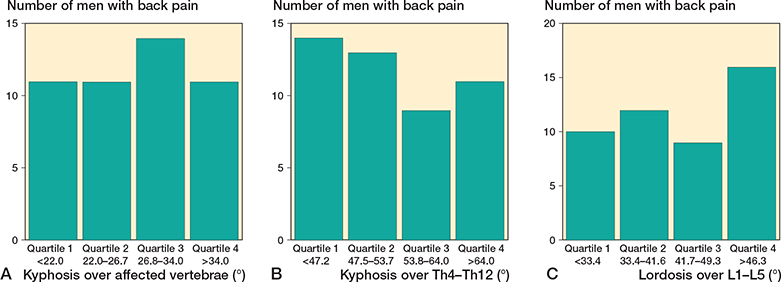
Figure 5. Number of individuals with back pain in relation to quartiles of deformity over the affected vertebrae (A), quartiles of kyphosis in thoracic spine (Th4 to Th12) (B), and quartiles of lordosis in lumbar spine (L1 to L5) (C). There was no statistical difference when comparing proportion of individuals with back pain in quartiles of deformity over the affected segments (p = 0.8), quartiles of thoracic kyphosis (p = 0.5) or quartiles of lumbar lordosis (p = 0.2)
Among the men who answered the questions on neck pain, 28/90 (31%) with and 401/1,315 (31%) without Scheuermann’s disease reported neck pain during the preceding 12 months (P = 0.9) (Table 2). Among the men with neck pain, we found similar severity, prevalence of rhizopathy, and prevalence of neurological deficits in men with and without Scheuermann’s disease (all p > 0.05) (Table 2). The most wedge-shaped vertebra in men with Scheuermann’s disease and neck pain was 10° (SD 3.2) and in men with Scheuermann’s disease but without neck pain 11° (SD 2.7) (P = 0. 6), the kyphosis over all wedge-shaped vertebrae was 33° (SD 12) and 28° (SD 10), respectively (P = 0.09), the thoracic kyphosis (T4 to T12) 60° (SD 11) and 53° (SD 11), respectively (P = 0.2), and the lumbar lordosis (L1 to L5) 47° (SD 11) and 40° (SD 11) respectively (P = 0.01).
Among the men who answered the questions on back pain, 47/92 (51%) with and 715/1,319 (55%) without Scheuermann’s disease reported back pain during the 12 months preceding the evaluation (P = 0.4) (Table 2). Among men with back pain, morbidity, prevalence of sciatica, and prevalence of neurological deficits were similar in individuals with and without Scheuermann’s disease (all P > 0.05) (Table 2). Fewer men with Scheuermann’s disease had back pain most of the time/constantly compared with men without Scheuermann’s disease (P < 0.05) (Table 2). The most wedge-shaped vertebra in men with Scheuermann’s disease and back pain was 11° (SD 2.7) and in men with Scheuermann’s disease but without back pain 10° (SD 2.9) (P = 0.5), the kyphosis over all wedgeshaped vertebrae was 30° (SD 10) and 30° (SD 12), respectively (P = 0.9), the thoracic kyphosis (T4 to T12) 52° (SD 12) and 55° (SD 10), respectively (P = 0.3), and the lumbar lordosis (L1 to L5) 44° (SD 12) and 40° (SD 10), respectively (P = 0.1).
Discussion
The prevalence of Scheuermann’s disease in elderly Swedish men is 6.5%. This should be compared with the prevalence reported in the literature ranging between 0.4% and 25.0% (1-3,11). The lowest prevalence (3) has been reported in young individuals, thereby hypothetically missing individuals who develop the disease later in life. A twin study that reported a prevalence of 2.8% used self-reported data on diagnosis (6), thereby probably missing patients without symptoms (11). Radiographic studies usually report higher prevalence (2), in one as high as 25% (1). However, results from radiographic studies may also be discussed. The normal wedge-shaped vertebral bodies in the thoraco-lumbar region may be misclassified as Scheuermann’s disease, as may multiple osteoporotic fractures, congenital abnormalities, hemi-vertebrae, or degenerative abnormalities (17). The use of different definitions of Scheuermann’s disease may also result in different prevalence data (18).
Scheuermann’s disease in old men seems not to be associated with more neck or back pain, pain severity, rhizopathy, or neurological deficits. The 1-year prevalence of neck pain in the general populations is reported as between 30% and 50% (19,20). We have, however, not been able to identify any neck pain prevalence study in individuals with Scheuermann’s disease. The 1-year prevalence of neck pain in men with Scheuermann’s disease in our cohort was 31%, similar to that in men without the disease, similar to the prevalence in Greeks aged 15–65 years (1-year neck pain prevalence of 29%) (19), and similar to a Spanish population > 70 years (1-year neck pain prevalence of 29%) (20). We also found greater lumbar lordosis in men with Scheuermann’s disease and neck pain than in men with Scheuermann’s disease but without neck pain. We speculate that the compensatory lordosis in the cervical spine may also have been greater in the men with neck pain, being 1 possible explanation for this, but as we have no cervical radiographs, we could not evaluate this hypothesis. We acknowledge that the findings could occur due to chance.
We found similar back pain prevalence when comparing men with and without Scheuermann’s disease. Other studies have reported a 1-year prevalence of 45% of lower back pain in individuals > 70 (21) and 45% in men between the ages of 69 and 81 (22). The higher prevalence in our study could be explained by the inclusion of thoracic pain in the back pain group, while cited studies included only lower back pain (22). It is therefore of interest to find a reported 1-year back and thoracic pain prevalence of 59% in individuals over age 65 (23). Our data further opposes data by Ristolainen et al., who reported that patients with Scheuermann’s disease have more than double the risk of constant back pain of those without the disease (13).
We found further that, among patients with back pain, the severity was similar in men with and without Scheuermann’s disease, being lower than being reported in individuals with back pain aged 45–91, where 23% had mild, 36% moderate, and 41% severe pain (24). However, even if studies imply that individuals with Scheuermann’s disease have no more severe back pain than patients without Scheuermann’s disease (14), others oppose this view (4). As pain severity data are subjective as well as context specific and most studies do not have a control population, it is difficult to compare data across populations. The similarity in ADL restrictions and the prevalence of severe back pain when comparing men with and without Scheuermann’s disease in our study strengthens the view that elderly men with Scheuermann’s disease have no more severe back pain than the general population. We found fewer men with Scheuermann’s disease who reported back pain most of the time or constantly and fewer who reported severe neck pain than old men without the disease. We speculate that as physical activity is inversely related to back and neck pain, and men with Scheuermann’s disease are usually prescribed physical activity, they may be more physically active than the general population. However, our findings could also occur due to chance.
We have been unable to identify any study on the prevalence of rhizopathy/neurological deficit in elderly men with Scheuermann’s disease. The prevalence of sciatica in individuals aged > 65 with back pain has in another population been reported at 33% (25). This is lower than the prevalence in our study. However, in our cohort we found no difference in prevalence of rhizopathy/neurological deficit in elderly men with and without Scheuermann’s disease.
We also found no indications that elderly men with Scheuermann’s disease and neck and/or back pain have greater thoracic deformity than elderly men with Scheuermann’s disease but no pain. It should, then, be noted that our study included individuals with a kyphosis over the affected segments exceeding 50° and a thoracic kyphosis exceeding 70°.
Our findings suggest that there is no need to change current recommendations (usually no restrictions but with the recommendation of being physically active) as these men have in the long-term perspective no higher morbidity of neck or back pain, rhizopathy, or neurological deficits in the extremities and no more restricted ADL function than men without the diseases. It is further no need to specifically follow men with Scheuermann’s disease as they even if having had the disease for over 5 decades have no higher morbidity than men without the disease.
Strengths and limitations
The strengths of our study include the large population-based sample, the high attendance rate, and a study design with a control population with the same ethnicity and with data collected in the same way during the same period as the target population. Nevertheless, there may still be selection bias. Unhealthy and sick men may have declined to participate. We have, however, no indication that there were different proportions of those who declined in men with and without Scheuermann’s disease. Study weaknesses include problems with selection and statistical uncertainty in the prevalence estimation, the inability to draw conclusions in non-ambulatory elderly, those with bilateral hip replacement, men of other age spans, men with other ethnic background, or men living in countries other than Sweden. It would also have been advantageous to have women included as there may exist sex differences in prevalence and severity of the disease. Furthermore, the study design makes it impossible to establish temporal or causal relationships. The retrospective study design includes a risk of recall bias. In addition, it would have been advantageous to have data on the number and duration of pain episodes, frequency of associated symptoms, as well as recurrence rate, and for a greater number of individuals with Scheuermann’s disease to have been included, to be able to conduct subgroup evaluations, such as grouping outcomes according to type of underlying disorder or if osteoporosis was present or not. It would also have been of great interest to have surgically treated patients included so as to be able to evaluate how operated patients did progress, as well as information on brace treatment because the SOSORT (International Society on Scoliosis Orthopaedic and Rehabilitation Treatment) panel of experts recommended when focusing on conservative treatment of idiopathic and Scheuermann’s kyphosis the use of rigid braces and physiotherapy to correct thoracic hyperkyphosis during adolescence (26).
Conclusion
The prevalence of Scheuermann’s disease in elderly Swedish men is 6.5%. At this age, the condition seems not to be a risk factor for neck and/or back pain, morbidity, rhizopathy, or neurological deficits. We found no indication that elderly men with Scheuermann’s disease and back pain had greater deformity of the Scheuermann kyphosis than elderly men with the disease but no back pain.
- Gaudé M, Chapurlat R, Pialat J B, Szulc P. Long term prognosis of Scheuermann’s disease: the association with fragility fracture—the MINOS cohort. Bone 2018; 117: 116-22. doi: 10.1016/j.bone.2018.09.016.
- Armbrecht G, Felsenberg D, Ganswindt M, Lunt M, Kaptoge S K, Abendroth K, et al. Vertebral Scheuermann’s disease in Europe: prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Osteoporos Int 2015; 26: 2509-19. doi: 10.1007/s00198-015-3170-6.
- Nissinen M. Spinal posture during pubertal growth. Acta Paediatr 1995; 84: 308-12. doi: 10.1111/j.1651-2227.1995.tb13634.x.
- Murray P M, Weinstein S L, Spratt K F. The natural history and long-term follow-up of Scheuermann kyphosis. J Bone Joint Surg Am 1993; 75: 236-48. doi: 10.2106/00004623-199302000-00011.
- Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine 2014; 81: 209-14. doi: 10.1016/j.jbspin.2013.11.012.
- Damborg F, Engell V, Andersen M, Kyvik K O, Thomsen K. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. J Bone Joint Surg Am 2006; 88: 2133-6. doi: 10.2106/JBJS.E.01302.
- Damborg F, Engell V, Nielsen J, Kyvik K O, Andersen M, Thomsen K. Genetic epidemiology of Scheuermann’s disease. Acta Orthop 2011; 82: 602-5, doi: 10.3109/17453674.2011.618919.
- Bezalel T, Carmeli E, Been E, Kalichman L. Scheuermann’s disease: current diagnosis and treatment approach. J Back Musculoskelet Rehabil 2014; 27: 383-90. doi: 10.3233/BMR-140483.
- Lowe T G. Scheuermann disease. J Bone Joint Surg Am 1990; 72: 940-5. doi: 10.2106/00004623-199072060-00026.
- Weiss H R, Dieckmann J, Gerner H J. Effect of intensive rehabilitation on pain in patients with Scheuermann’s disease. Stud Health Technol Inform 2002; 88: 254-7. doi: 10.3233/978-1-60750-932-5-254.
- Huq S, Ehresman J, Cottrill E, Ahmed A K, Pennington Z, Westbroek E M, et al. Treatment approaches for Scheuermann kyphosis: a systematic review of historic and current management. J Neurosurg Spine 2019; 32: 235-47. doi: 10.3171/2019.8.SPINE19500.
- Ristolainen L, Kettunen J A, Kujala U M, Heinonen A, Schlenzka D. Progression of untreated mild thoracic Scheuermann’s kyphosis: radiographic and functional assessment after mean follow-up of 46 years. J Orthop Sci 2017; 22: 652-7. doi: 10.1016/j.jos.2017.03.009.
- Ristolainen L, Kettunen J A, Heliövaara M, Kujala U M, Heinonen A, Schlenzka D. Untreated Scheuermann’s disease: a 37-year follow-up study. Eur Spine J 2012; 21: 819-24. doi: 10.1007/s00586-011-2075-0.
- Bezalel T, Carmeli E, Kalichman L. Scheuermann’s disease: radiographic pathomorphology and association with clinical features. Asian Spine J 2019; 13: 86-95. doi: 10.31616/asj.2018.0025.
- Blank J B, Cawthon P M, Carrion-Petersen M L, Harper L, Johnson J P, Mitson E, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 2005; 26: 557-68. doi: 10.1016/j.cct.2005.05.005.
- Jutberger H, Lorentzon M, Barrett-Connor E, Johansson H, Kanis J A, Ljunggren O, et al. Smoking predicts incident fractures in elderly men: Mr OS Sweden. J Bone Miner Res 2010; 25: 1010-16 doi: 10.1359/jbmr.091112.
- Genant H K, Wu C Y, van Kuijk C, Nevitt M C. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993; 8: 1137-48. 10.1002/jbmr.5650080915.
- Makurthou A A, Oei L, El Saddy S, Breda S J, Castaño-Betancourt M C, Hofman A, et al. Scheuermann disease: evaluation of radiological criteria and population prevalence. Spine (Phila Pa 1976) 2013; 38: 1690-4 doi: 10.1097/BRS.0b013e31829ee8b7.
- Stranjalis G, Kalamatianos T, Stavrinou L C, Tsamandouraki K, Alamanos Y. Neck pain in a sample of Greek urban population (fifteen to sixty-five years): analysis according to personal and socioeconomic characteristics. Spine (Phila Pa 1976) 2011; 36: E1098-1104. doi: 10.1097/BRS.0b013e3182054add.
- Andersson H I, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain 1993; 9: 174-82 doi: 10.1097/00002508-199309000-00004.
- Reid M C, Williams C S, Gill T M. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci 2005; 60: 793-7. doi: 10.1093/gerona/60.6.793.
- Ghanei I, Rosengren B E, Hasserius R, Nilsson J, Mellström D, Ohlsson C, et al. The prevalence and severity of low back pain and associated symptoms in 3,009 old men. Eur Spine J 2014; 23: 814-20. doi: 10.1007/s00586-013-3139-0.
- Cecchi F, Debolini P, Lova R M, Macchi C, Bandinelli S, Bartali B, et al. Epidemiology of back pain in a representative cohort of Italian persons 65 years of age and older: the InCHIANTI study. Spine (Phila Pa 1976) 2006; 31: 1149-55. doi: 10.1097/01.brs.0000216606.24142.e1.
- Wong A, Hyde Z, Smith K, Flicker L, Atkinson D, Skeaf L, et al. Prevalence and sites of pain in remote-living older Aboriginal Australians, and associations with depressive symptoms and disability. Intern Med J 2020. doi: 10.1111/imj.14870 10.1111/imj.14870.
- Ludwig C, Luthy C, Allaz A F, Herrmann F R, Cedraschi C. The impact of low back pain on health-related quality of life in old age: results from a survey of a large sample of Swiss elders living in the community. Eur Spine J 2018; 27: 1157-65. doi: 10.1007/s00586-017-5427-6.
- de Mauroy J, Weiss H, Aulisa A, Aulisa L, Brox J, Durmala J, et al. 7th SOSORT consensus paper: conservative treatment of idiopathic & Scheuermann’s kyphosis. Scoliosis 2010; 5: 9. doi: 10.1186/1748-7161-5-9.