Article
Risk factors for revision due to prosthetic joint infection following total knee arthroplasty based on 62,087 knees in the Finnish Arthroplasty Register from 2014 to 2020
Hannes KEEMU 1,a, Kasperi J ALAKYLÄ 1,a, Riku KLÉN 2, Valtteri J PANULA 1, Mikko S VENÄLÄINEN 3,4, Jaason J HAAPAKOSKI 5, Antti P ESKELINEN 6, Konsta PAMILO 6, Jukka S KETTUNEN 7, Ari-Pekka PUHTO 8, Anna I VASARA 9, Laura L ELO 3, and Keijo T MÄKELÄ 1
1 Department of Orthopaedics and Traumatology, Turku University Hospital and University of Turku, Turku; 2 Turku PET Centre, University of Turku and Turku University Hospital, Turku; 3 Turku Bioscience Centre, University of Turku and Åbo Akademi University, Turku; 4 Department of Medical Physics, Turku University Hospital, Turku; 5 National Institute for Health and Welfare, Helsinki; 6 Coxa Hospital for Joint Replacement and Faculty of Medicine and Health Technologies, University of Tampere, Tampere; 7 Department of Orthopaedics and Traumatology, Kuopio University Hospital, Kuopio; 8 OYS Centre for Musculoskeletal Surgery, Oulu University Hospital, Oulu; 9 Department of Orthopaedics and Traumatology, Helsinki University Hospital and University of Helsinki, Finland
a Shared first authorship
Background and purpose — Periprosthetic joint infection (PJI) is the commonest reason for revision after total knee arthroplasty (TKA). We assessed the risk factors for revision due to PJI following TKA based on the Finnish Arthroplasty Register (FAR).
Patients and methods — We analyzed 62,087 primary condylar TKAs registered between June 2014 and February 2020 with revision for PJI as the endpoint. Cox proportional hazards regression was used to estimate hazard ratios (HR) with 95% confidence intervals (CI) for the first PJI revision using 25 potential patient- and surgical-related risk factors as covariates.
Results — 484 knees were revised for the first time during the first postoperative year because of PJI. The HRs for revision due to PJI in unadjusted analysis were 0.5 (0.4–0.6) for female sex, 0.7 (0.6–1.0) for BMI 25–29, and 1.6 (1.1–2.5) for BMI > 40 compared with BMI < 25, 4.0 (1.3–12) for preoperative fracture diagnosis compared with osteoarthritis, and 0.7 (0.5–0.9) for use of an antimicrobial incise drape. In adjusted analysis the HRs were 2.2 (1.4–3.5) for ASA class III–IV compared with class I, 1.7 (1.4–2.1) for intraoperative bleeding ≥ 100 mL, 1.4 (1.2–1.8) for use of a drain, 0.7 (0.5–1.0) for short duration of operation of 45–59 minutes, and 1.7 (1.3–2.3) for long operation duration > 120 min compared with 60–89 minutes, and 1.3 (1.0–1.8) for use of general anesthesia.
Conclusion — We found increased risk for revision due to PJI when no incise drape was used. The use of drainage also increased the risk. Specializing in performing TKA reduces operative time and thereby also the PJI rate.
Citation: Acta Orthopaedica 2023; 94: 215–223. DOI: https://doi.org/10.2340/17453674.2023.12307.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-06-23. Accepted: 2023-03-13. Published: 2023-05-03.
Correspondence: Hannes.Keemu@tyks.fi
HK, KA, and KM designed the study. RK and JH collected the data and VP edited the manuscript. KA, VP, and HK wrote the manuscript, and the analyses were done by MV and LE. KP, JK, AV, AE, and A-PP proofread the manuscript and provided meticulous comments. All authors have read and approved the final manuscript.
Handling co-editors: Eivind Witsø and Robin Christensen
Acta thanks Richard De Steiger and Johan Kärrholm for help with peer review of this study.
Prosthetic joint infection (PJI) is currently the commonest reason for revision after total knee arthroplasty (TKA) (1). PJI may have disastrous consequences, and revision as a result of this invariably reduces the patient’s quality of life (2). Several revision procedures are often required, with prolonged antibiotic treatment and hospital stay. The incidence of PJI after TKA varies from 0.5% to 3.3% (3,4). Male sex, severe obesity, rheumatoid arthritis, and fracture as an underlying diagnosis for TKA are known risk factors for PJI (5).
The number of TKAs overall is growing along with the rise in life expectancy (6,7). The incidence of TKA has increased steadily in all the Nordic countries, with the highest incidence in Finland (8). Presumably, a proportionate rise can be expected in the number of patients requiring revision due to PJI (9). The risk of PJI after total hip arthroplasty in the Nordic countries is increasing (10,11), and there is some evidence of a similar trend for TKA (12).
The content of the Finnish Arthroplasty Register (FAR) (1) was revised in 2014 to include new parameters such as BMI, anesthesia mode, use of kinematic alignment, use of an antimicrobial incise drape, and surgical approach. We have previously reported on risk factors for PJI after THA in Finland (13), but PJI risk factors after primary condylar TKA have not previously been assessed systematically. In 2020 the total number of primary TKAs in Finland was 12,692 and of revision TKAs 1,015 (including repeated surgery), 30% of which were for PJI (1). In 2020 there were 25 public hospitals performing most primary TKAs and 10 minor private hospitals. Our aim was to determine the risk factors for first revision due to PJI after primary TKA based on the revised FAR data.
Patients and methods
This study is reported according to REporting of studies Conducted using Observational Routinely-collected Data (RECORD) guidelines.
Finnish healthcare units are obliged to report arthroplasties to the FAR, which is maintained by the National Institute of Health and Welfare (14). Patient- and surgery-related data is reported on a standard online sheet completed during and immediately after the operation. Dates of death are obtained from the Population Register Centre. Primary and revision arthroplasties are linked to each other with a personal identification number. In 2020, the reporting coverage of all primary and revision TKAs to the FAR was 97% and 92%, respectively (1).
Definition of risk factors
The following 25 risk factors are included in the revised data contents of the FAR and were therefore included: age (≤ 62, 63–69, 70–75, > 75), sex, ASA class (I–IV), BMI (< 25, 25–29, 30–34, 35–40, > 40), preoperative diagnosis (primary osteoarthritis (OA), fracture, inflammatory arthritis, other), previous contributing operation (no, yes), simultaneous bilateral operation (no, yes), surgical approach (medial parapatellar, lateral, midvastus, subvastus, other), intraoperative bleeding (< 100, ≥ 100 mL), duration (< 45, 45–59, 60–89, 90–119, ≥ 120 minutes), anesthesia mode (spinal, epidural, general—the most invasive method was chosen if several), local infiltrative anesthesia (LIA) anesthesia (no, yes), complications during surgery (no, yes), fixation (cemented, uncemented, hybrid), drain (no, yes), kinematic alignment (no, yes), hospital annual volume (< 700, ≥ 700), surgeon’s level of education (orthopedic specialist, resident), assistant’s level of education (orthopedic specialist, resident), antimicrobial incise drape (no, yes), antibiotic prophylaxis (cefuroxime, clindamycin, vancomycin, other), antithrombotic prophylaxis (enoxaparin, other), antifibrinolytic medication (tranexamic acid, not used, other), mechanic antithrombotic prophylaxis (no, calf muscle pump), and surgical stocking (no, yes).
Definition of outcome
The survival endpoint was a revision for which the indication was marked as PJI and where 1 or more components were removed or exchanged within 1 year of the primary operation. Diagnosis of PJI was based on the patient’s clinical presentation and preoperative evaluation according to common diagnostic guidelines (15). Follow-up time varied between 0 and 5.7 years.
Statistics
Revisions for reasons other than PJI were censored at the time of operation, with a similar approach used for death. The overall mortality was 3.1%. We did not perform a competing risk analysis even though death can be considered a competing risk, as our main objective was to estimate relative risks for which the Cox regression model has been shown to be more accurate (16). There were 2,255 patients who had undergone a simultaneous bilateral TKA operation. Although these cannot be regarded as separate independent observations, we included them in the analysis due to their potential clinical importance (17). Potential risk factors were adjusted based on their relation to other factors on the directed acyclic graph (DAG) (Figure 1).
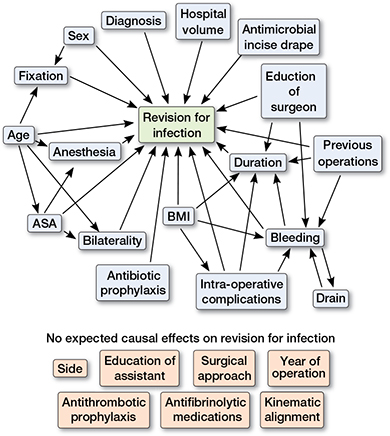
Figure 1. Directed acyclic graph (DAG) constructed under following assumptions:
- TKA “revision for infection” is dependent on “patient age,” “sex,” “bilaterality,” “ASA class,” “BMI,” “diagnosis,” “hospital volume,” “education of surgeon,” “bleeding,” “drain,” “duration,” “intraoperative complications,” “previous operations,” “antimicrobial incise drape,” “anesthesia,” “antibiotic prophylaxis,” and type of TKA “fixation.” Choice of “side,” “education of assistant,” “surgical approach,” “antithrombotic prophylaxis,” “antifibrinolytic medications,” and “kinematic alignment” are not expected to affect “revision for infection” due to clinical suspicion.
- “Fixation” is dependent on “age” and “sex” because older and female patients have probably received a cemented or hybrid TKA due to poorer bone quality. ASA class is partly dependent on age by definition. “Bilaterality” is dependent on “age” and “ASA class,” because both knees are seldom operated on in elderly or high ASA class patients.
- “BMI” may affect “duration” and “intraoperative complications” due to more difficult operation with high BMI. “Duration” and “bleeding” may be dependent on “education of surgeon” due to the experience factor. “Bleeding,” “duration,” and “previous operations” may be dependent on clinical basis.
- “Anesthesia” is dependent on “ASA class” and “age,” because general anesthesia is usually avoided in elderly patients. “Drain” is dependent on “bleeding” and vice versa.
The unadjusted rate of revision for PJI with 95% confidence intervals (CI) was estimated with Kaplan–Meier analysis. Based on previous literature and clinical practice, we performed a directed DAG analysis (Figure 1) to organize variables and their supposed relation to PJI and other variables. The unadjusted Cox proportional hazards regression model was then used to estimate potential risk factors and hazard ratios with CIs to the first revision due to PJI (Table 2). All variables with potential confounding bias were further scrutinized with Cox adjusted analysis in which adjustment was done according to the DAG analysis. Potential risk factors were adjusted based on their relation to other factors on the DAG. The following 8 risk factors were adjusted with associated covariates on the DAG: anesthesia (age and ASA class), ASA class (age), bilaterality (age and ASA class), intraoperative complications (BMI), bleeding (BMI, intraoperative complications, drain, previous operations, surgeon’s education), drain (bleeding), duration (BMI, intraoperative complications, bleeding, previous operations, surgeon’s education), and fixation (age and sex).
The proportional hazards (PH) assumption for Cox models was assessed from Kaplan–Meier curves graphically and with testing on scaled Schoenfeld residuals (18,19). A p-value of < 0.05 indicates non-proportional hazards. In the unadjusted model, antithrombotic-only medication did not fulfill the proportional risks assumption and was then analyzed for 2 time periods in both of which the proportional hazard assumption was fulfilled.
We also performed a sensitivity analysis with a “standard patient” to check for overfitting, including only patients with primary OA as a diagnosis, and cemented fixation only with a medial parapatellar approach. The data used for sensitivity analysis is listed as Supplementary data.
All the statistical analyses were done using R statistical computing environment version 3.6.0 (R Core Team, 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Ethics, funding, and disclosures
Ethical approval was obtained at the National Institute of Health and Welfare (Dnro THL/506/5.05.00/2016). Sharing of the raw data is not permitted. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. MSV reports funding from the Academy of Finland (grant number 322123). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.12307
Results
Data was extracted concerning 62,087 primary TKAs, of which 484 were revised for the first time for PJI during the first post-operative year between June 2014 and February 2020 (Figure 2 and Table 1, see Appendix). The number of TKA revisions for any reason was 925.
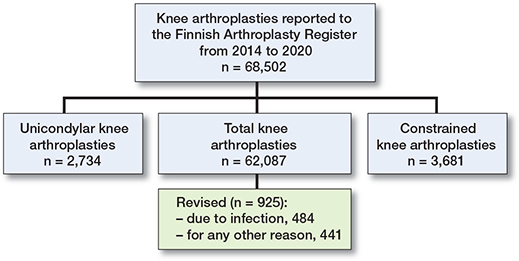
Figure 2. Flowchart of patients.
In our study cohort, most of the patients were women (39,146; 63%). All age groups were almost equally represented: ≤ 62, 63–69, 70–75, and > 75 years (17,478, 28%; 15,719, 25%; 14,150, 23%; and 14,740, 24%, respectively). Most of the treated patients had ASA class II (31,655, 51%) and BMI 25–29 or 30–34 (22,130, 38% and 18,471, 32%, respectively) and their primary diagnosis for TKA was OA (58,398, 94%). The operation was mostly performed under spinal anesthesia (56,793, 91%) and lasted 60–89 minutes (26,232, 48%). The commonest approach to the knee joint was medial parapatellar (60,533, 98%) and most of the patients received TKA with cemented fixation (59,238, 96%) without the use of a drain (47,396, 76%). (Table 1)
The overall Kaplan–Meier probability of no revision for PJI at the end of the study period with 0–5.7-year follow-up was 99% (CI 99.1–99.3).
Unadjusted analyses identified an increased risk of revision due to PJI for patients with a high BMI (BMI ≥ 35 vs. < 25, HR 1.4, CI 1.0–1.9). Patients with a BMI of 25–29 had a lower infection risk than patients with a BMI of < 25 (HR 0.7, CI 0.6–1.0). Male sex increased the risk of revision due to PJI (female vs. male HR 0.5, CI 0.4–0.6). Patients whose primary indication for TKA was fracture had a higher risk of revision due to PJI than patients with a diagnosis of OA (HR 4.0, CI 1.3–12). Also, high hospital volume (≥ 700) was associated with an increased risk of revision for PJI compared with low hospital volume (< 700) (HR 1.4, CI 1.2–1.7). Further, a decreased risk of revision for PJI was found with the use of an antimicrobial incise drape (HR 0.7, CI 0.5–0.9). A short duration of surgery < 60 minutes decreased the risk of revision for PJI compared with a duration of 60–89 minutes (< 45 vs. 60–89 minutes: HR 0.6, CI 0.4–1.0; and 45–59 vs. 60–89 minutes: HR 0.7, CI 0.6–1.0). A long duration of surgery was associated with a higher risk of revision for PJI than a duration of 60–89 minutes (≥ 120 vs. 60–89 minutes: HR 1.9, CI 1.4–2.5) (Table 2).
In adjusted analysis, advanced ASA class increased the risk of revision for PJI (ASA class III–IV vs. I: HR 2.2, CI 1.4–3.5). Intraoperative bleeding > 100 mL also increased the PJI risk (bleeding ≥ 100 mL vs. < 100 mL: HR 1.7, CI 1.4–2.1). Using a drain increased the risk of revision for PJI (HR 1.5, CI 1.2–1.8), as did the use of general anesthesia (HR 1.3, CI 1.0–1.8).
Long duration of surgery was associated with an increased risk of revision for PJI compared with an operative time of 60–89 minutes (≥120 vs. 60–89 minutes: HR 1.7, CI 1.3–2.3). We also found that a short duration of surgery of 45–59 minutes decreased the risk of revision for PJI compared with a duration of 60–89 minutes (HR 0.7, CI 0.5–1.0) (Table 3).
For the first 30 postoperative days after TKA, antithrombotic prophylaxis with a group of anticoagulants other than enoxaparin decreased the PJI risk compared with enoxaparin (HR 0.6, CI 0.4–0.9) (Table 4).
Discussion
We found that male sex, advanced ASA class, high BMI, bleeding > 100 mL, fracture diagnosis, general anesthesia, and duration of operation > 120 minutes compared with 60–89 minutes increased the risk of revision. In addition, we found that duration of operation of 45–59 minutes decreased the risk of revision compared with 60–89 minutes, the reference group. The results of the sensitivity analysis were essentially the same. The overall Kaplan–Meier survival estimate of no revision due to PJI at the end of the study period of 0–5.7 years’ follow-up was 99.2% (CI 99.1–99.3), which is comparable to or slightly lower than previous reports (3,20,21).
Male sex was a twofold risk factor for revision due to PJI compared with female sex in our study, which is slightly higher than the 1.4-fold risk presented previously (22) but similar to that reported earlier based on FAR (7). The reason for increased PJI risk in males may lie in confounding factors that are not included in the FAR, such as smoking and alcohol abuse, both of which are more common among males (23,24). Especially in Finland, the habit among males of drinking strong alcohol may predispose to higher PJI risk compared with many other countries. Detailed preoperative patient counseling is advisable to identify and treat silent alcohol abuse before TKA. We also found that high ASA class was associated with increased risk of revision for PJI. ASA class is a crude estimate of a patient’s comorbidity. ASA class III–IV had twice the risk of revision due to PJI compared with ASA class I in our study, which is similar to earlier reports (4). It has also been suggested earlier that the risk of PJI in TKA patients with diabetes versus non-diabetic patients is 1.7-fold and that of cancer versus non-cancer patients 1.5-fold (22). These findings are also in accordance with our results, although we were not able to assess specific medical diagnoses.
We found that long duration of operation (≥ 2 hours) was associated with a 1.7-fold risk of revision for PJI compared with the reference group (60–89 minutes). This finding supports previous evidence (4,25,26). However, we also found that short (45–59 minutes) operation time was associated with a decreased revision due to PJI rate compared with the reference group. To our knowledge, this association has not been clearly established previously. The finding is intuitive; the shorter the time the wound is open the lesser contamination will occur. Unfortunately, we did not have data on surgeon operative volume.
Surprisingly, high hospital volume (≥ 700) was associated with an increased risk of revision due to PJI, probably due to comorbidity-related factors. More complex cases are probably referred to high-volume hospitals. Also, the reporting completeness on revision operations in large hospitals is higher than in smaller ones, which very likely further explains our finding.
Kinematically aligned TKA has emerged as an alternative method to the mechanically aligned procedure, basing bony cuts off the patient’s pre-arthritic anatomy while limiting the need for soft tissue and ligamentous releases (28,29). While this less scarring and invasive technique could potentially affect PJI risk, data on revision rates using kinematically aligned TKA is still scarce. There were 1,081 TKAs performed using kinematic alignment in our database, with no difference in PJI rate compared with mechanically aligned TKAs. Similar results have been presented recently based on registry data from Australia and New Zealand including 416 kinematically aligned TKAs (30). For the time being, less soft tissue balancing has not brought about a decreased PJI rate.
High BMI was associated with an increased risk of revision for PJI in our study. Patients with a BMI of 35–39 and > 40 had an HR of 1.3 and 1.6, respectively, compared with the reference group. The correlation of obesity and risk of PJI has been documented previously (22,4,31,21). However, the risk of revision due to PJI is much higher in very obese THA patients (5-fold risk if BMI > 35 vs. normal weight) than in very obese TKA patients (13). Morbid obesity with a prolonged operation time and technical challenges in the groin region may predispose to PJI, especially after hip surgery. Our findings support previous studies whereby obesity seems to have an effect on PJI risk after TKA, but with a higher BMI threshold than for THA (32).
In our study, preoperative fracture diagnosis was associated with a 4-fold PJI rate compared with OA, although previous knee operations overall were not a risk factor. Earlier reports are partly contradictory. Kunutsor et al. (22) stated in their meta-analysis that patients with post-traumatic vs. non-post-traumatic arthritis had a 1.4-fold PJI risk, whereas previous joint surgery vs. no previous joint surgery was a 3-fold risk factor. However, the definition of previous surgery may vary between studies. Previously, only major surgery such as osteotomy (odds ratio [OR] 2.0) and ligament reconstruction (OR 2.7) to same knee has been found to be associated with increased PJI risk, but no such association was found for minor operations (33). Previous open meniscectomies are recorded as previous surgery in Finland, with probably very little effect on PJI rates. Inflammatory arthritis was not associated with greater PJI risk compared with OA in our study (HR 1.5, CI 0.9–2.5), although earlier data suggests it is (21,22). Previous studies on the use of drainage in TKA suggest that it might be a protective factor against PJI (4), but our findings contradict this. Based on current data we cannot recommend using a drain routinely.
Bleeding of more than 100 mL during surgery was associated with an HR of 1.7 compared with less bleeding. Previous studies have not assessed whether bleeding is a significant risk factor for PJI but have reported the need for blood transfusion to be one of them. The reason for bleeding and transfusion being associated with infectious complications could be immunomodulation caused by loss and transfusion of blood cells (34). Our results on general anesthesia being associated with risk of revision for PJI is in accordance with previous studies (21,35).
The use of antimicrobial incise drapes has been shown to reduce colonization during TKA operation (36), but studies have been inconclusive on whether this actually reduces the risk of PJI (37). We found that using an antimicrobial incise drape is associated with decreased risk of revision for PJI and therefore we recommend using it routinely. Using other antithrombotic prophylaxis than enoxaparin was associated with a decreased risk of revision due to PJI during the first 30 days after the primary operation with an HR of 0.6. No such effect was found after the first 30 days. In previous studies, antithrombotic medication was not associated with an increased PJI risk, but use of tranexamic acid in association with joint replacement has been found to be protective against PJI (21,35). Our findings on tranexamic acid do not confirm earlier findings.
We acknowledge that our study has several limitations. We do not have data on patients’ socioeconomic status, smoking status, alcohol abuse, or comorbidities, although ASA class is a crude estimate of medical condition. Furthermore, the FAR only captures revision operations in which one or more of the components is exchanged or removed. Superficial infections without any liner exchange were unfortunately not included. A further limitation is that we were not able to analyze all surgical details like bearing surface material. It has been proposed previously, based on the Australian register, that minimally stabilized TKA with non-crosslinked polyethylene-bearing surfaces have a greater revision risk for PJI than TKA with cross-linked polyethylene-bearing surfaces (38).
The completeness of FAR data on revision surgery during the study period was 80–85% compared with the discharge register; therefore we are probably missing some PJI revisions (1). Most probably, revisions done during on-call hours are those not reported to the FAR. Furthermore, our data is recorded in operating theatres based on clinical diagnosis and is not complemented afterwards based on, e.g., microbiology data, which may influence the estimated incidence of PJIs.
Conclusion
We found that high BMI, advanced ASA class, male sex, fracture around the knee as the reason for joint replacement, long operation duration, and the use of general anesthesia or enoxaparin as antithrombotic prophylaxis increased the risk of revision for PJI, whereas a shorter operation time and use of an antimicrobial incise drape decreased the revision risk.
- FAR: Finnish arthroplasty register. Finnish Inst. Heal. Welf. [Internet]. 2022. Available from: www.thl.fi/FAR.
- Cahill J L, Shadbolt B, Scarvell J M, Smith P N. Quality of life after infection in total joint replacement. J Orthop Surg 2008; 16(1): 58-65. doi: 10.1177/230949900801600115.
- Kurtz S M, Ong K L, Lau E, Bozic K J, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1): 52. doi: 10.1007/s11999-009-1013-5.
- Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J 2017; 14(3): 529-36. doi: 10.1111/iwj.12640.
- Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty: a register-based analysis of 43,149 cases. J Bone Joint Surg Am 2009; 91(1): 38-47. doi: 10.2106/JBJS.G.01686.
- Kurtz S M, Ong K L, Schmier J, Mowat F, Saleh K, Dybvik E, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(SUPPL. 3): 144-51. doi: 10.2106/JBJS.G.00587.
- Pamilo K J, Haapakoski J, Sokka-Isler T, Remes V, Paloneva J. Rapid rise in prevalence of knee replacements and decrease in revision burden over past 3 decades in Finland: a register-based analysis. Acta Orthop 2022; 93: 382-9. doi: 10.2340/17453674.2022.2266.
- Niemeläinen M J, Mäkelä K T, Robertsson O, W-Dahl A, Furnes O, Fenstad A M, et al. Different incidences of knee arthroplasty in the Nordic countries. Acta Orthop 2017; 88(2): 173-8. doi: 10.1080/17453674.2016.1275200.
- Patel A, Pavlou G, Mújica-Mota R E, Toms A D. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the national joint registry dataset. Bone Joint J 2015; 97-B(8): 1076-81. doi: 10.1302/0301-620X.97B8.35170.
- Dale H, Fenstad A M, Hallan G, Havelin L I, Furnes O, Overgaard S, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 2012; 83(5): 449-58. doi: 10.3109/17453674.2012.733918.
- Schrama J C, Fenstad A M, Dale H, Havelin L, Hallan G, Overgaard S, et al. Increased risk of revision for infection in rheumatoid arthritis patients with total hip replacements. Acta Orthop 2015; 86(4): 469-76. doi: 10.3109/17453674.2015.1017793.
- Springer B D, Cahue S, Etkin C D, Lewallen D G, McGrory B J. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplasty Today 2017; 3(2): 137-40. doi: 10.1016/j.artd.2017.05.003.
- Panula V J, Alakylä K J, Venäläinen M S, Haapakoski J J, Eskelinen A P, Manninen M J, et al. Risk factors for prosthetic joint infections following total hip arthroplasty based on 33,337 hips in the Finnish Arthroplasty Register from 2014 to 2018. Acta Orthop 2021; 92(6): 665. doi: 10.1080/17453674.2021.1944529
- Puolakka T J, Pajamäki K J, Halonen P J, Pulkkinen P O, Paavolainen P, Nevalainen J K. The Finnish Arthroplasty Register: report of the hip register. Acta Orthop Scand 2001; 72(5): 433-41. doi: 10.1080/000164701753532745.
- Parvizi J, Fassihi S C, Enayatollahi M A. Diagnosis of periprosthetic joint infection following hip and knee arthroplasty. Orthop Clin North Am 2016; 47(3): 505-15. doi: 10.1016/j.ocl.2016.03.001.
- Ranstam J, Robertsson O. The Cox model is better than the Fine and Gray model when estimating relative revision risks from arthroplasty register data. Acta Orthop 2017; 88(6): 578-80. doi: 10.1080/17453674.2017.1361130.
- Ranstam J, Robertsson O. Statistical analysis of arthroplasty register data. Acta Orthop 2010; 81(1): 10-14. doi: 10.3109/17453671003587168.
- Grambsch P M, Therneau T M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81(3): 515-26.
- Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen A B, et al. Statistical analysis of arthroplasty data, II: Guidelines. Acta Orthop 2011; 82(3): 258-67. doi: 10.3109/17453674.2011.588863.
- Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections. Acta Orthop 2015; 86(3): 321-5. doi: 10.3109/17453674.2015.1035173
- Lenguerrand E, Whitehouse M R, Beswick A D, Kunutsor S K, Foguet P, Porter M, et al. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis 2019; 19(6): 589. doi: 10.1016/S1473-3099(18)30755-2.
- Kunutsor S K, Whitehouse M R, Blom A W, Beswick A D. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. Virdi AS, editor. PLoS One 2016; 11(3): e0150866. doi: 10.1371/journal.pone.0150866.
- WHO. WHO global report on trends in prevalence of tobacco smoking 2015. Geneva, WHO; 2015.
- WHO. Global status report on alcohol and health 2018. Geneva, WHO; 2018.
- Teo B J X, Yeo W, Chong H C, Tan A H C. Surgical site infection after primary total knee arthroplasty is associated with a longer duration of surgery. J Orthop Surg 2018; 26(2). doi: 10.1177/2309499018785647.
- Ravi B, Jenkinson R, O’Heireamhoin S, Austin P C, Aktar S, Leroux T S, et al. Surgical duration is associated with an increased risk of periprosthetic infection following total knee arthroplasty: a population-based retrospective cohort study. EClinicalMedicine 2019; 16: 74-80. doi: 10.1016/j.eclinm.2019.09.015.
- Patel K, Judd H, Harm R G, Nolan J R, Hummel M, Spanyer J. Robotic-assisted total knee arthroplasty: is there a maximum level of efficiency for the operating surgeon? J Orthop 2022; 31: 13-16. doi: 10.1016/j.jor.2022.02.015.
- Theodore W, Twiggs J, Kolos E, Roe J, Fritsch B, Dickison D, et al. Variability in static alignment and kinematics for kinematically aligned TKA. Knee 2017; 24(4): 733-44. doi: 10.1016/j.knee.2017.04.002.
- Stake S, Fassihi S, Gioia C, Gu A, Agarwal A, Akman A, et al. Kinematic versus mechanically aligned total knee arthroplasty: no difference in frequency of arthroscopic lysis of adhesions for arthrofibrosis. Eur J Orthop Surg Traumatol 2021; 31(4): 763-8. doi: 10.1007/s00590-020-02836-7.
- Klasan A, de Steiger R, Holland S, Hatton A, Vertullo C J, Young S W. Similar risk of revision after kinematically aligned, patient-specific instrumented total knee arthroplasty, and all other total knee arthroplasty: combined results from the Australian and New Zealand Joint Replacement Registries. J Arthroplasty 2020; 35(10): 2872-7. doi: 10.1016/j.arth.2020.05.065.
- Kurtz S M, Lau E C, Son M-S, Chang E T, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty 2018; 33(10): 3238-45. doi: 10.1016/j.arth.2018.05.042.
- Si H B, Zeng Y, Shen B, Yang J, Zhou Z K, Kang P D, et al. The influence of body mass index on the outcomes of primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2015; 23(6): 1824-32. doi: 10.1007/s00167-014-3301-1.
- Tayton E R, Frampton C, Hooper G J, Young S W. The impact of patient and surgical factors on the rate of infection after primary total knee arthroplasty. Bone Joint J 2016; 98B(3): 334-40. doi: 10.1302/0301-620X.98B3.36775.
- Ko M S, Choi C H, Yoon H K, Yoo J H, Oh H C, Lee J H, et al. Risk factors of postoperative complications following total knee arthroplasty in Korea: a nationwide retrospective cohort study. Medicine 2021; 100 (48): e28052. doi: 10.1097/MD.0000000000028052.
- Yazdi H, Klement M R, Hammad M, Inoue D, Xu C, Goswami K, et al. Tranexamic acid is associated with reduced periprosthetic joint infection after primary total joint arthroplasty. J Arthroplasty 2020; 35(3): 840-4. doi: 10.1016/j.arth.2019.10.029.
- Hesselvig A B, Arpi M, Madsen F, Bjarnsholt T, Odgaard A. Does an antimicrobial incision drape prevent intraoperative contamination? A randomized controlled trial of 1187 patients. Clin Orthop Relat Res 2020; 478(5): 1007. doi: 10.1097/CORR.0000000000001142.
- Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev 2015; 2015(4). doi: 10.1002/14651858.CD006353.pub4.
- Vertullo C J, Lewis P L, Peng Y, Graves S E, de Steiger R N. The effect of alternative bearing surfaces on the risk of revision due to infection in minimally stabilized total knee replacement: an analysis of 326,603 prostheses from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am 2018; 100(2): 115-23. doi: 10.2106/JBJS.17.00269.