Acceptable migration of a fully cemented rotating hinge-type knee revision system measured in 20 patients with model-based RSA with a 2-year follow-up
Simon N van LAARHOVEN 1, Malou E M TE MOLDER 2, Gijs G VAN HELLEMONDT 1, and Petra J C HEESTERBEEK 2
1 Department of Orthopaedic Surgery, Sint Maartenskliniek, Nijmegen; 2 Department of Research, Sint Maartenskliniek, Nijmegen, the Netherlands
Background and purpose — Rotating hinged knee implants are highly constrained prostheses used in cases in which adequate stability is mandatory. Due to their constraint nature, multidirectional stresses are directed through the bone–cement–implant interface, which might affect fixation and survival. The goal of this study was to assess micromotion of a fully cemented rotating hinged implant using radiostereometric analysis (RSA).
Patients and methods — 20 patients requiring a fully cemented rotating hinge-type implant were included. RSA images were taken at baseline, 6 weeks, and 3, 6, 12, and 24 months postoperatively. Micromotion of femoral and tibial components referenced to markers in the bone was assessed with model-based RSA software, using implant CAD models. Total translation (TT), total rotation (TR), and maximal total point motion (MTPM) were calculated (median and range).
Results — At 2 years, TTfemur was 0.38 mm (0.15–1.5), TRfemur was 0.71° (0.37–2.2), TTtibia was 0.40 mm (0.08–0.66), TRtibia was 0.53° (0.30–2.4), MTPMfemur was 0.87 mm (0.54–2.8), and MTPMtibia was 0.66 mm (0.29–1.6). Femoral components showed more outliers (> 1 mm, > 1°) compared with tibial components.
Conclusion — Fixation of this fully cemented rotating hinge-type revision implant seems adequate in the first 2 years after surgery. Femoral components showed more outliers, in contrast to previous RSA studies on condylar revision total knee implants.
Citation: Acta Orthopaedica 2023; 94: 185–190. DOI https://doi.org/10.2340/17453674.2023.12305.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-11-16. Accepted: 2023-03-16. Published: 2023-04-24.
Correspondence: s.vanlaarhoven@maartenskliniek.nl
SvL: wrote the manuscript, interpreted the data. MtM: wrote study protocol, analyzed and computed the data, gave critical feedback on the final manuscript. GvH: conceived the original idea and designed the study, discussed the results, and gave critical feedback on the final manuscript. PH: conceived the original idea and designed the study, wrote study protocol, analyzed and computed the data, wrote the manuscript.
Handling co-editors: Ivan Hvid and Robin Christensen
Acta thanks Leif Ryd and Stephan Röhrl for help with peer review of this study.
Rotating hinged knee implants are the most constrained type of knee prosthesis. They are mostly used in complex revision knee surgery with insufficient ligaments or extensive bone loss. Due to the hinge mechanism, relatively high multidirectional stresses will be transferred across the bone–implant interface. These forces, in combination with impaired bone, make appropriate fixation of hinge implants in revision knee surgery challenging.
Radiostereometric analysis (RSA) can be used to evaluate the stability of an implant, with early detection of micromotion between the implant and the surrounding bone (1). The degree of micromotion assessed by RSA in the first 2 years after surgery is associated with late revision for aseptic loosening in primary total knee arthroplasty (TKA) (2,3). From the sparse data available on condylar revision knee implants, it can be observed that higher degrees of micromotion do not result in later aseptic loosening (4-6).
For rotating hinged knee implants, no RSA data is currently available. This information is required to determine the acceptable limit of micromotion for the long-term survival of a stable implant. Furthermore, RSA data can aid in evaluating the pattern of migration and potential failure modes, which might be different from primary or condylar revision implants due to forces directed through the rigid hinge mechanism.
The primary objective of our study was to investigate the stability of the fixation of a fully cemented rotating hinged knee implant in revision surgery within the first 2 years postoperative. The secondary objective was to assess clinical and functional performance in these patients.
Patients and methods
Design and patients
We conducted a single-center, cohort study from 2017 to 2021 at the Sint Maartenskliniek, Nijmegen, the Netherlands. 20 patients requiring a revision total knee replacement with a hinged-type knee system were included in this study. Exclusion criteria were BMI > 40, active infection (systemic/local), disorders that could compromise compliance with the follow-up period, known sensitivity to materials in the device, and no visible markers in both the femoral and tibial components on the first postoperative RSA radiograph.
The present study was reported according to STROBE guidelines.
Intervention
All patients received the Legion Hinge Knee (HK) System (Smith & Nephew, Memphis, TN, USA) and were operated on by orthopedic surgeons specialized in knee revision surgery. We used a medial approach and a fully cemented technique for all patients. Previous implants were carefully removed, and 6 interface cultures were taken as per routine care. Realigned refresh cuts were performed, and the bone canal was prepared for stem fixation. The canal was reamed until cortical contact was obtained and a 2-mm downsized stem was chosen for a sufficient cement mantle. Stem length (120 or 160 mm) was dependent on the surgeon’s preference to obtain sufficient fixation. Bone loss was assessed by the Anderson Orthopaedic Research Institute (AORI) bone stock classification and defects were treated with metal augments, cones, and/or bone grafting. The bone surface was cleaned with pulse lavage irrigation after placement of a polyethylene cement plug. Vacuum-mixed antibiotic-impregnated polymethyl methacrylate (Copal G+C, Biomet Merck, Darmstadt, Germany) was loaded onto the components and retrogradely injected into the canal after tantalum beads for RSA were placed in the femur and tibia. The final components (tibia, femur) were cemented sequentially. All patellae were resurfaced or revised. A standard postoperative care protocol with direct full weight-bearing and 5-day antibiotic treatment was followed for all patients.
Primary outcome
The primary outcome parameter was micromotion of both the femoral and tibial components, measured with model-based RSA using a uniplanar setup with 1 ceiling-mounted X-ray tube and 1 mobile device. Patients were lying in a supine position, with standardized foot rotation to enable marker visibility throughout the follow-up period. Micromotion of the implant component was evaluated at predetermined time points (6 weeks, 3 months, 6 months, 1 year, and 2 years postoperatively) compared with the baseline RSA radiograph taken after initial weight-bearing shortly after surgery. At 6 weeks, double RSA radiographs were performed in all patients to assess the precision with measurement error statistics (mean with standard deviation) (7). Accuracy was determined with a phantom study prior to patient inclusion. Model-based RSA (MBRSA) measurements with CAD models were performed with MBRSA software (Modelbased RSA 4.2, RSAcore, Leiden, the Netherlands) to calculate translation (T) and rotation (R) of the component with reference to the bone markers. Translation was expressed in millimeters and rotation in degrees along or around the transverse (x), longitudinal (y), and sagittal (z) axes. Total translation (TT) was calculated as TT = √(Tx2 + Ty2 + Tz2) and total rotation (TR) was calculated as TR = √(Rx 2+ Ry2 + Rz2) (8). Outliers in relation to TT and TR were defined as components moving > 1 mm or > 1° as described by Heesterbeek et al. (5). Maximum total point motion (MTPM) was the length of the translation vector of the (virtual) marker on the implant that showed the greatest migration. All measurements were evaluated according to the condition number and rigid body error. ISO 16087:2013 was followed, which recommends a maximum condition number of 150 and a rigid body error < 0.35 to have reliable results.
Other outcome scores
During follow-up visits, clinical and functional outcome measures were collected in a standardized way by a research nurse. Outcome measures were the Knee Society Score (KSS), Knee injury and Osteoarthritis Outcome Score – Physical Function Short form (KOOS-PS), Oxford Knee Score (OKS), Oxford Knee Score – Activity and Participation (OKS-APQ), and visual analog scales (VASs) for pain and satisfaction. VAS pain scores ranged from 0 (no pain) to 100 (worst pain) and VAS satisfaction ranged from 0 (dissatisfied) to 100 (satisfied). Additionally, knee flexion was measured with a longarm goniometer and all (severe) adverse device-related events were registered.
Statistics
Descriptive statistics were used to present patient characteristics. Micromotion and outcome measures were given as medians with ranges and presented graphically to demonstrate micromotion patterns over time. Data were analyzed using STATA 13.0 (StataCorp, College Station, TX, USA).
Ethics, registration, funding, and disclosures
The study protocol was approved by the hospital’s investigational review board and the Medical Ethical Review Board of Slotervaart and Reade (NL58887.048.16). This study was conducted in accordance with the Declaration of Helsinki and RSA guidelines (1). Written informed consent was obtained from all participating patients. The hospital received funding from Smith & Nephew to pay for staff and materials for conducting this study. Smith & Nephew had no role in the design or conduct of the study, the collection, management, analyses and interpretation of the data, or the preparation of the manuscript. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.12305
Results
Preoperative patient characteristics such as age, sex, and surgical details can be found in Table 1.
At final follow-up, 3 patients were lost to follow-up. 1 patient died due to an unrelated cause and 2 refused further participation (1 due to dementia, 1 due to pain). Micromotion could not be assessed in 2 femoral components and 7 tibial components due to insufficient RSA marker visibility and distribution. This resulted in complete RSA measurements of 16 femoral components and 11 tibial components at 2-year follow-up (Figure 1). The median number of matching markers was 4 (3-9) and in 8 femora and 8 tibias marker configuration models were used. In 1 femoral and 3 tibial components, the markers were found to be in an equilateral triangle, which led to a higher condition number (> 150) which is beyond the ISO guideline recommendations. The analyses were reviewed by independent RSA experts (RSAcore, Leiden, the Netherlands) and found to be reliable to use. Measurement error statistics of the 6 degrees of freedom, TT, and TR are given in Table 2.
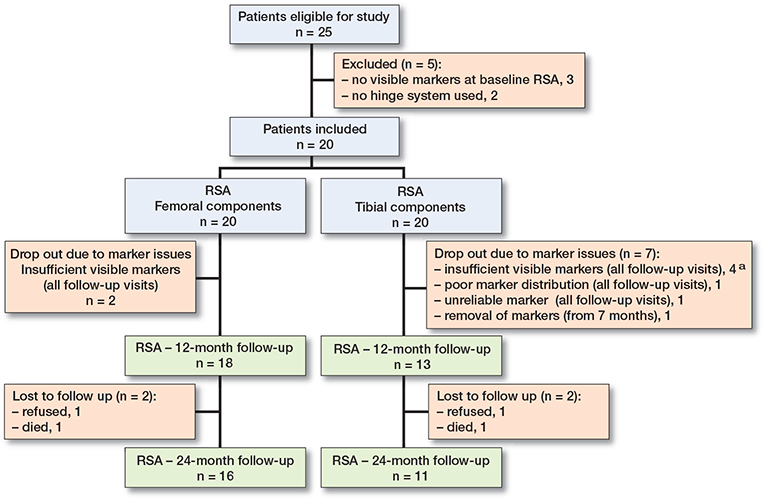
Figure 1. Flowchart of patients and components. At final follow-up clinical data was available for 17 patients (2 patients refused, 1 died). a In 1 patient also lost to follow up > 12 months.
At 2-year follow-up, median TTfemur, TRfemur, and MTPMfemur were 0.38 mm (0.15–1.5), 0.71° (0.37–2.2), and 0.87 mm (0.54–2.8), respectively. Median TTtibia, TRtibia, and MTPMtibia were 0.40 mm (0.08–0.66), 0.53° (0.30–2.4), and 0.66 mm (0.29–1.6), respectively (Figure 2). The majority of micromotion occurred from baseline to 6 weeks postoperatively, followed by stabilization of micromotion (Figure 2). Individual translation and rotation trajectories did not show uniform migration patterns (towards a specific direction), with median translation and rotation values close to zero. However, individual outliers with higher migrations were most prominent in the anterior–posterior translation, flexion–extension, and internal–external rotation in femoral components and in the flexion–extension rotation of tibial components (Table 3). At 2 years, 5 femoral components translated with a TT > 1 mm and 5 femoral components rotated with a TR > 1°, whereas no tibial components translated with a TT > 1 mm and 2 tibial components rotated with a TR > 1°. Moreover, MTPM trajectories of the femoral component showed more continuous migration over time compared with tibial components (Figure 3). Translation and rotation of the femoral and tibial components in all 6 degrees of freedom throughout the entire follow-up are presented in Table 3.
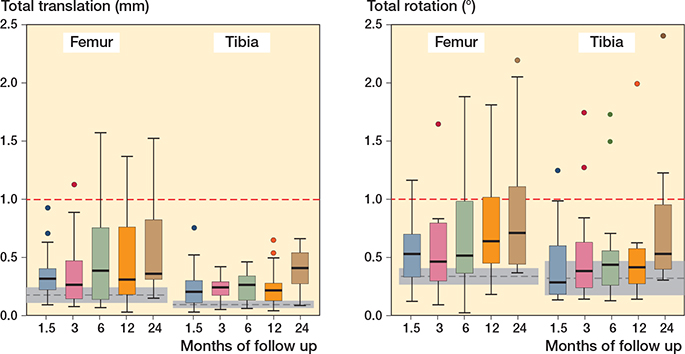
Figure 2. Total translation (left panel) and total rotation (right panel) of the femoral and tibial components at follow-up intervals. Top and bottom of box are the 25th and 75th percentiles, horizontal line within box is the median, whiskers are the lower and upper adjacent values (1.5 x IQR), and markers are outside values. Grey dashed lines are the measurement error statistics (mean ± 95% CI).
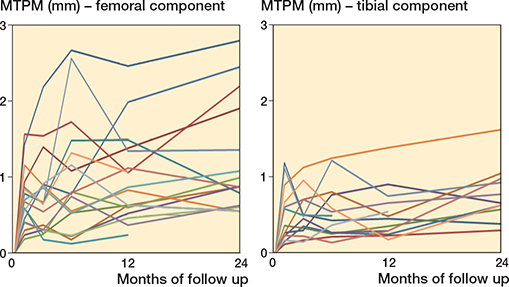
Figure 3. Individual RSA trajectories of the MTPM of the femoral (left panel) and tibial (right panel) components.
Most outcome scores at 2 years showed an improvement from baseline (Table 4). Pain scores decreased significantly over time both in the subscore of the OKS as well as in the VAS pain score (Table 4). 11 patients encountered a complication (recurrence of arthrofibrosis [4], [neuropathic] pain [5, of whom 3 with worsening of pre-existing pain], deep venous thrombosis [1], recurrence of quadriceps tendon rupture [1], pneumonia [1], extended wound leakage [1]), with only 1 patient requiring reoperation (full allograft extensor mechanism reconstruction). None of the implants were revised or suspected of loosening at 2-year follow-up.
Discussion
This is the first RSA study to investigate the stability of a revision rotating hinged-knee implant. Most implants showed some degree of early micromotion followed by stabilization of micromotion between 6 weeks and 2 years. At 2 years, a significant number of components showed a TT > 1 mm or TR > 1°, especially on the femoral side. However, none of the implants failed or showed radiological signs of loosening. Most clinical and functional scores showed an improvement from baseline, and pain decreased.
Implant stability has been studied widely for tibial components in primary TKA (3). Acceptable migration has been defined as MTPM < 0.5 mm in the first 6 months and MTPM < 0.2 mm from 6–12 months and 12–24 months. Although these values cannot be applied to complex knee revision surgery with rotating hinged implants, the micromotion results of the current study are within or close to these safe zones, with a median MTPM of 0.46 mm at 6 months and an increase of 0.21 mm between 12 and 24 months. For femoral components, there is no reference data for acceptable micromotion. Although there was higher early migration, with an MTPM of 0.78 mm at 6 months, this was followed by satisfactory stabilization up to 2 years (0.06 mm 6–12 months, 0.03 mm 12–24 months). A possible explanation for higher early migration in revision TKA compared with primary TKA might be existing bone loss with compromised primary fixation and subsequent remodeling in stemmed revision TKA (9). No difference in migration was found between the groups AORI F1/T1 and AORI >F1/>T1.
While on the group level acceptable levels of micromotion were observed, individual RSA trajectories did show outliers outside the previously mentioned safe zones. 4 femoral components could be identified as having continuous migration (Figure 3). Further analysis showed mainly anterior–posterior translation, and rotation around the transverse (flexion–extension), and (to a lesser extent) longitudinal (internal–external rotation) axis. 1 tibial component showed increasing rotation around the transverse (flexion–extension) axis. This particular migration for both the femoral and tibial components might be due to increased momentum in the sagittal plane caused by the fixed-hinge mechanism and extension stop of the implant system.
In the literature there is limited RSA data for revision TKA: Heesterbeek et al. reported median TT (TTfemur 0.31 mm, TTtibia 0.40 mm) and median TR (TRfemur 0.62°, TRtibia 0.86°) for fully cemented revision TKA with condylar revision implants at 2 years, which are comparable with the current findings (5). In contrast to this, the number of outliers (TT > 1 mm or TR > 1°) seems higher for femoral components compared with the tibial components in the current study (7/16 vs. 2/11) and in contrast to the study of Heesterbeek et al. with cemented condylar revision TKA (4/15 vs. 5/14) (5). Higher rates of aseptic loosening of femoral components in rotating hinged knee implants have been reported, for which different explanations have been provided (10,11). Farid et al. believed that the femoral component in rotating hinged knee implants is subject to more torsion and bending stresses due to differences in the anatomic femoral and mechanical axis (10). We presumed that the femoral component and stem are subject to higher torsional stresses due to the design of the rotating hinge mechanism. The tibial component is relatively free from these stresses because the rotational free axis is always in line with the longitudinal axis of the tibial component and stem. Due to these findings, our previous focus on tibial fixation in revision knee surgery with rotating hinged implants has been changed into increased attention to fixation of the femoral component.
Although a significant number of implants showed continuous migration or a high degree of micromotion (> 1 mm or > 1°), longer follow-up RSA in revision knee surgery with condylar implants did not show signs of aseptic loosening for this potential group at risk (4,6). In addition, a retrospective analysis of a fully cemented Legion HK cohort showed only 1 femoral component loosening in 147 cases with a mean follow-up of 3.8 years (16). This might confirm appropriate stability of the present fully cemented rotating hinged implants and might indicate that higher degrees of micromotion are acceptable in revision TKA along with the use of hinged implants. Moreover, long-term survival data of fully cemented rotating hinged implants showed excellent and superior survival over hybrid fixated implants (11). Extended clinical follow-up of the current patients will be needed to ascertain whether the degree of migration will lead to early re-revisions and, if so, to investigate a relationship with the migration patterns.
Most clinical and functional scores showed a significant improvement at 24 months (OKS, KSS clinical, OKS-APQ, and KOOS-PS) and this improvement is of clinical importance in knee revision surgery (12,13). However, a slight decrease was seen for most of the scores after 12 months. This was especially true for patients with recurrent arthrofibrosis and increased (neuropathic) pain. This is in line with previous studies on revision knee surgery for arthrofibrosis, which showed clinical deterioration after 12 months (14). Recurrence of arthrofibrosis was the most frequent complication, which is similar to previous reports with moderate outcomes after revision surgery (14,15). Nevertheless, a total of 8 patients had a preoperative stiff knee (range of motion < 90°) and improved at least 20° in range of motion at 2 years’ follow-up. Only 1 reoperation occurred (full allograft extensor mechanism reconstruction), which is very reasonable compared with previous studies on revision knee surgery with hinged implants (11,16).
The main limitation of the present study is the substantial loss of analyzable tibial components due to marker invisibility. Because of the extensive size of the implant and the limited cancellous bone for marker placement, RSA measurements were not possible in 4 tibial components and 2 femoral components. This could not be solved with marker-configuration models. Furthermore, poor distribution of markers and unreliable measurements in another 2 tibial components resulted in failure to analyze the micromotion in these patients. In 1 tibial component, some markers were removed during a full allograft reconstruction of the extensor apparatus, which made analysis impossible at 12 and 24 months. In 4 components, the condition number was > 150, which may have impeded the quality of the RSA measurements.
Conclusion
Micromotion in fully cemented rotating hinged knee implants was comparable to previous findings in satisfactory fixated condylar revision knee implants with long-term follow-up. Therefore, fixation of these implants seems adequate in the first 2 years after surgery. Femoral components showed more outliers, in contrast to previous RSA studies.
- Valstar E R, Gill R, Ryd L, Flivik G, Börlin N, Kärrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76(4): 563-72. doi: 10.1080/17453670510041574.
- Ryd L, Albrektsson B E J, Carlsson L, Dansgard F, Herberts P, Lindstrand A, et al. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br 1995; 77(3): 377-83. doi: 10.1302/0301-620x.77b3.7744919.
- Pijls B G, Plevier J W M, Nelissen R G H H. RSA migration of total knee replacements. Acta Orthop 2018; 89(3): 320-8. doi: 10.1080/17453674.2018.1443635.
- Mills K, Wymenga A B, van Hellemondt G G, Heesterbeek P J C. No difference in long-term micromotion between fully cemented and hybrid fixation in revision total knee arthroplasty: a randomized controlled trial. Bone Joint J 2022; 104 B(7): 875-83. doi: 10.1302/0301-620X.104B7.BJJ-2021-1600.R1.
- Heesterbeek P J C, Wymenga A B, Van Hellemondt G G. No difference in implant micromotion between hybrid fixation and fully cemented revision total knee arthroplasty: a randomized controlled trial with radiostereometric analysis of patients with mild-to-moderate bone loss. J Bone Joint Surg 2016; 98(16): 1359-69. doi: 10.2106/JBJS.15.00909.
- Kosse N M, van Hellemondt G G, Wymenga A B, Heesterbeek P J C. Comparable stability of cemented vs press-fit placed stems in revision total knee arthroplasty with mild to moderate bone loss: 6.5-year results from a randomized controlled trial with radiostereometric analysis. J Arthroplasty 2017; 32(1): 197-201. doi: 10.1016/j.arth.2016.06.003.
- Niesen A E, Hull M L. Measurement error versus repeated measurements: a guide describing two methods for computing bias and precision of migration measurements from double examinations using radiostereometric analysis. J Biomech Eng 2022; 144(6). doi: 10.1115/1.4054375.
- Selvik G. Roentgen stereophotogrammetry: a method for the study of the kinematics of the skeletal system. Acta Orthop 1989; 60(S232): 1-51. doi: 10.3109/17453678909154184.
- van Lenthe G H, Willems M M M, Verdonschot N, de Waal Malefijt M C, Huiskes R. Stemmed femoral knee prostheses. Acta Orthop Scand 2002; 73(6): 630-7. doi: 10.3109/17453670209178027.
- Farid Y R, Thakral R, Finn H A. intermediate-term results of 142 single-design, rotating-hinge implants: frequent complications may not preclude salvage of severely affected knees. J Arthroplasty 2015; 30(12): 2173-80. doi: 10.1016/j.arth.2015.06.033.
- van Laarhoven S N, van Eerden A H J, van Hellemondt G G, Schreurs B W, Wymenga A B, Heesterbeek P J C. Superior survival of fully cemented fixation compared to hybrid fixation in a single design rotating hinge knee implant. J Arthroplasty 2022; 37(3): 482-7. doi: 10.1016/J.ARTH.2021.11.037.
- Khow Y Z, Liow M H L, Goh G S, Chen J Y, Lo N N, Yeo S J. Defining the minimal clinically important difference for the Knee Society score following revision total knee arthroplasty. Knee Surgery, Sport Traumatol Arthrosc 2021; 30(8): 2744-52. doi: 10.1007/s00167-021-06628-2.
- Khow Y Z, Liow M H L, Goh G S, Chen J Y, Lo N N, Yeo S J. The Oxford Knee Score minimal clinically important difference for revision total knee arthroplasty. Knee 2021; 32: 211-17. doi: 10.1016/j.knee.2021.08.020.
- Van Kempen R W T M, Schimmel J J P, Van Hellemondt G G, Vandenneucker H, Wymenga A B. Reason for revision TKA predicts clinical outcome: prospective evaluation of 150 consecutive patients with 2-years followup. Clin Orthop Relat Res 2013; 471(7): 2296-302. doi: 10.1007/S11999-013-2940-8.
- Heesterbeek P J C, Goosen J H M, Schimmel J J P, Defoort K C, van Hellemondt G G, Wymenga A B. Moderate clinical improvement after revision arthroplasty of the severely stiff knee. Knee Surgery, Sport Traumatol Arthrosc 2015; 24(10): 3235-41. doi: 10.1007/s00167-015-3712-7.
- Yeroushalmi D, Van Laarhoven S, Tang A, Heesterbeek P J C, Van Hellemondt G, Schwarzkopf R. Short- to midterm outcomes of a novel guided-motion rotational hinged total knee arthroplasty. J Knee Surg 2021; 35(10): 1153-8. doi: 10.1055/s-0040-1722349.