Health-related quality of life after segmental pedicle screw instrumentation: a matched comparison of patients with neuromuscular and adolescent idiopathic scoliosis
Venla SOINI 1,2, Johanna SYVÄNEN 1, Linda HELENIUS 3, Arimatias RAITIO 1, and Ilkka HELENIUS 4,5
1 Department of Paediatric Surgery and Paediatric Orthopaedic Surgery, University of Turku, and Turku University Hospital Turku; 2 Department of Surgery, Vaasa Central Hospital, Wellbeing Services County of Ostrobothnia, Vaasa; 3 Department of Anaesthesiology and Intensive Care, Turku University Hospital and University of Turku, Turku; 4 Department of Paediatric Orthopaedic Surgery, Helsinki New Children’s Hospital, Helsinki; 5 Department of Orthopaedics and Traumatology, University of Helsinki, and Helsinki University Hospital, Finland
Background and purpose — Progressive neuromuscular scoliosis (NMS) often requires a long instrumented spinal fusion to improve health-related quality of life (HRQoL) and sitting balance. Segmental pedicle screw instrumentation improves HRQoL in patients with adolescent idiopathic scoliosis (AIS), but data on NMS is limited. We aimed to assess the impact of spinal fusion on HRQoL in NMS patients.
Patients and methods — We conducted a retrospective case-control study with prospective data collection of NMS patients undergoing posterior spinal fusion at a tertiary level hospital in 2009–2021. 2 controls with AIS matched for sex and age were selected for each NMS patient. The Scoliosis Research Society-24 (SRS-24) questionnaire was utilized for pre- and postoperative HRQoL assessment. Follow-up time was a minimum of 2 years.
Results — 60 NMS and 120 AIS patients were included in the analysis, and the mean age (SD) at operation was 14.6 (2.7) in NMS and 15.7 (2.5) in AIS groups. Total SRS score and all domains showed a significant improvement in NMS patients (p < 0.05). Total SRS score improved more (p < 0.001), while pain score improved less (p = 0.04) in NMS (change [95% CI], 0.31 [0.05–0.58] and 0.55 [0.27–0.81]) compared with AIS (0.01 [–0.10 to 0.12] and 0.88 [0.74–1.03]). Postoperative self-image was significantly better in NMS than in AIS at 2-year follow up (p = 0.01). Pelvic instrumentation reduced improvements in the SRS domains.
Conclusion — HRQoL in NMS patients improved significantly after spinal fusion, and these benefits are comparable to those of AIS patients.
Citation: Acta Orthopaedica 2023; 94: 165–170. DOI https://doi.org/10.2340/17453674.2023.11962.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-09-29. Accepted: 2023-03-16. Published: 2023-04-17.
Correspondence: veviso@utu.fi
Study design: all authors. Study conduct: JS, AR, IH. Data collection: all authors. Data analysis: VS. Data interpretation: all authors. Drafting of manuscript: VS. Revising manuscript: all authors.
Handling co-editor: Taco Gosens
Acta thanks Benny Dahl, Marc Nieuwenhuijse, and Anne Versteeg for help with peer review of this study.
Neuromuscular scoliosis (NMS) is defined as scoliosis caused by any neuromuscular disease, such as cerebral palsy (CP) or Duchenne muscular dystrophy (1). It is characterized by a rapid progression of curvature during growth depending on the neurological involvement (2) and this progression may continue also after skeletal maturity (3). NMS results in reduced pulmonary function and poor sitting balance secondary to scoliosis and increased pelvic obliquity (4). With curves exceeding 50°, surgical intervention is usually considered (5,6). In contrast to adolescent idiopathic scoliosis (AIS), the fusion area in NMS often comprises the whole thoracolumbar spine, which permanently diminishes spinal mobility (7-10).
Previous studies suggest that NMS patients benefit from spinal fusion (11,12). Health-related quality of life (HRQoL) in AIS patients has been studied comprehensively and reports suggest that pedicle screw instrumentation improves HRQoL more than observation in long-term follow-up (13-15). The extensive spinal surgery necessary to address NMS requires efforts to evaluate not only radiographic parameters and complications, but also assessment of HRQoL. The possible changes in the SRS-24 questionnaire were compared with minimum clinically important difference (MCID) reported for SRS outcome questionnaire and with changes observed in otherwise healthy individuals undergoing pedicle screw instrumentation for AIS. This comparison is complex, because otherwise healthy AIS patients do not use similar outcome parameters to patients with neuromuscular comorbidities.
The data comparing HRQoL of NMS and AIS patients undergoing spinal fusion is limited. Therefore, we aimed to add a single-center case-control study with a standardized perioperative protocol comparing NMS and AIS patients’ improvement in HRQoL after spinal fusion with the existing literature. We hypothesized that the quality of life in NMS patients after spinal fusion would be inferior to that of patients with AIS, yet superior to their own preoperative HRQoL levels.
Patients and methods
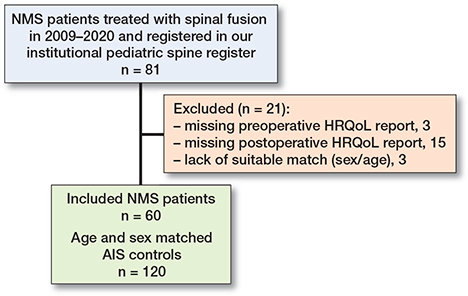
This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline. We conducted a retrospective case-control study in children undergoing segmental pedicle screw instrumentation for NMS in a tertiary-level hospital with prospective data collection from our institutional pediatric spine register. The operations were conducted in 2009–2020. 81 NMS patients underwent spinal fusion, and all NMS patients with available HRQoL scores were enrolled; 18 patients were excluded due to missing HRQoL data, and 3 due to lack of match (Figure). For each NMS patient, 2 control patients with AIS operated on at the same institution were matched manually for sex and age (±3.5 years) at the last follow-up (2:1). All patients had a minimum of 2-year follow-up.
Perioperative management was standardized for both cohort and control groups. Preoperatively, this included upright spine radiographs, laboratory testing and clinical examination. AIS patients underwent MRI imaging.
The anesthesia protocol consisted of intravenous propofol, remifentanil, and dexmedetomidine infusions. Normothermia and a mean arterial pressure of 65–75 mmHg was maintained during the operation. Before the incision all patients received a bolus of tranexamic acid (30 mg/kg, max 1,500 mg), which was followed by an infusion (10 mg/kg/h, max 500 mg/h) during the surgical procedure. Cell-saver was utilized with autologous blood transfusions and for allogenic blood cell transfusions preset criteria were used (16).
Surgical planning including type of implants and need for posterior column osteotomies was performed. In NMS, the fusion level was chosen based on ambulation and pelvic oblique. Indications for pelvic instrumentation in NMS patients included non-ambulatory status (8), pelvic obliquity of > 10°, lack of independent sitting or standing, and lumbar location of the curve apex. In ambulatory patients, the level was chosen based on the presence of pelvic oblique, with pelvic oblique spinal fusion continued to S2 alar iliac (S2AI) or ileum, and those without oblique to level L4 or L5 based on the central sacral vertical line. In non-ambulatory patients the fusion extended from T2 or T3 to pelvis (S2AI or pelvic screws). In the AIS patients, fusion level was determined by the Lenke classification (17). For Lenke 1 and 2 curves the last substantially touched vertebra was used as the lowest instrumented level, and for Lenke 3–6 curves spinal fusion was continued to L3 or L4 (18).
Both groups underwent segmental, bilateral pedicle screw instrumentation aiming at 2.0 screw density per instrumented vertebra. Corrective maneuvers were performed to obtain a horizontal pelvis (NMS) with well-corrected (aiming to > 70% correction) spinal deformity in both the coronal and sagittal plane (NMS and AIS). All procedures were carried out by a single experienced pediatric orthopedic spine surgeon together with another attending pediatric orthopedic surgeon. Bilateral segmental pedicle screw instrumentation (6.35 Legacy, Solera 6.0, Medtronic Spinal and Biologics, Fridley, MN, USA; Mesa2, Stryker Spine, Kalamazoo, MI, USA) was used for spinal deformity correction. The operations were performed in prone position and electrocautery was used for exposing the posterior elements. Pedicle screws were inserted using the freehand technique. Autograft from facetectomies and osteotomies was used. The NMS patients received an allograft from morselized femoral head, and the AIS patients a local autograft with bone graft extenders (tricalcium phosphate, Nanostim, Medtronics; iFactor, Cerapedics, Westminster, CO, USA). A single closed suction subfascial drain was used for all patients for the first 24 postoperative hours.
Intraoperative neurophysiological monitoring including motor evoked potentials, somatosensory evoked potentials, and lumbar nerve root EMG was performed at specific timepoints (incision, exposure, pedicle screw insertion, correction complete, wound closure).
SRS-24 questionnaire
The Scoliosis Research Society-24 (SRS-24) questionnaire was used to assess the pre- and postoperative HRQoL in the case and control patients. SRS-24 contains 24 questions, which are scaled from 1–5 (1 = severe pain, 5 = no pain), and the maximum score of the questionnaire is 120 (divided by 24 questions equaling 5.0). The higher the score, the better the outcome.
SRS-24 domains are divided into 7 categories: pain, general and postoperative function, general and postoperative self-image, general activity level, and satisfaction. The questionnaire was filled out by the patient or the caregiver depending on the patient’s age and ability, at 3 timepoints: preoperatively, 6 months, and 24 months after surgery. Preoperatively the patients answered only the first 15 preoperative questions.
Statistics
All statistical analyses were performed using JMP Pro 16.2.0 for Macintosh (SAS Institute in, Cary, NC, USA 1989–2022). HRQoL changes between preoperative and postoperative follow-up points were determined and the significance was tested with a matched pairs t-test. Demographic factors for postoperative HRQoL were tested with bivariate analysis. The normal distribution assumption was tested, linear fit correlations were analyzed for continuous data, one-way ANOVA was performed for categorical data, and Wilcoxon rank-sum test was used for non-parametric data. P-values < 0.05 were considered statistically significant.
Our primary outcome measure was postoperative mean differences in HRQoL domains between the groups from preoperative to the 2-year follow-up. Secondary outcome measures included the change of means inside and between the groups and comparison with other patient factors, such as Cobb angle and fusion level.
Ethics, data sharing, funding, and disclosures
Ethical committee approval was obtained for our study (Turku, ETMK 96/1801/2020). All the patients and their parents provided written consent preoperatively. The data that support the findings is available on request. Personal research funds were received for the following authors: VS has received grants from Vappu Uuspään säätiö, Turku University research funding, and Finnish Pediatric Research Foundation; JS from the Clinical Research Institute HUCH, and LH funding from Finnish Paediatric Research Foundation and Finska Läkaresällskapet. IH has received scientific funding from Industry to Institutions from Medtronic, Stryker, Nuvasive, and Cerapedics. IH has been working as a consultant for Medtronic. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.11962
Results
Patient characteristics
60 NMS and 120 AIS patients were included in the analysis (Table 1). Mean (SD) age at 2-year follow-up was 18.1 (3.9) in the NMS group and 17.6 (2.6) in the AIS group, and 55% of patients were female. Cerebral palsy was the most common diagnosis among the NMS patients (16 patients) and other diagnoses included Duchenne muscular atrophy (2 patients), myelomeningocele (2 patients), and multiple other diagnoses such as spinal muscular atrophy, and congenital myopathy. The mean (SD) preoperative major curve was 69° (18°) in NMS and 52° (8°) in AIS, p < 0.001. Postoperatively, the mean major curves at 2-year follow-up were 19° (11°) in the NMS patients and 13° (5°) in the AIS patients, p = 0.002. Mean (SD) scoliosis correction was 74% (13%) in the NMS group and 75% (10%) in the AIS group (p = 0.4). Mean (SD) number of levels fused was 16.3 (1.6) for NMS and 11.4 (1.7) for AIS.
Improvement of HRQoL within groups
HRQoL improved significantly after the surgical treatment in both groups (Table 2). For the NMS group, the total SRS score increased significantly from preoperative 3.8 (0.6) to 4.1 (0.5) at 2-year follow-up, p = 0.02. There was a statistically significant improvement in all SRS-24 domains from preoperatively to the 2-year follow-up: mean change on the SRS scale for pain (0.55), function (0.29), general self-image (0.46), and general activity (0.29). In the AIS patients, statistically significant improvement in pain (mean change of 0.87), general self -image (0.42), and function domain (0.15) was observed from preoperative to 2-year follow up. The general activity domain did not improve in the AIS group (Table 2).
Comparison of HRQoL between the study groups
The NMS patients had significantly lower preoperative general function (mean [SD] 3.6 [0.8] in NMS vs. 4.0 [0.5] in AIS, p = 0.005) and general activity scores (3.7 [1.1] and 4.5 [0.7], for NMS and AIS respectively, p < 0.001) compared with the AIS patients (Table 2). NMS patients reported significantly less preoperative pain, with mean pain scores of 3.9 (0.7) for NMS and 3.5 (0.6) for AIS, p < 0.001. The total preoperative SRS score was also significantly lower in the NMS patients 3.8 (0.6) compared with the AIS patients 4.1 (0.5), p = 0.02.
At the 6-month follow-up, the preoperative SRS score differences had disappeared, with the exception of postoperative function being significantly better in the NMS than in AIS patients (2.7 [1.4] vs. 2.1 [1.0], p = 0.03).
At the 2-year follow-up the general activity (4.0 [0.8] vs. 4.7 [0.6], p < 0.001) and function scores (3.9 [0.6] vs. 4.2 [0.4], p = 0.002) were significantly better in the AIS group. The NMS patients reported significantly better postoperative self-image than the AIS patients (3.6 [0.6] vs. 3.3 [0.5], p = 0.01) (Table 2). The total SRS score was similar in NMS and AIS (mean 4.1 [0.5] vs. 4.1 [0.4], p = 0.2).
The improvement in total SRS score from preoperative to 2-year follow-up was larger in the NMS patients than in AIS patients (p = 0.02). The improvement in the pain domain score was significantly smaller in the NMS compared with the AIS patients during the 2-year follow-up (p = 0.04).
Predictors of postoperative HRQoL
Neither age nor sex influenced any of the HRQoL domains. In NMS patients more extensive spinal instrumentation was associated with worse pain scores at 2-year follow-up, correlation coefficient –0.35, p < 0.05 and postoperative total SRS score correlated negatively with fusion level, –0.40, p = 0.02. These findings were not observed in the AIS group: fusion level had no impact on HRQoL domains. Residual major curve and curve correction had no impact on HRQoL domains or total score in the NMS patients. In contrast, a negative correlation between the postoperative residual curve and domains was observed in AIS for the total SRS score (–0.43, p = 0.001) as well as pain (–0.29, p = 0.03), general activity (–0.40, p = 0.002), and satisfaction (–0.30, p = 0.03) domains. The correction percentage correlated positively with total SRS score in AIS, correlation 0.31, p = 0.008.
In 36 NMS patients the fusion was extended to the pelvis, 24 using iliac screws and 12 using S2AI screws. The postoperative function domain in NMS patients without pelvic fixation was superior to patients with pelvic instrumentation using S2AI screws (mean 3.3 [1.1] vs. 1.9 [1.1], p = 0.02). Patients with iliac had better function domain compared with S2AI screw implantation (mean 3.2 [1.1] vs. 1.9 [1.1], p = 0.01).
Complications
The number of complications was significantly larger in the NMS group (6/60) than in the AIS group (1.7%, 2/120) (p = 0.02) (Table 3). 1 patient in each group required reoperation: 1 patient with AIS for a postoperative neurologic deficit and 1 patient with NMS had irrigation and debridement for deep surgical site infection.
Discussion
Based on our results, there was a significant improvement in multiple HRQoL domains after an instrumented posterior spinal fusion for NMS patients including SRS-24 total score and its domains. When compared with AIS patients, the SRS domain scores for function and general activity remained at a statistically lower level in the NMS patients. NMS patients reported better postoperative pain and self-image than the AIS patients. Although the total score was similar, the overall increase in health-related quality of life in NMS patients was significantly larger than in AIS.
Our hypothesis was that the HRQoL after a spinal fusion in NMS patients would be inferior compared with the AIS patients, which was not confirmed. At 2-year follow-up an improvement in all domains was observed in the NMS group, similar to the AIS group. In addition, the total SRS-score increase was significantly larger in the NMS than in the AIS group. The only significant finding in the change of separate domain between the groups was in pain, with the improvement being more significant in AIS patients. Given the superior preoperative pain status of NMS patients, this significance may not be relevant for postoperative quality of life, especially with no difference in postoperative pain between the groups. In addition, pain perception in NMS patients can be more difficult to evaluate (due to lack of self-expression), and for some patients it is based on parental assessment. These findings suggest that the perceived HRQoL in NMS patients after pedicle screw instrumentation is not inferior compared with the AIS patients.
The curvature correction influenced the HRQoL only in the AIS group. This may be explained by the differences in indications and preoperative quality of life concerns between the 2 patient groups. The main concerns for idiopathic patients might be back pain and its consequences for daily life, as well as fear of developing a severe curvature, whereas in NMS patients challenges related to breathing, posture, and daily routines may also be more frequent. In the NMS patients the fusion level correlated negatively with postoperative pain and total SRS scores, compared with AIS where no differences could be seen.
Comparison with previous data
Ersberg and Gerdhem conducted a similar retrospective register analysis, using the SRS-22r questionnaire. They found an increase in HRQoL in 13 children with NMS and 123 in the AIS groups, but no significant differences between the groups. When comparing the postoperative domain changes, neuromuscular patients experienced improved function and idiopathic patients less pain and both groups experienced postoperatively improved self-image. The preoperative Cobb angles were equivalent to our cohort, yet the curve correction was not reported and remains unclear (19). In accordance with these findings, our NMS patients’ HRQoL also increased postoperatively. We found a similar difference in pain domains, but in contrast the function domain improvements of our patients were similar between groups. The general self-image improved also in our data for both groups.
Obid et al. reported similar correction rates, yet a larger preoperative curve (83°), and increased quality of life postoperatively for patients with NMS evaluated with PEDI (pediatric disability inventory) and GMFS (gross motor function score). There was no control group in this study, and the preoperative status was not reported (20). Suk et al. conducted a retrospective study in NMS patients comparing the difference between 2 questionnaires (12). In their study better sitting balance did not impact on quality of life. The mean postoperative Cobb angle in their data (39° [20°]) was almost twice as large as our data, which might have an impact on the results.
Tondevold et al. observed that use of pelvic instrumentation was beneficial in terms of radiographic deformity correction and maintenance for non-ambulatory children with NMS. In the current study pelvic instrumentation was associated with worse HRQoL, making the clinical decision for fusion levels more complicated (8). Suresh et al. described the benefit of the S2AI technique in their article as there is less subcutaneous muscle dissection, more deeply seated implants, and no need for connectors, all diminishing the risk of implant prominence (5). In our data, the function domain score was better with iliac screws than with the S2AI technique, yet no differences in other domains were noted. This finding might be incidental; however, the longer lever arm of iliac screws for pelvic obliquity correction might be beneficial if soft tissue complications can be avoided.
As no levels for minimum clinically important difference MCID have been established for the SRS-24 scores, the clinical significance of the results of these 3 studies containing preoperative and postoperative values cannot be determined, but only estimated by using the existing SRS-22R MCIDs. Carreon et al. defined the MCID after surgical correction for the SRS-22R questionnaire, and the MCID for pain, activity, and appearance domains was 0.20, 0.08, and 0.98 respectively (21). In the NMS group, both pain and general activity means changes exceed the MCID thresholds, and in AIS pain exceeds the limit. In NMS 65% of the patients were above the threshold concerning pain, and 59% concerning general activity. In AIS patients, 86% of the patients exceeded the MCID threshold. These results should be considered clinically relevant.
Strengths and limitations
The strengths of our study include the prospective data collection and that the control groups underwent a similar type of operation. Also, all the operations were performed by the same experienced pediatric orthopedic spine surgeon and the perioperative protocols were standardized in both case and control groups.
The preoperative baseline function of neuromuscular patients does not match that of AIS patients, as many of them are non-ambulatory and cannot walk or function in the same way. Constraints have been part of patients’ lives for a longer time, so it is likely that the requirements of the NMS patients are not similar to that of AIS patients. This may blur the differences between the groups. However, this uncertainty can be reduced by looking at within-group differences. Also, the operations performed for NMS are more extensive and the fusions cover the whole thoracolumbar spine. Therefore, the differences should also be more apparent: an inferior baseline situation compared with a larger change should lead to a bigger difference.
The main limitation of this study is its retrospective nature based on a prospective database. Also, in some neuromuscular patients the assessment of quality of life was carried out by the patient’s caregiver due to the patients’ disabilities. NMS includes a heterogeneous group of medical comorbidities and for CP a special HRQoL assessment tool has been developed (CPCHILD). The use of a SRS-24 questionnaire brings its own problems: first, as the patient enrollment to our study has been ongoing for a long time, during the study period better validated questionnaires have become available. Second, it was developed to evaluate the HRQoL in patients treated for AIS (20) and is not ideal for NMS patients. The use of a similar outcome questionnaire for our study groups allowed us to perform a direct comparison.
Conclusions
The health-related quality of life in NMS patients improved significantly after segmental pedicle screw instrumentation from preoperatively to 2-year follow-up. This improvement in total SRS score was significantly larger in the NMS than in the AIS patients. Spinal fusion to the pelvis was associated with worse pain scores, while the residual curve had no impact on the HRQoL in the NMS group.
- Vialle R, Thevenin-Lemoine C, Mary P. Neuromuscular scoliosis. Orthop Traumatol Surg Res 2013; 99(1 Suppl.): S124-S139. doi: 10.1016/j.otsr.2012.11.002.
- Hägglund G, Pettersson K, Czuba T, Persson-Bunke M, Rodby-Bousquet E. Incidence of scoliosis in cerebral palsy. Acta Orthop 2018; 89(4): 443-7. doi: 10.1080/17453674.2018.1450091.
- Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet 1998; 351(9117): 1687-92. doi: 10.1016/S0140-6736(98)01302-6.
- Helenius I J, Viehweger E, Castelein R M. Cerebral palsy with dislocated hip and scoliosis: what to deal with first? J Child Orthop 2020; 14(1): 24-9. doi: 10.1302/1863-2548.14.190099.
- Suresh K V, Ikwuezunma I, Margalit A, Sponseller P D. Spinal fusion with sacral alar iliac pelvic fixation in severe neuromuscular scoliosis. JBJS Essent Surg Tech 2021; 11(3):e20.00060 doi: 10.2106/JBJS.ST.20.00060.
- Thometz J G, Simon S R. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am 1988; 70(9): 1290-6.
- Wishart B D, Kivlehan E. Neuromuscular scoliosis: when, who, why and outcomes. Phys Med Rehabil Clin N Am 2021; 32(3): 547-56. doi: 10.1016/j.pmr.2021.02.007.
- Tondevold N, Lastikka M, Andersen T, Gehrchen M, Helenius I. Should instrumented spinal fusion in nonambulatory children with neuromuscular scoliosis be extended to L5 or the pelvis? Bone Joint J 2020; 102-B(2): 261-7. doi: 10.1302/0301-620X.102B2.BJJ-2019-0772.R2.
- El-Hawary R, Chukwunyerenwa C. Update on evaluation and treatment of scoliosis. Pediatr Clin North Am 2014; 61(6): 1223-41. doi: 10.1016/j.pcl.2014.08.007.
- El-Bromboly Y, Hurry J, Padhye K, Johnston C, McClung A, Samdani A, et al. The effect of proximal anchor choice during distraction-based surgeries for patients with nonidiopathic early-onset scoliosis: a retrospective multicenter study. J Pediatr Orthop 2021; 41(5): 290-5. doi: 10.1097/BPO.0000000000001784.
- Miyanji F, Nasto L A, Sponseller P D, Shah S A, Samdani A F, Lonner B, et al. Assessing the risk–benefit ratio of scoliosis surgery in cerebral palsy: surgery is worth it. Bone Joint Surg Am 2018; 100(7): 556-63. doi: 10.2106/JBJS.17.00621.
- Suk K S, Baek J H, Park J O, Kim H S, Lee H M, Kwon J W, et al. Postoperative quality of life in patients with progressive neuromuscular scoliosis and their parents. Spine J 2015; 15(3): 446-53. doi: 10.1016/j.spinee.2014.09.030.
- Danielsson A J. What impact does spinal deformity correction for adolescent idiopathic scoliosis make on quality of life? Spine 2007; 32(19 Suppl.): S101-8. doi: 10.1097/BRS.0b013e318134ed0e.
- Danielsson A J, Wiklund I, Pehrsson K, Nachemson A L. Health-related quality of life in patients with adolescent idiopathic scoliosis: a matched follow-up at least 20 years after treatment with brace or surgery. Eur Spine J 2001; 10(4): 278-88. doi: 10.1007/s005860100309.
- Fan H, Wang Q, Huang Z, Sui W, Yang J, Deng Y, et al. Comparison of functional outcome and quality of life in patients with idiopathic scoliosis treated by spinal fusion. Medicine 2016; 95(19): e3289. doi: 10.1097/MD.0000000000003289.
- Soini V, Raitio A, Helenius I, Helenius L, Syvanen J. A retrospective cohort study of bleeding characteristics and hidden blood loss after segmental pedicle screw instrumentation in neuromuscular scoliosis as compared with adolescent idiopathic scoliosis. N Am Spine Soc J 2022; 12: 100190. doi: 10.1016/j.xnsj.2022.100190.
- Lenke L, Edwards C C, Bridwell K H. The Lenke classification of adolescent idiopathic scoliosis: how it organizes curve patterns as a template to perform selective fusions of the spine. Spine 2003; 28: S199-S207.
- Beauchamp E C, Lenke L G, Cerpa M, Newton P O, Kelly M P, Blanke K M, et al. Selecting the “touched vertebra” as the lowest instrumented vertebra in patients with Lenke type-1 and 2 curves: radiographic results after a minimum 5-year follow-up. J Bone Joint Surg Am 2020; 102(22): 1966-73. doi: 10.2106/JBJS.19.01485.
- Ersberg A, Gerdhem P. Pre- and postoperative quality of life in patients treated for scoliosis. Acta Orthop 2013; 84(6): 537-43. doi: 10.3109/17453674.2013.854667.
- Obid P, Bevot A, Goll A, Leichtle C, Wulker N, Niemeyer T. Quality of life after surgery for neuromuscular scoliosis. Orthop Rev 2013;5 (1):e1. doi: 10.4081/or.2013.e1.
- Carreon L Y, Sanders J O, Diab M, Sucato D J, Sturm P F, Glassman S D, et al. The minimum clinically important difference in Scoliosis Research Society-22 Appearance, Activity, and Pain domains after surgical correction of adolescent idiopathic scoliosis. Spine 2010; 35(Issue): 2079-83. doi: 10.1097/BRS.0b013e3181c61fd7.