Revision risk by using the direct superior approach (DSA) for total hip arthroplasty compared with postero-lateral approach: early nationwide results from the Dutch Arthroplasty Register (LROI)
Bart VAN DOOREN 1, Rinne M PETERS 1,2, Harmen B ETTEMA 3, B Willem SCHREURS 4,5, Liza N VAN STEENBERGEN 5, Stefan B T BOLDER 6, and Wierd P ZIJLSTRA 1
1 Department of Orthopaedics, Medical Center Leeuwarden, Leeuwarden; 2 Department of Orthopaedics, University Medical Center Groningen, Groningen; 3 Department of Orthopaedics, Isala, Zwolle; 4 Department of Orthopaedics, Radboudumc, Nijmegen; 5 Dutch Arthroplasty Register (LROI), ‘s Hertogenbosch; 6 Department of Orthopaedics, Amphia Hospital, Breda, The Netherlands
Background and purpose — The direct superior approach (DSA) is a modification of the classic posterolateral approach (PLA) for total hip arthroplasty (THA), in which the iliotibial band and short external rotators are spared. The revision rate of the DSA has not been investigated previously using arthroplasty registry data. We examined the reasons and risk of revision of the DSA, compared with the direct anterior approach (DAA) and PLA.
Patients and methods — In this population-based cohort study we included 175,543 primary THAs performed between 2014 and 2020 (PLA, n = 117,576; DAA, n = 56,626; DSA, n = 1,341). Competing risk survival analysis and multivariable Cox proportional hazard analyses, adjusted for potential confounders, were performed.
Results — After 3 years, crude revision rates due to any reason were 2.1% (95% confidence interval [CI] 1.3–3.3) for DSA, and 2.9% (CI 2.8–3.0) for PLA. Crude dislocation revision rates were 0.3% (CI 0.1–0.8) for DSA, versus 1.0% (CI 0.9–1.0) for PLA. Dislocation revision rate for DSA did not differ from DAA (0.3% [CI 0.2–0.3]). Multivariable Cox regression analysis demonstrated no overall difference in revision rates for the DSA (HR 0.6 [CI 0.4–1.09) compared with the PLA. Lower risk of revision due to dislocation was found in patients operated on through the DSA (HR 0.3 [0.1–0.9]) compared with the PLA.
Conclusion — Early nationwide results suggest that the DSA for total hip arthroplasty seems to show a tendency towards a lower risk of revision for dislocation but no overall reduced revision risk compared with the PLA.
Citation: Acta Orthopaedica 2023; 94: 158–164. DOI https://doi.org/10.2340/17453674.2023.11959.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-09-29. Accepted: 2023-03-13. Published: 2023-04-13.
Correspondence: bart.van.dooren@mcl.nl
Authors contributed to: (1) study design and study protocol, (2) gathered data, (3) analyzed data, (4) initial draft, and (5) final draft. BvD contributed to 3–5; RMP contributed to 1, 3, 4 and 5; WPZ, HBE, BWS, and SBTB contributed to 1, 4 and 5; LNvS contributed to 3, 4 and 5.
Handling co-editors: Keijo Mäkelä and Philippe Wagner
Acta thanks José Cordero-Ampuero, Leif Ryd, and Sarunas Tarasevicius for help with peer review of this study.
Recurrent dislocation is the most common cause of early revision in primary total hip arthroplasty (THA) (1,2). Risk factors associated with recurrent dislocation are surgical approach and femoral head size (1,3-5). In the Netherlands, the posterolateral approach (PLA) is the most frequently used approach (50%) in hip replacement, although the direct anterior approach (DAA) has gained considerable popularity over the past years (2). The DAA has been associated with a reduced risk of revision for dislocation, compared with the PLA, but a higher risk of femoral stem revisions has previously been reported (1,6-7).
To reduce dislocation rates and enhance recovery of patients operated on through the classic PLA, the direct superior approach (DSA) was developed. The DSA is an adaptation of the PLA, in which the iliotibial band and short external rotators (except for the piriformis or conjoint tendon) are preserved (8-10). The main goals of a muscle-sparing approach are to enhance early recovery and decrease complications. In comparison with the PLA, the DSA should therefore improve implant stability. Outcomes of THA with the DSA have shown that reliable implant positioning can be obtained with a low early complication rate (11-13). Shorter length of hospital stay and enhanced recovery with higher functional scores were recorded in comparison with the PLA (14-19). Contrary to the DAA, the learning curve seems to be limited (10-11,20). Whether a limited learning curve will be found outside specialist centers remains to be proven. In addition, there is need for stronger evidence to support the claim for a reduced dislocation risk with the DSA.
We examined the early outcomes of the DSA, using Dutch nationwide arthroplasty data. Specifically, we examined the risk of revision for dislocation, as well as the risk of revision for any other reason than dislocation.
Patients and methods
Study design
This is a population-based cohort study of all primary THAs using the DAA, PLA, or DSA in a Dutch hospital. Data from January 1, 2014 to December 31, 2020, was retrieved from the Dutch Arthroplasty Register (LROI). The study is reported according to the STROBE/RECORD guideline.
Setting and data source
The Dutch Arthroplasty Register (LROI) is a nationwide population-based register that has prospectively collected data on joint arthroplasties in the Netherlands since 2007. The LROI was initiated by the Dutch Orthopaedic Association (NOV). Data from arthroplasties performed in the Netherlands can be entered directly into the LROI databases using the LROI webforms or by uploads from the electronic patient file of the healthcare provider. In this manner patient, procedure (e.g., surgical approach), and prosthesis characteristics are uniformly and completely collected. Internal checks and defaults are included in the system to stimulate valid and optimal registration. Completeness of records is validated every year by comparing the number of procedures in the LROI with the number of procedures in the hospital information system (HIS). Therefore, high validity and data quality is retained for both primary and revision THAs (21,22). The register covers 100% of Dutch hospitals with a completeness of 99% for primary THAs and over 97% for hip revision arthroplasty in the last 5 years (2,21).
Participants
Eligible patients who received a primary non-metal-on-metal (MoM) THA using the DAA, PLA, or DSA in a Dutch hospital between 2014 and 2020 and registered in the LROI were included. Demographic data, procedure, prosthesis characteristics, and outcome measures were provided by the LROI.
Outcome
The primary outcome is the short-term risk of revision for any reason, for dislocation, and for any other reason except dislocation. Revision was defined as a change, addition, or removal of 1 or more components of the prosthesis (2). All revision procedures and reasons for revision are registered during revision surgery. Multiple reasons for revision can be registered (e.g., infection, dislocation, periprosthetic fracture, liner wear, and/or loosening). An overview of revision procedures was provided by the LROI. Afterwards, reasons for revision were categorized by the authors as dislocation and non-dislocation including all reasons for revision except dislocation.
Surgical technique
DSA data has been collected in the LROI since 2014. The orthopedic surgeon fills out which surgical approach is used during surgery using mandatory LROI webforms. DSA is performed by selected dedicated hospitals in the Netherlands with a strong interest in this technique. Registration for DSA is therefore optimal. Validity for the registration of surgical approaches for primary THA is checked annually and was 99.3% in the past year (22). The DSA has been described in detail by Roger and Hill (18). In short, the patient is in lateral decubitus position. From the posterosuperior corner of the greater trochanter extending proximally in line with the gluteus maximus fibers, the skin, subcutis, and gluteus maximus fascia are minimally incised, sparing the iliotibial band. The gluteus maximus is split and the piriformis and, if conjoined, the obturator internus tendon are detached, tagged, and reflected posteriorly. The gluteus minimus is elevated and, after a capsulotomy in line with the collum femoris, the hip is dislocated, followed by femoral neck resection, acetabulum reaming, and implantation of acetabular and femoral components using long DSA Hohmann retractors (GerMedUSA, Garden City Park, NY, USA) and specialized reamers. The capsule is closed side-to-side, the piriformis reattached, and the fascia, subcutaneous tissue, and skin closed in layers.
Statistics
Group comparisons were made using a chi-square test. Survival time was calculated as the time from primary THA to first revision arthroplasty for any reason, death of the patient, or the end of the follow-up period (January 1, 2021) using competing risk analysis. We calculated the 1-, 3-, and 5-year crude cumulative incidence of revision for any reason, dislocation, and other reason except dislocation.
Multivariable Cox proportional hazard analyses were performed to test for differences in risk of revision for any reason, revision for dislocation, and revision for all other causes than dislocation, adjusted for possible confounding variables (i.e., age, sex, ASA score, body mass index (BMI), diagnosis, previous surgery, femoral head size, and fixation and articulation type). For all categorical covariates added to the model, the proportional hazards assumption was checked and met by inspecting log-minus-log curves. P-values below 0.05 were considered statistically significant. Results were reported as hazard ratios (HR) with 95% confidence intervals (CI). All analyses were performed using SPSS version 24.0 (IBM Corp, Armonk, NY, USA).
Sensitivity analyses
Separate post-hoc sensitivity analyses were conducted to assess whether altering any of our assumptions or inclusions within the model may lead to different final interpretations of our data (23). First, the multivariable Cox proportional hazard analysis was repeated for the period 2007–2020 without BMI as this confounder was available in the LROI only from 2014 onward. Second, we repeated the multivariable Cox proportional hazard analysis for the period 2016–2020 to obtain more equal follow-up time among approaches, since the DSA was introduced more recently compared with the DAA and PLA. Furthermore, we performed a sensitivity analysis where all dual mobility cups were excluded from the analysis. Finally, we performed a sensitivity analysis for THA procedures for osteoarthritis (OA), because a higher risk of revision is seen in THAs for acute fracture (24).
Ethics, funding, data sharing, and disclosures
The study was approved by the LROI board and scientific advisory board of the LROI and the Medical Ethical Committee of the University Medical Center Groningen (No. METc 2021/280). The dataset was processed in compliance with the regulations of the LROI governing research on registry data. Restrictions apply to the availability of this data, which was used under license for the current study. This project was supported by an unrestricted grant from a non-profit foundation, Stichting MCL fonds. No benefits in any form have been received or will be received related directly or indirectly to the subject of this article. No conflicting interests were declared. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.11959
Results
175,543 primary non-MoM THAs were analyzed (Figure 1).
Figure 1. Flow chart included patients
Patient characteristics
175,543 primary THAs performed between 2014 and 2020 were included (PLA 117,576; DAA 56,626; DSA 1,341). The median length of follow-up was 2.9 years (range 0–7), with a shorter follow-up for the DSA (1.6 years; range 0–6) compared with the PLA (3.3 years; range 0–7). An overview of patient characteristics is set out in Table 1.
| PLA n = 117,576 67% | DSA n = 1,341 0.8% | DAA n = 56,626 32% | Total n = 175,543 | |
| Age | b | a | ||
| < 60 | 19,605 (17) | 253 (19) | 9,789 (17) | 29,647 (17) |
| 60–74 | 60,049 (51) | 703 (52) | 31,062 (55) | 91,814 (52) |
| ≥ 75 | 37,741 (32) | 385 (29) | 15,759 (29) | 53,885 (31) |
| Sex | a | |||
| Male | 41,758 (36) | 496 (37) | 19,202 (34) | 61,456 (35) |
| Female | 75,702 (64) | 845 (63) | 37,413 (66) | 113,960 (65) |
| ASA score | a | |||
| I | 18,773 (16) | 241 (18) | 11,372 (20) | 30,386 (17) |
| II | 74,300 (63) | 858 (64) | 36,204 (64) | 111,362 (64) |
| III–IV | 24,368 (21) | 242 (18) | 9,002 (16) | 33,612 (19) |
| Diagnosis | a | |||
| OA | 99,379 (85) | 1,220 (91) | 52,167 (92) | 152,766 (87) |
| Non-OA | 17,964 (15) | 120 (9.0) | 4,398 (7.8) | 22,482 (13) |
| Previous operation | a | |||
| Yes | 6,728 (5.8) | 32 (2.4) | 1,117 (2.0) | 7,877 (4.6) |
| No | 108,933 (94) | 1,304 (98) | 54,692 (98) | 164,929 (95) |
| Operation year | a | |||
| 2014–2016 | 52,244 (44) | 88 (6.6) | 14,434 (26) | 66,766 (38) |
| 2017–2020 | 65,332 (56) | 1,253 (93) | 42,192 (74) | 108,777 (62) |
| Smoking | a | |||
| Yes | 12,748 (11) | 153 (11) | 5,490 (9.8) | 18,391 (11) |
| No | 99,580 (89) | 1,186 (87) | 50,559 (90) | 151,325 (89) |
| BMI | b | a,b | ||
| < 18.5 | 1,124 (1.0) | 19 (1.4) | 420 (0.8) | 1,563 (0.9) |
| 18.5–25 | 37,128 (32) | 535 (40) | 20,739 (37) | 58,402 (34) |
| 25–30 | 48,103 (42) | 554 (41) | 23,686 (42) | 72,343 (42) |
| 30–40 | 27,436 (24) | 229 (17) | 10,698 (19) | 38,363 (22) |
| > 40 | 1,596 (1.4) | 4 (0.3) | 359 (0.6) | 1,959 (1.1) |
| Charnley | b | a | ||
| A | 49,756 (45) | 602 (46) | 24,779 (45) | 75,137 (45) |
| B1 | 31,714 (29) | 374 (29) | 17,227 (32) | 49,315 (30) |
| B2 | 25,187 (23) | 318 (24) | 11,520 (21) | 37,025 (22) |
| C | 3,510 (3.2) | 19 (1.4) | 1,067 (2.0) | 4,596(2.8) |
| Fixation | b | b | b | |
| Cemented | 35,946 (31) | 695 (52) | 6,136 (11) | 42,777 (24) |
| Cementless | 68,724 (59) | 470 (35) | 45,411 (80) | 114,605 (65) |
| Reversed hybrid | 4,299 (3.7) | 138 (10) | 2,205 (3.9) | 6,642 (3.8) |
| Hybrid | 7,883 (6.7) | 27 (2.0) | 2,474 (4.4) | 10,384 (5.9) |
| Unknown | 522 (0.4) | 11 (0.8) | 316 (0.6) | 849 (0.5) |
| Head size | b | a,b | ||
| 22–28 mm | 23,211 (20) | 380 (29) | 6,121 (12) | 29,712 (18) |
| 32 mm | 66,672 (58) | 794 (61) | 34,499 (65) | 101,965 (60) |
| 36 mm | 24,367 (21) | 131 (10) | 12,072 (23) | 36,570 (22) |
| > 38 mm | 681 (0.6) | 0 (0) | 41 (0.1) | 722 (0.4) |
| Articulation | a | |||
| CoC | 5,494 (4.9) | 0 (0) | 4,748 (9.2) | 10,242 (6.2) |
| CoM | 39 (0) | 1 (0.1) | 1 (0) | 41 (0) |
| CoP | 66,108 (59) | 1,014 (83) | 32,296 (63) | 99,418 (60) |
| MoC | 1 (0) | 0 (0) | 1 (0) | 2 (0) |
| MoP | 29,664 (27) | 92 (7.5) | 11,077 (21) | 40,833 (25) |
| ZoP | 10,598 (9.5) | 118 (9.6) | 3,585 (6.9) | 14,301 (8.7) |
| ASA score = American Society of Anesthesiology score, | ||||
| BMI = Body Mass Index, | ||||
| OA = Osteoarthritis, | ||||
| Articulation: CoC = ceramic-on-ceramic, CoM = ceramic-on-metal, | ||||
| CoP = ceramic-on-polyethylene, MoC = metal-on-ceramic, | ||||
| MoP = metal-on-polyethylene, ZoP: oxidized-zirconium-on -polyethylene. | ||||
| a p < 0.001. | ||||
| b Numbers do not add up to total due to unknown or missing values. | ||||
Reasons for revision
4,608 (2.6%) THAs were revised during the follow-up period. 21 revisions were performed in the DSA group. Reasons for revision differed between the surgical approach groups. PLA THAs were more often revised for dislocation, while DAA and DSA THAs were revised more often because of loosening of the femoral component and periprosthetic fractures (Table 2, see Appendix).
Overall revision
The overall crude cumulative incidence of revision for all causes after 1, 3, and 5 years for THAs using DSA was 0.8% (CI 0.5–1.5), 2.1% (CI 1.3–3.3), and 2.6% (CI 1.5–4.6) respectively. After 1 year the crude revision rate was lower for the DSA compared with the PLA (1.8% [CI 1.7–1.9]). At 3 and 5 years, the overall crude revision rate was comparable for the DSA compared with the PLA (respectively 2.9% [CI 2.9–3.0]; 3.5% [CI 3.4–3.6]). The crude revision rates for the DSA were comparable with the DAA (Table 3, Figure 2a).
| PLA | DSA | DAA | |
| Revision for any reason (n = 4,608 revisions) | |||
| 1 year | 1.8 (1.7–1.9) | 0.8 (0.5–1.5) a | 0.9 (0.9–1.0) b |
| 3 years | 2.9 (2.8–3.0) | 2.1 (1.3–3.3) | 1.7 (1.5–1.8) |
| 5 years | 3.5 (3.4–3.6) | 2.6 (1.5–4.6) | 1.9 (1.8–2.1) |
| Revision for dislocation (n =1,360 revisions) | |||
| 1 year | 0.5 (0.5–0.6) | 0.15 (0.04–0.6) | 0.19 (0.16–0.23) b |
| 3 years | 1.0 (0.9–1.0) | 0.26 (0.1–0.8) a | 0.27 (0.23–0.32) b |
| 5 years | 1.2 (1.1–1.3) | 0.84 (0.2–3.4) | 0.29 (0.24–0.34) b |
| Revision for any other reason than dislocation (n = 3,248 revisions) | |||
| 1 year | 1.3 (1.2–1.3) | 0.7 (0.4–1.3) | 0.8 (0.7–0.8) b |
| 3 years | 2.0 (1.9–2.1) | 1.8 (1.1–3.0) | 1.4 (1.3–1.5) b |
| 5 years | 2.4 (2.3–2.5) | 1.8 (1.1–3.0) | 1.6 (1.5–1.8) b |
| a p < 0.05 (DSA vs PLA) | |||
| b p < 0.05 (DAA vs PLA) | |||
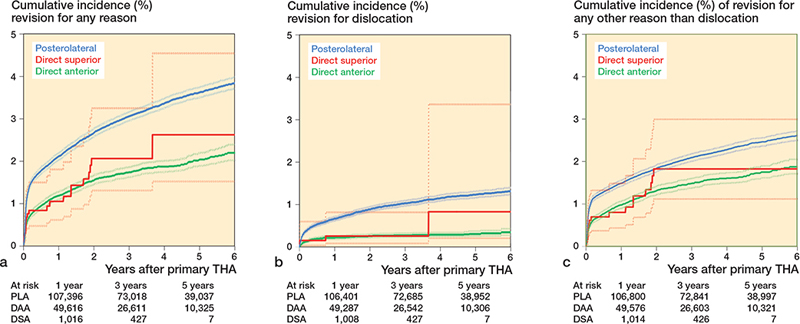
Figure 2. Crude cumulative incidence of revision for any reason (a), for dislocation (b), and for any other reason than dislocation (c) in primary THAs according to surgical approach performed in 2014–2020 in the Netherlands including number at risk by time in years (N = 175,543)
Multivariable Cox regression analysis, adjusting for potential confounders, demonstrated no difference in overall revision rates for the DSA (HR 0.6 (CI 0.4–1.0) compared with the PLA (Table 4).
| Cases | Revisions | Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) a | |
| Revisions for any reason | ||||
| DSA | 1,341 | 21 | 0.6 (0.4–1.0) b | 0.6 (0.4–1.0) b |
| DAA | 56,626 | 899 | 0.5 (0.5–0.6) b | 0.6 (0.56–0.64) b |
| PLA | 117,576 | 3,688 | 1 | 1 |
| Revision for dislocation | ||||
| DSA | 1,341 | 4 | 0.4 (0.1–1.0) b | 0.3 (0.1–0.9) b |
| DAA | 56,626 | 147 | 0.3 (0.2–0.3) b | 0.3 (0.2–0.4) b |
| PLA | 117,576 | 1,209 | 1 | 1 |
| Revision for any other reason than dislocation | ||||
| DSA | 1,341 | 17 | 0.8 (0.5–1.2) | 0.8 (0.5–1.3) |
| DAA | 56,479 | 752 | 0.7 (0.6–0.7) b | 0.8 (0.7–0.8) b |
| PLA | 117,576 | 2,479 | 1 | 1 |
| a Adjusted for gender, age, ASA-score, diagnosis, previous operations, femur head diameter, fixation articulation and BMI. | ||||
| b p < 0.05. | ||||
Revision due to dislocation
4 revisions due to dislocation were performed in the DSA group. The crude cumulative incidence of revision due to dislocation after 1, 3, and 5 years for the DSA group was 0.2% (CI 0.04–0.6), 0.3% (CI 0.1–0.8), and 0.8% (CI 0.2–3.4) and was comparable with the DAA group (0.2% (CI 0.16–0.23), 0.3% (CI 0.2–0.3), and 0.3% (CI 0.2–0.3) respectively). PLA THAs showed a higher 3-year crude cumulative incidence of revision due to dislocation compared with the DSA (1.0% [CI 0.9–1.0] vs 0.3% [CI 0.1–0.8]). At 1 and 5 years, the crude revision rates for dislocation were comparable for the DSA compared with the PLA (Table 3, Figure 2b).
Multivariable Cox regression analysis demonstrated, after adjusting for potential confounders, a lower risk of revision due to dislocation for the DSA and the DAA compared with PLA (respectively HR 0.3 [CI 0.1–0.9] and HR 0.3 [CI 0.2– 0.4] vs. HR 1.0) (Table 4).
Revision due to any other reason except dislocation
3,248 THAs were revised due to any other reason than dislocation. The crude 1-year revision rate was lower for the DAA (0.8% [CI 0.7–0.8]) and comparable for the DSA (0.7% [CI 0.4–1.3]), compared with the PLA (1.3% [CI 1.2–1.3]) (Table 3, Figure 2c). Multivariable Cox regression analyses showed no statistically significant difference in risk of revision due to any other reason than dislocation between the different approaches (Table 4).
Sensitivity analyses
First, we repeated the multivariable Cox proportional hazard analysis for the period 2016–2020 to obtain more equal follow-up time between the 3 approaches, with a median follow-up of respectively 2, 1.6, and 2.4 years for the PLA, DSA, and DAA. Thereafter, the DSA showed similar HRs for risk of revision due to any reason (HR 0.6 [CI 0.4–1.0]) and dislocation (HR 0.4 [CI 0.1–1.0]) compared with the previous analysis (respectively HR 0.6 [CI 0.4–1.0] and 0.3 [CI 0.1–0.9]). Although HRs were similar for dislocation between the approaches, the difference was not statistically significant, which can be possibly explained by the reduced sample size.
Second, the multivariable Cox proportional hazard analysis was repeated for the period of 2007–2020 (n = 266,005). BMI was excluded from the analysis as BMI was only registered from 2014. Hereafter, the analyses showed that the hazard ratios for the DSA were comparable for the overall risk of revision (HR 0.6 [CI 0.4–1.0]) and for revision due to dislocation (HR 0.3 [CI 0.1–0.9]), compared with the previous analysis.
Furthermore, we performed a sensitivity analysis without dual mobility bearings. This analysis showed similar HRs for the DSA for risk of revision due to any reason (HR 0.7 [CI 0.4–1.0]) and dislocation (HR 0.4 [0.1–0.9]) compared with the previous analysis.
Finally, after excluding THAs for non-OA, the DSA showed slightly higher HRs for risk of revision due to any reason (HR 0.8 [CI 0.5–1.2]) and dislocation (HR 0.4 [CI 0.2–1.1]) compared with the previous analysis. Revision for any reason and for dislocation did not remain significant compared with the initial analysis.
Discussion
We found similar crude revision rates for the DSA compared with the PLA at 3 years postoperatively (2.1%, 2.9%, respectively). Crude dislocation revision rates after 3 years were lower after DSA than after PLA (0.3%, 1%, respectively). After correction for confounders, there was no longer a lower risk of revision for any reason but lower risk of revision due to dislocation for the DSA compared with the PLA. The outcomes of the DSA were similar to the outcomes of the DAA. To our knowledge, this is the first nationwide registry study to report on the outcomes of the DSA.
To date, most DSA studies have been case series or casecontrol studies from specialized centers with limited followup. Based on these retrospective series it may be concluded that the DSA can reduce the risk for dislocation and enhance early recovery (14). One prospective single surgeon study of 200 cases did not find any dislocations in the DSA group within the first year (13). Overall, 2 complications were reported: 1 acute deep and 1 superficial wound infection. Roger and Hill in 2012 reported 3 complications in 135 patients operated through the DSA with a mean follow-up of 22 months (range 14–33) (18). No dislocations were observed. In contrast, 1 recent randomized controlled trial compared self-reported and clinical measurements between subjects after DSA (n = 22) compared with PLA (n = 23). The authors reported 1 periprosthetic fracture and 2 dislocations due to falls in the DSA group compared with 1 complication in the PLA group in the first 3 months after surgery (19). Both dislocations were treated with closed reduction, without further consequences.
Multiple large registry studies have been performed to compare the revision rate of primary THAs related to surgical approach (1,6). Hoskins et al. in 2020 examined the revision rates between surgical approaches with data from the Australian Orthopaedic Association National Joint Replacement Registry between 2015 and 2018 (6). They reported a higher revision rate for femoral-sided revisions for the DAA compared with the PLA. Furthermore, a higher rate of dislocation was found for the PLA, which is in line with our results. Likewise, Zijlstra et al. in 2017 reported that the DAA has been associated with a reduced risk of revision for dislocation, compared with the PLA, but a higher risk of femoral-sided revisions was seen (1). To our knowledge, no registry studies have previously reported on the mid-term survival of the DSA.
Our results may be affected by case-mix factors. Several studies demonstrated, based on registry data, that high ASA scores and severe obesity are the strongest predictors for short-term revision after a primary THA in patients with osteoarthritis (25,26). In our study, patients in the PLA and DSA cohort were more likely to be ASA III–IV compared with the DAA group and therefore to have a potentially higher risk of early revision. In addition, our data showed a lower BMI in the DSA cohort compared with the PLA and DSA cohorts, which might partly explain the low number of dislocations. This may represent an inherent selection bias of the study, as DSA is generally performed only in patients with a BMI below 35. Therefore, we corrected for BMI (and other potential confounders) using multivariable Cox proportional hazard analyses and found similar outcomes to the unadjusted results. In addition, sensitivity analyses showed similar HRs for revision due to dislocation for the DSA after excluding BMI from the analysis.
Revision for dislocation
At 3 years, crude revision rates for dislocation were lower for the DSA compared with the PLA. However, at 1 and 5 years we found comparable crude risk of revision for dislocation with the DSA approach, compared with the PLA. After correction for confounders, we found a lower risk of revision due to dislocation for the DSA compared with the PLA. A previous study using Dutch arthroplasty data demonstrated an increased risk of revision due to dislocation for THAs using 22–28-mm femoral head components (1). Our study demonstrated a higher number of small femoral heads (22–28 mm) for the DSA compared with the PLA (respectively 29% and 20% of registered THAs). Compared with the PLA, however, the DSA demonstrated a lower adjusted risk of dislocation revision, even with a relatively higher number of small femoral heads. This suggests the DSA shows promising results with regard to dislocation risk, even in the presence of small femoral heads. This is further stressed by the relatively low number of 36-mm heads with DSA, compared with DAA THA (10% versus 23%), while demonstrating a similar risk for revision due to dislocation.
Limitations
The generalizability of these results is subject to certain limitations. For instance, this is a non-randomized, observational study and is therefore impacted by selection bias. Second, this study has a limited number of patients in the DSA subgroup (n = 1,341) and a limited median follow-up of 19 months. This is reflected in the relatively broad confidence intervals seen in our data, some of which border on their significance with respect to the PLA. Furthermore, based on data from the LROI annual reports from the last years, we can conclude that the DSA is used in 5 large-volume centers, which is rather limited (2). Surgeon experience and annual case volume is not registered in the LROI (for privacy reasons), hence these factors may act as possible confounders, for which we cannot adjust. Moreover, data on non-surgically treated dislocations after THA is not available in the LROI, as only revision procedures are registered. Likewise, there is no data on THAs that were treated for postoperative complications by reoperation using open reduction and internal fixation in the case of a periprosthetic fracture. Furthermore, some surgical factors that can potentially affect the risk of revision (e.g., choice of prosthetic system and implant positioning) were not available. Lastly, some DSAs may erroneously have been registered as PLAs, depending on the ICT systems in the hospital, because not every digital system had the option to separately register the DSA in addition to the usual approach options. Moreover, the exact distinctions between DSA, minimally invasive PLA, and classical PLA are not clearly defined and these approaches may be used by surgeons as a sliding scale. We cannot fully rule out that some surgeons may have registered a DSA approach for what other surgeons would consider a minimally invasive PLA. A key strength of the present study was that no exclusion for learning curve was performed. As the DSA is a relatively new approach the early phases of the learning curve can potentially affect our results. In our study all THAs operated on through the DSA were included, strengthening the generalizability of the DSA results.
The DSA may be promising for the future as this approach shows similarities to the PLA. The same anatomical landmarks and lateral decubitus position are used and therefore the DSA seems to be easy to adopt for surgeons trained with the PLA. If required, intraoperative conversion to the PLA is possible.
Conclusion
We found that DSA had a lower risk of revision for dislocation but not for overall revision. For orthopedic surgeons experienced in the PLA, the DSA offers an attractive opportunity to modify the PLA in order to improve future outcomes after THA.
- Zijlstra W P, De Hartog B, Van Steenbergen L N, Scheurs B W, Nelissen R G H H. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty. Acta Orthop 2017; 88(4): 395-401. doi: 10.1080/17453674.2017.1317515
- Dutch Arthroplasty Register (LROI). Annual report. LROI report, 2021. Numbers – LROI Report - Information on orthopaedic prosthesis procedures in the Netherlands (lroireport.nl). Last accessed: March, 2022.
- Charney M, Paxton E W, Stradiotto R, Lee J J, Hinman A D, et al. A comparison of risk of dislocation and cause-specific revision between direct anterior and posterior approach following elective cementless total hip arthroplasty. J Arthroplasty 2020; 35(6): 1651-7. doi: 10.1016/j.arth.2020.01.033.
- Meermans G, Konan S, Das R, Volpin A, Haddad F S. The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J 2017; 99-B(6): 732-40. doi: 10.1302/0301620X.99B6.38053.
- Kuijpers M F L, Hannink G, Vehmeijer S B W, van Steenbergen L N, Schreurs B W. The risk of revision after total hip arthroplasty in young patients depends on surgical approach, femoral head size and bearing type; an analysis of 19,682 operations in the Dutch arthroplasty register. BMC Musculoskelet Disord 2019; 20(1): 385. doi: 10.1186/s12891-0192765-z.
- Hoskins W, Bingham R, Lorimer M, de Steiger R N. Early rate of revision of total hip arthroplasty related to surgical approach. J Bone Joint Surg 2020; 102(21): 1874-82 doi: 10.2106/JBJS.19.01289
- Docter S, Philpot H T, Godkin L, Bryant D, Somerville L, et al. Comparison of intra and post-operative complication rates among surgical approaches in Total Hip Arthroplasty: a systematic review and meta-analysis. J Orthop 2020; (20): 310-25. doi: 10.1016/j.jor.2020.05.008
- Nam D, Meyer Z, Rames RD, Nunley R M, Barrack R L, et al. Is the direct superior, iliotibial band-sparing approach associated with decreased pain after total hip arthroplasty? J Arthroplasty 2017; 32(2): 453-7. doi: 10.1016/j.arth.2016.07.045.
- Barrett A A, Ezzibdeh R M, Horst P K, Roger D J, Amanatullah D F. Direct superior approach to the hip for total hip arthroplasty. JBJS Essent Surg Tech 2019; 9(2):e17. doi: 10.2106/JBJS.ST.18.00078.
- Kayani B, Konan S, Chandramohan R, Haddad F S. The direct superior approach in total hip arthroplasty. Br J Hosp Med 2019; 80(6): 320-4. doi: 10.12968/hmed.2019.80.6.320.
- Duijnisveld B J, van den Hout J A A M, Wagenmakers R, Koenraadt K L M, Bolder S B T. No learning curve of the direct superior approach in total hip arthroplasty. Orthop Surg 2020; 12(3): 852-60. doi:10.1111/os.12689.
- Korth M J S, Ezzibdeh R, Arora P, Amanatullah D F. Excellent clinical and radiographic outcomes with direct superior total hip arthroplasty with a minimum of two-year follow up. Surg Technol Int 2021; 37: 342-6.
- Tsiridis E, Kenanidis E, Potoupnis M, Sayegh F E. Direct superior approach with standard instrumentation for total hip arthroplasty: safety and efficacy in a prospective 200-case series. Hip Int 2020; 30(5): 552-8. doi: 10.1177/1120700019843120.
- Ezzibdeh R, Korth M J S, Arora P, Amanatullah D F. Case-controlled analysis of the direct superior and mini-posterior approach for total hip arthroplasty at a minimum of two years of follow up. Surg Technol Int 2021; 37: 353-9.
- LeRoy T E, Hayden B L, Desmarais J, Menendez M E, Ward D. Early outcome comparison of the posterior approach and the superior approach for primary total hip arthroplasty. Arthroplast Today 2020; 6(3): 508-12. doi: 10.1016/j.artd.2020.05.005.
- Kenanidis E, Paparoidamis G, Pegios VF, Anagnostis P, Potoupnis M, et al. Earlier functional recovery and discharge from hospital for THA patients operated on via direct superior compared to standard posterior approach: a retrospective frequency-matched case-control study. Hip Int 2022:11207000221086506. doi: 10.1177/11207000221086506.
- Leonard H J, Ohly N E. Direct superior approach for total hip arthroplasty. Bone Joint J 2021;103-B(3):500-6. doi: 10.1302/0301620X.103B3.BJJ-2020-0916.R1.
- Roger D J, Hill D. Minimally invasive total hip arthroplasty using a transpiriformis approach: a preliminary report. Clin Orthop Relat Res 2012; 470(8): 2227-34. doi: 10.1007/s11999-011-2225-z.
- Ulivi M, Orlandini L, Vitale JA, Meroni V, Prandoni L, et al. Direct superior approach versus posterolateral approach in total hip arthroplasty: a randomized controlled trial on early outcomes on gait, risk of fall, clinical and self-reported measurements, Acta Orthop 2021; 92(3): 274-9. doi: 10.1080/17453674.2020.1865633.
- de Steiger R N, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 2015; 473(12): 3860-6. doi: 10.1007/s11999-015-4565-6.
- van Steenbergen L N, Denissen G A W, Spooren A, van Rooden S M, van Oosterhout F J, et al. More than 95% completeness of reported procedures in the population-based Dutch Arthroplasty Register. Acta Orthop 2015; 86(4): 498-505. doi: 10.3109/17453674.2015.1028307
- Dutch Arthroplasty Register (LROI).– LROI Report - Information on data quality and validity. (https://www.lroi-report.nl/data-quality/validity). Last accessed: March, 2023.
- Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol 2013; 13: 92. doi: 10.1186/1471-2288-13-92.
- Enocson A, Hedbeck C J, Tidermark J, Pettersson H, Ponzer S, Lapidus L J. Dislocation of total hip replacement in patients with fractures of the femoral neck. Acta Orthop 2009; 80(2): 184-9. doi: 10.3109/17453670902930024.
- Peters R M, van Steenbergen L N, Stewart R E, Stevens M, Rijk P C, et al. Patient characteristics influence revision rate of total hip arthroplasty: American Society of Anesthesiologists score and body mass index were the strongest predictors for short-term revision after primary total hip arthroplasty. J Arthroplasty 2020; 35(1): 188-92.e2. doi: 10.1016/j.arth.2019.08.024.
- Wagner E R, Kamath A F, Fruth K M, Harmsen W S, Berry D J. Effect of body mass index on complications and reoperations after total hip arthroplasty. J Bone Joint Surg 2016; 98(3): 169-79. doi: 10.2106/JBJS.O.00430.
Appendix
| Reasons for revision | PLA n = 117,576 | DSA n = 1,341 | DAA n = 56,626 | Total N = 175,543 |
| Revisions, total no. a | 3,688 (3.1) | 21 (1.6) | 899 (1.6) | 4,608 (2.6) |
| Infection | 1,121 (30) | 4 (19) | 267 (30) | 1,392 (30 |
| Periprosthetic fracture | 501 (14) | 4 (19) | 190 (21) | 695 (15) c |
| Dislocation | 1,209 (33) | 4 (19) | 147 (16) | 1,360 (30) c |
| Loosening of femoral component | 447 (12) | 4 (19) | 174 (19) | 625 (14) c |
| Loosening of acetabular component | 303 (8.2) | 3 (14) | 87 (9.7) | 393 (8.5) |
| Cup/ liner wear | 58 (1.6) | 0 (0) | 18 (2.0) | 76 (1.6) |
| Periarticular ossification | 31 (0.8) | 0 (0) | 14 (1.6) | 45 (1.0) |
| Girdlestone | 55 (1.5) | 0 (0) | 24 (2.7) | 79 (1.7) b |
| Other | 490 (13) | 2 (9.5) | 139 (16) | 631 (14) |
| a Percentages of total number of THAs according to approach. A revision may have more than 1 reason. As such, the total number of reasons is over 100%. | ||||
| b p < 0.05 between different groups. | ||||
| c p < 0.001 | ||||