Assessing osteonecrosis of the femoral head after internal fixation of femoral neck fractures: MARS MRI versus conventional radiography and patient-reported outcomes
Mikael KINDT 1,a, Maria L JÖNSSON 2,a, Trine TORFING 3, Sebastian STRØM RÖNNQUIST 1,4, Bjarke VIBERG 4, Søren OVERGAARD 2,5, and Cecilia ROGMARK 1
1 Department of Orthopaedics, Lund University, Skåne University Hospital, Malmö, Sweden; 2 Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg; 3 Department of Radiology, Odense University Hospital, Odense; 4 Department of Orthopaedic Surgery and Traumatology, Odense University Hospital, Odense; 5 Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
a Shared first authorship.
Background and purpose — Little is known on the use of metal artifact reduction sequence (MARS) MRI to diagnose osteonecrosis of the femoral head (ONFH) after fixation of femoral neck fractures (FNF) with conventional metal implants present. We compared MARS MRI with radiography in diagnosing ONFH. Secondarily, we determined whether signs of ONFH on MARS MRI correlate with patient-reported outcomes (PROs) via Oxford Hip Score (OHS) and pain (VAS).
Patients and methods — 30 adults under 60 years treated with internal fixation after FNF were prospectively included (2015–2018) at 2 hospitals. They were followed up with radiography and PROs at 4, 12, and 24 months and MARS MRI at 4 and 12 months. OHS < 34 or VAS pain > 20 was considered significant.
Results — At 12 months, 14 patients had a pathological MRI. 3 of 14 had ONFH on radiographs at 12 months, increasing to 5 at 24 months, and 4 had unfavorable PROs. 2 of 5 patients with ONFH signs on both MRI and radiography had unfavorable PROs. 1 of 10 patients with normal MRI and radiography had unfavorable 2-year PROs. 4 patients had inconsistent MRI results, of which 1 developed ONFH. 1 patient dropped out.
Conclusion — Information from a pathological MRI was not useful, as a majority remained free from symptoms and ONFH signs on radiographs. Furthermore, PROs did not correlate with imaging results. MARS MRI findings must be better understood before being taken into clinical practice. However, a normal MARS MRI seems to be a good prognostic finding.
Citation: Acta Orthopaedica 2023; 94: 135–140. DOI https://doi.org/10.2340/17453674.2023.11658.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-10-17. Accepted: 2023-03-03. Published: 2023-03-31.
Correspondence: mikael.kindt@skane.se
CR, SSR, BV, and SO designed and conducted the main cohort study. TT contributed expert knowledge and evaluated all images. MK did the statistical analyses. MK and MJ organized the data and contributed equally to the manuscript writing. All authors participated in revision of the manuscript and approved the submitted version of the manuscript.
The authors thank the Open Patient data Explorative Network (OPEN) for access to REDCap. Also, they express their gratitude to the HFU research group and to HFU participants for their contribution to the study. They thank Marika Bergman for her coordinator role and Jack Besjakov for radiological expertise.
Handling co-editors: Bart Swierstra
Acta thanks Martin van Amerongen and Marina Obradov for help with peer review of this study.
Osteonecrosis of the femoral head (ONFH) is a well-known complication after internal fixation of a femoral neck fracture (FNF) (1). For young patients and patients with undisplaced fractures, internal fixation is still the most common treatment (2). Development of ONFH can be devastating, leading to pain, disability, and need for secondary surgery (3,4). In a progressive state, ONFH can be diagnosed using conventional radiography, but the diagnosis is often delayed due to its late appearance on conventional radiography, and the patient may have to wait weeks or months for the final diagnosis.
A method for earlier diagnosis could be MRI, as it is the most reliable non-invasive diagnostic tool for non-traumatic ONFH (5-7). MRI can also be used to predict further progression of this condition (8,9). MRI has previously been shown to be superior to conventional radiography in detecting early signs of ONFH after FNFs. Due to metal artifacts, it has demanded either removal of implants or the routine use of titanium implants (5,10). The metal artifact reduction sequence (MARS) technique for MRI may be the future diagnostic tool to diagnose post-traumatic ONFH in hips with conventional metallic implants present. Current literature is insufficient to determine the clinical usefulness of MARS MRI in diagnosing post-traumatic ONFH, since it does not take the symptoms of the patients and patient-reported outcomes (PROs) into consideration. Early detection of ONFH is important for conservative and surgical treatment (8,11).
Our primary aim was to compare MARS MRI with conventional radiography in diagnosing ONFH following internal fixation of femoral neck fractures. The secondary aim was to determine whether signs of ONFH on MARS MRI correlated to Oxford Hip Score (OHS) (12) and pain reported using a visual analog scale (VAS) and, third, whether it correlates with reoperation.
Patients and methods
Patient selection
The study population is a subgroup of the cohort in the HFU-60 project (Hip Fracture in Adults Under 60 Years of Age), a prospective multi-center cohort study of 218 adults under 60 years who suffered a non-pathological hip fracture during 2015 to 2018. 4 orthopedic departments contributed to the general cohort: Sygehus Lillebælt Kolding, Odense University Hospital and Hvidovre Hospital in Denmark, and Skåne University Hospital Malmö in Sweden, but not all hospitals had the resources to perform MARS MRI. Patients were included regardless of medical, cognitive, and functional pre-fracture status. Both intra- and extracapsular hip fractures were included and were treated as per regimen at the local department. The overarching aim of the HFU-60 project was to describe the epidemiology of hip fractures, clinical results, and PRO in non-elderly patients (13).
Inclusion and exclusion
The current study includes patients treated in Odense or Malmö with femoral neck fracture, defined by ICD code S72.00 (n = 50). The implants used for internal fixation were all stainless-steel implants: Swemac Hansson Pin System, Swemac Hansson Twin Hook, Swemac Hip Plate, DePuy Synthes 7.3 mm cannulated screws, DePuy Synthes DHS screw, DePuy Synthes LCP DHS plate, and DePuy Synthes Olmed screw. Patients primarily treated with arthroplasty (n = 5) or Girdlestone procedure (n = 1) were excluded. Patients not attending MARS MRI according to the study protocol were also excluded (Figure 1). The total follow-up time was 24 months.
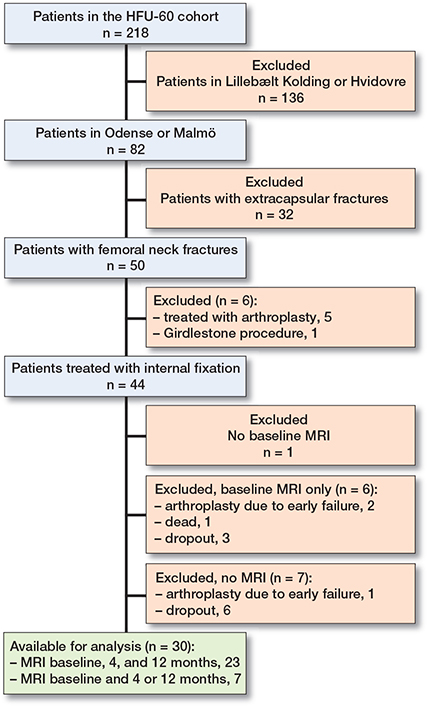
Figure 1. Flowchart for the study.
MARS MRI
MARS MRI was planned before discharge and after 4 and 12 months. Some participants did not attend all MARS MRI appointments, and there was a variation in time for the examinations (Table 1). In Odense MARS MRI was performed with Philips 1.5T Achieva scanners (Philips Healthcare, Amsterdam, the Netherlands). No contrast agent was administered. The following sequences were performed: coronal STIR, T2W and T1W, and axial T2W and T1W. For some patients axial STIR was also performed. In Malmö MARS MRI was performed using a Siemens 1.5 Tesla MRI. Dotarem (279.3 mg/mL, Guerbet, France), a gadolinium containing contrast agent, was administered intravenously (0.2 mL/kg bodyweight). Before Dotarem was administered the following sequences were performed using view-angle tilting (VAT) for artifact reduction: coronal T1 (Figure 2), coronal STIR (Figure 3), axial T1, and sagittal T2. After Dotarem was administered, axial T1 was performed and axial subtraction images were obtained. Both MRI scanners used commercially available standard sequences for metal artifact reduction, utilizing high bandwidth.
| Planned FU | Actual FU |
| MARS a MRI | |
| Postoperatively | 4.0 (10.5) days |
| 4 months | 4.0 (0.6) months |
| 12 months | 12.1 (0.7) months |
| Radiography | |
| Postoperatively | 2.0 (3.0) days |
| 4 months | 4.1 (0.7) months |
| 12 months | 11.9 (0.8) months |
| 24 months | 24.2 (0.5) months |
| a Metal artifact reduction sequence. | |
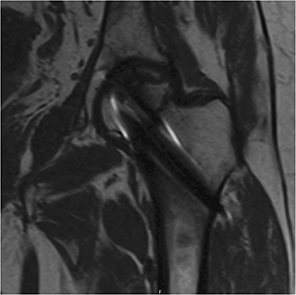
Figure 2. Example of coronal T1 MARS MRI image showing signs of ONFH 12 months after surgery.
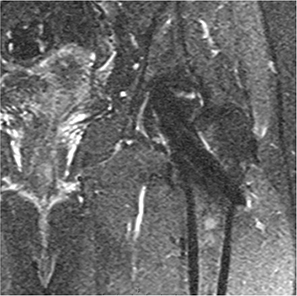
Figure 3. Example of coronal STIR MARS MRI image showing signs of ONFH 12 months after surgery.
Conventional radiography
Radiographs were obtained before discharge and after 4, 12, and 24 months. In the case of new or worsened symptoms between scheduled appointments, additional radiographs were obtained. Non-weight-bearing anteroposterior (AP) and lateral projections of the proximal femur and AP projection of the pelvis were used.
Radiological evaluation
MARS MRI and radiographs were reviewed by 1 of the authors, TT, a senior musculoskeletal radiologist. ONFH on MARS MRI was defined as the presence of the “double line sign” on T2 weighted images without fat saturation, consisting of demarcation of a subchondral segment with a distinct line, appearing as a fluid equivalent, on the necrotic side and a parallel hypointense line representing sclerosis. MRI examinations with such findings were referred to as “pathological.” When signs of ONFH were seen on MARS MRI at 4 months but not at 12 months the findings were considered transient. Some images showed subtle changes or changes uncharacteristic of ONFH, and in some instances image quality was too poor to make a conclusion—these images were considered inconclusive. When no pathological changes were found, images were considered normal. When reviewing MARS MRI scans the radiologist was blinded for radiographs, PROs, and clinical outcome. On radiographs, ONFH was defined as a combination of features including interruption of femoral head contour, sclerosis, subchondral cysts, crescent sign, and cortical collapse. Signs of ONFH seen on MARS MRI scans and radiographs were then compared with clinical outcome, i.e., reoperation and PRO data.
Patient-reported outcome (PROs)
Patients reported their symptoms via postal questionnaires at 6 weeks, 4, 12, and 24 months after fracture. Disability was described using OHS, a questionnaire designed to assess disability in patients undergoing total hip replacement. In the absence of consensus on which PRO is best for patients suffering hip fractures, OHS was chosen because it is joint specific and has been demonstrated to be both consistent and sensitive (14). A score of 48 represents the best outcome whereas 0 represents the worst. A score of 33 or less was considered unfavorable, according to established cut-offs (15). In any case where a question was left unanswered, the mean values representing all other responses in the questionnaire were used. Pain was described using a visual analog scale (VAS). Answers were converted to a number between 0 and 100 where “no pain” corresponds to 0 and “very severe pain” corresponds to 100. A score of more than 20 was considered unfavorable (16).
Statistics
Data was collected locally and stored online using REDCap (https://www.project-redcap.org), available to participating researchers. Data considered normally distributed was presented with mean and standard deviation (SD). Data not considered normally distributed was presented with median and interquartile range (IQR). Descriptive analyses were performed using Microsoft Excel (Microsoft Corp, Redmond, WA, USA).
Ethics, registration, data sharing plan, funding, and disclosures
HFU-60 was approved by ethical review boards in Sweden (Regionala etikprövningsnämnden Lund, Dnr: 2015/28) and Denmark (Videnskabsetisk Komité for Region Syddanmark, projekt ID: s-20150137), registered at ClinicalTrials.gov (NCT03848195), and conducted in accordance with the Helsinki declaration. All participating patients gave their written informed consent. Data may be shared upon reasonable request. The HFU-60 project was supported by grants from the Greta and Johan Kock Foundation, A. Påhlsson Foundation, H. Järnhardt foundation, Skåne University Hospital Research Fund, the Research and Development Council of Region Skåne, the Swedish Research Council funding for clinical research in medicine, and “Region Syddanmarks forskningsfond” from the Region of Southern Denmark. None of the funders had influence on the scientific work of this study. None of the authors have any financial or competing interests. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.11658
Results
Of the 44 patients eligible for inclusion 14 did not undergo MARS MRI scan according to protocol and were therefore excluded (Figure 1). This left 30 patients available for analysis, 20 men and 10 women. The median age at surgery was 52 years (range 28–58, IQR 9.7). A majority were healthy (Table 2). 18 patients were treated with parallel pins, 9 with parallel screws, and 3 with sliding hip screw devices. The median time from admission to surgery was 16 hours (range 3–33, IQR 12). 1 patient dropped out after 4 months.
| Item | Number |
| Age, median (IQR) | 52 (9.7) |
| Men/women | 20/10 |
| ASA category a | |
| I | 17 |
| II | 9 |
| III | 4 |
| IV | 0 |
| Smokers b | 6 |
| Diabetics | 4 |
| Any regular medication | |
| last 5 years | 16 |
| Glucocorticoid treatment | 2 |
| DXA results c | |
| Osteoporosis | 7 |
| Osteopenia | 17 |
| BMI, mean (SD) | 24 (4.2) |
| a American Society of Anesthesiologists physical status classification system. | |
| b Either active smokers or smokers until less than 2 years before inclusion. | |
| c According to the WHO definition of osteoporosis (normal: T-score ≥ −1; osteopenia: T-score between −1 and −2.5; osteoporosis: T-score ≤ −2.5). Dual-energy X-ray absorptiometry (DXA) screening was performed within 3 months after injury. 1 patient did not attend for DXA screening. | |
Correlation between MARS MRI and conventional radiography (Table 3)
Group 1—pathological MARS MRI (n = 14): At 4 months 9 of these patients showed signs of ONFH on MARS MRI, increasing to 14 at 12 months. 3 patients developed signs of ONFH on radiographs at 12 months, increasing to 4 at 24 months.
Group 2—normal MARS MRI (n = 10): All 10 patients with all normal MRI also had normal radiographs.
Group 3—transient pathology or inconclusive MARS MRI (n = 6): 3 of these patients had inconclusive MARS MRI results, of whom 1 developed signs of ONFH on radiographs. 3 had MARS MRI signs of ONFH at 4 months, of whom 2 had normal MARS MRI at 12 months and 1 dropped out.
Correlation between MARS MRI and PROs (Table 3)
At 6 weeks most patients, 24 of 27, had unfavorable PROs, i.e., OHS < 34 or VAS pain > 20.
Group 1: Among the 14 patients with signs of ONFH on MARS MRI, 7 had unfavorable PROs at 4 months, decreasing to 5 at 12 months, and 4 at 24 months.
Group 2: Among the 10 patients without signs of ONFH on MARS MRI, 5 had unfavorable PROs at 4 months, decreasing to 2 at 12 months. At 24 months only 1 had unfavorable PROs in this group.
Group 3: Among the 6 patients with transient pathology or inconclusive MARS MRI, none had unfavorable PROs at 4 months, but 1 at 12 months and 2 at 24 months.
Reoperations
2 patients from group 1 underwent secondary surgery with arthroplasty due to ONFH during the 2-year follow-up; 1 of these had the internal fixation removed in a separate operation prior to arthroplasty. 5 other patients had their internal fixation removed due to localized pain. None of the included patients underwent any other major orthopedic surgeries during follow-up.
Discussion
MARS MRI detected ONFH-associated pathology in every 2nd patient during the 1-year follow-up, whilst only a few developed radiographically verified ONFH. As for the patient-reported outcome, the early OHS and VAS results were distributed evenly among the groups with or without signs of ONFH on MARS MRI. From an imaging point of view, all 5 cases with ONFH found with radiography did have ONFH on MARS MRI, and all 10 who had normal MARS MRI also had normal radiographs. Nevertheless, 9 had pathological MARS MRI and normal radiographs. At 12 months, PRO results were slightly worse in the group with signs of ONFH on MARS MRI. Based on clinical experience, pain and disability at 4 months is to be expected due to the relatively long healing time of FNFs, regardless of MARS MRI findings. The association between imaging findings and symptoms needs to be better understood. On the other hand, a normal MARS MRI seems to be a good sign.
Few studies examined the use of MRI to detect ONFH after FNF. Within a cohort of 58 participants, Kim et al. used radiography and MRI in a search for hidden ONFH in healed FNFs after internal fixation implants were removed (10), but neither performed MRI at scheduled times, nor reported on when, after implant removal, MRI was performed. The mean time from initial fracture surgery to implant removal was 24 months. Their findings are similar to ours, as 38 participants had no signs of ONFH on radiographs, but 13 of these had signs of ONFH on the subsequent MRI (10). An older study on 31 patients with titanium implants found MRI after 6 months useful in predicting collapse of the femoral head caused by ONFH following internal fixation (5). A study of 21 adults compared the MARS MRI types fast spin-echo (FSE) and multi-acquisition variable-resonance image combination (MAVRIC) sequences in assessing ONFH following internal fixation of FNFs with 2 stainless steel pins and a fibular cortical bone allograft placement. With a schedule for radiography and MARS MRI similar to ours, they also found a high MARS MRI prevalence of ONFH, 14/21 of the patients with FSE, and 16/21 of the MAVRIC images. Radiographs showed ONFH in nil patients at 3 months, and 6 at 12 months (17). None of the above-mentioned studies took clinical presentation or PROs into account. Neither did they have an upper age limit, and they displayed a long or unspecified time to initial fracture surgery (5,10,17), which is not regarded acceptable in modern orthopedic care.
Strengths and limitations
Our study is the first to combine the ONFH-related findings on conventional radiography and MARS MRI and compare them with PROs after FNF. We consider our cohort to represent the most relevant age group for this study, as FNFs in this age group are treated primarily with internal fixation (2).
The small number of subjects available for analysis does not allow any solid conclusions or relevant statistical data analyses to be performed. 14 of the 44 eligible study participants did not follow the MARS MRI protocol and could not be included. We considered an initial and a late MARS MRI to be sufficient to follow any pathological changes, which was available in 30 patients.
We chose the design of 1 observer of the image diagnostics (TT), who was a senior musculoskeletal radiologist with more than 25 years of experience. This set-up precludes interobserver variability analysis. While reviewing MARS MRI images we sought to classify signs of ONFH using the JIC classification (18). As 9 of the 27 pathological MARS MRI scans were non-classifiable using this classification, we decided to state only whether signs of ONFH were present or not.
The use of 2 different MARS MRI protocols can be regarded as a limitation. In addition, one might see a theoretical problem with the different fixation implants, as they may cause different artifacts on MARS MRI. However, because the implants were all made of stainless steel, we considered this a minor factor.
Lastly, from a radiological perspective the lack of 24-month follow-up MRI may be considered a limitation. However, the study is constructed to evaluate the practicable use of MARS MRI as a faster diagnostic tool than conventional radiography to detect early signs of ONFH. As the focus of this study is the clinical perspective, the 24-month follow-up MARS MRI was omitted.
Clinical perspective and future research
Some of the pathological MARS MRIs at 4 months progress, others do not, and the correlation with PRO is weak. We consider our results to speak against MARS MRI for routine use in patients after internal fixation of FNFs. There is no existing literature on how progression of osteonecrosis, as well as normal healing, looks on MARS MRI after FNFs with retained fixation material. Therefore, MARS MRI findings after internal fixation must be further elaborated and better understood. For further studies, larger cohorts are needed, with repeated MARS MRI scans to evaluate the most advantageous timing to detect signs of ONFH. As pathological findings on image diagnostics do not consistently correlate with PROs, extended cohort variables, such as psychological factors, could also be taken into consideration, to clarify which patients do better, or worse, after FNF surgery.
Conclusion
Our results suggest that information from a pathological MARS MRI is not useful per se, as a majority remained free from symptoms and ONFH on follow-up radiographs. In a theoretical clinical scenario, where routine radiography shows signs suggesting ONFH but the patient is free of symptoms, a MARS MRI would only be useful to disregard the diagnosis. However, a normal MARS MRI seems to be a good prognostic finding.
- Ehlinger M, Moser T, Adam P, Bierry G, Gangi A, de Mathelin M, et al. Early prediction of femoral head avascular necrosis following neck fracture. Orthop Traumatol Surg Res 2011; 97(1): 79-88. doi: 10.1016/j.otsr.2010.06.014.
- Rönnquist S S, Lagergren J, Viberg B, Möller M, Rogmark C. Rate of conversion to secondary arthroplasty after femoral neck fractures in 796 younger patients treated with internal fixation: a Swedish national register-based study. Acta Orthop 2022; 93: 547-53. doi: 10.2340/17453674.2022.3038.
- Rogmark C, Kristensen M T, Viberg B, Rönnquist SS, Overgaard S, Palm H. Hip fractures in the non-elderly: who, why and whither? Injury 2018; 49(8): 1445-50. doi: 10.1016/j.injury.2018.06.028.
- Stockton D J, O’Hara L M, O’Hara N N, Lefaivre K A, O’Brien P J, Slobogean G P. High rate of reoperation and conversion to total hip arthroplasty after internal fixation of young femoral neck fractures: a population-based study of 796 patients. Acta Orthop 2019; 90(1): 21-5. doi: 10.1080/17453674.2018.1558380.
- Kawasaki M, Hasegawa Y, Sakano S, Sugiyama H, Tajima T, Iwasada S, et al. Prediction of osteonecrosis by magnetic resonance imaging after femoral neck fractures. Clin Orthop Relat Res 2001; (385): 157-64. doi: 10.1097/00003086-200104000-00024.
- Blum A, Raymond A, Teixeira P. Strategy and optimization of diagnostic imaging in painful hip in adults. Orthop Traumatol Surg Res 2015; 101(1 Suppl.): S85-99. doi: 10.1016/j.otsr.2014.11.002.
- Choi H R, Steinberg M E, Y. Cheng E. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med 2015; 8(3): 210-20. doi: 10.1007/s12178-015-9278-7.
- Kuroda Y, Tanaka T, Miyagawa T, Kawai T, Goto K, Tanaka S, et al. Classification of osteonecrosis of the femoral head: who should have surgery? Bone Joint Res 2019; 8(10): 451-8. doi: 10.1302/2046-3758.810.BJR-2019-0022.R1.
- Väänänen M, Tervonen O, Nevalainen M T. Magnetic resonance imaging of avascular necrosis of the femoral head: predictive findings of total hip arthroplasty. Acta Radiol Open 2021; 10(4): 2058460121100839. doi: 10.1177/20584601211008379.
- Kim C H, Shin M, Lee D, Choi S J, Moon D H. Hidden osteonecrosis of the femoral head after healed femoral neck fractures: magnetic resonance imaging study of 58 consecutive patients. Arch Orthop Trauma Surg 2022; 142(7): 1443-50. doi: 10.1007/s00402-021-03802-6.
- Larson E, Jones L C, Goodman S B, Koo K H, Cui Q. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int Orthop 2018; 42(7): 1723-8. doi: 10.1007/s00264-018-3917-8.
- Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br 1996; 78(2): 185-90.
- Strøm Rönnquist S, Viberg B, Kristensen M T, Palm H, Jensen J E B, Madsen C F, et al. Frailty and osteoporosis in patients with hip fractures under the age of 60: a prospective cohort of 218 individuals. Osteoporos Int 2022; 33(5): 1037-55. doi: 10.1007/s00198-021-06281-y.
- Hutchings L, Fox R, Chesser T. Proximal femoral fractures in the elderly: how are we measuring outcome? Injury 2011; 42(11): 1205-13. doi: 10.1016/j.injury.2010.12.016.
- Kalairajah Y, Azurza K, Hulme C, Molloy S, Drabu K J. Health outcome measures in the evaluation of total hip arthroplasties: a comparison between the Harris Hip Score and the Oxford Hip Score. J Arthroplasty 2005; 20(8): 1037-41. doi: 10.1016/j.arth.2005.04.017.
- Duncan G H, Bushnell C M, Lavigne G J. Comparison of verbal and visual analogue scales for measuring the intensity and unpleasantness of experimental pain. Pain 1989; 37(3): 295-303. doi: 10.1016/0304-3959(89)90194-2.
- Farshad-Amacker N A, Koff M F, Dyke J P, Lazaro L E, Shah P, Lorich D G, et al. Assessment of osteonecrosis in the presence of instrumentation for femoral neck fracture using contrast-enhanced MAVRIC sequence. HSS J 2016; 12(1): 51-8. doi: 10.1007/s11420-015-9475-3.
- Takashima K, Sakai T, Hamada H, Takao M, Sugano N. Which classification system is most useful for classifying osteonecrosis of the femoral head? Clin Orthop Relat Res 2018; 476(6): 1240-9. doi: 10.1007/s11999.0000000000000245.