Change in CT-measured acetabular bone density following total hip arthroplasty: a systematic review and meta-analysis
Thomas S ROBERTSON 1,2, Bart G PIJLS 3, Zachary MUNN 4, L Bogdan SOLOMON 1,2, Rob G H H NELISSEN 3, and Stuart A CALLARY 1,2
1 Department of Orthopaedics and Trauma Royal Adelaide Hospital, Adelaide, SA, Australia; 2 Centre for Orthopaedic and Trauma Research, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia; 3 Department of Orthopaedics, Leiden University Medical Center, Leiden, Netherlands; 4 JBI, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA Australia
Background and purpose — Assessing peri-acetabular bone quality is valuable for optimizing the outcomes of primary total hip arthroplasty (THA) as preservation of good quality bone stock likely affects implant stability. The aim of this study was to perform a meta-analysis of peri-acetabular bone mineral density (BMD) changes over time measured using quantitative computer tomography (CT) and, second, to investigate the influence of age, sex, and fixation on the change in BMD over time.
Methods — A systematic search of Embase, Scopus, Web of Science, and PubMed databases identified 19 studies that measured BMD using CT following THA. The regions of interest (ROI), reporting of BMD results, and scan protocols were extracted. A meta-analysis of BMD was performed on 12 studies that reported measurements immediately postoperatively and at follow-up.
Results — The meta-analysis determined that periacetabular BMD around both cemented and uncemented components decreases over time. The amount of BMD loss increased relative to proximity of the acetabular component. There was a greater decrease in cortical BMD over time in females and cancellous BMD for young patients of any sex.
Conclusion — Peri-acetabular BMD decreases at different rates relative to its proximity to the acetabular component. Cancellous BMD decreases more in young patients and cortical bone decreases more in females. Standardized reporting parameters and suggested ROI to measure peri-acetabular BMD are proposed, to enable comparison between implant and patient variables in the future.
Citation: Acta Orthopaedica 2023; 94: 191–199. DOI https://doi.org/10.2340/17453674.2023.11635.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-04-26. Accepted: 2023-01-19. Published: 2023-04-27.
Correspondence: thomas.robertson@sa.gov.au
TR, BS, RN, and SC contributed to study design, data extraction, analysis, and manuscript production. ZM and BP performed data analysis, data extraction, and manuscript production.
The authors would like to acknowledge research librarian Vikki Langton for her assistance with the literature search (University of Adelaide); Peter Robertson for his statistical assistance and figure production (University of Stanford); and Thomas Reynolds for his assistance in production of figures. Stuart Callary held a Research Fellowship from the Hospital Research Foundation Group during the time of this study.
Handling co-editors: Søren Overgaard and Robin Christensen
Acta thanks Eduardo García-Rey and Philip Hansen for help with peer review of this study.
Despite the improved results of primary THA involving highly cross-linked polyethylene bearings (1), revision surgery is still required in 5–10% of patients within the first 10 years and revision for loosening/lysis may be challenging (2). Bone quality parameters measured as bone mineral density (BMD) likely affect implant stability. In a recent survey of orthopedic surgeons, 77% stated they would change their implant choice based on osteoporosis, but only 4% of the same surgeons performed formal assessments of bone quality (3).
Peri-prosthetic BMD has commonly been investigated using dual-emission X-ray absorptiometry (DEXA), computerized tomography (CT), or a combination of the 2 imaging methods. Acetabular BMD changes post-THA have not been as extensively studied as femoral due to multiple difficulties, although are known to be reduced post-THA (4-6). Most DEXA studies use the commonly known radiological zones of DeLee and Charnley (7) or the Wilkinson modification into 4 axial regions of interest (ROI) (8). DEXA studies have shown an initial reduction of peri-acetabular BMD, which stabilizes at the level of the implant by early follow-up (5,9,10). This conflicts with CT studies, which have shown progressive BMD reduction particularly in the important retroacetabular zone, which is not visualized by DEXA (11,12). This reduction in BMD over time is due to a combination of factors including the expected BMD reduction with ageing, implant-related stress shielding (13,14), differing vectors of force following implantation (15), and variable patient activity and use of the limb postoperatively (16).
Evaluation of BMD using DEXA has the advantage of a lower patient radiation dose; however, it interprets only a 2D image, is unable to distinguish between cortical and cancellous bone (17), and cannot be used clinically to assess bone stock or osteolysis. The major limitation of CT is the presence of metal artefact streaking across the peri-acetabular bone, which can make BMD interpretation difficult, particularly in implants of more radio-opaque material such as tantalum and when the scans are performed for alternate pathologies and metal artefact reduction (MAR) software is not utilized. The development of improved MAR CT protocols has significantly improved the ability to assess periprosthetic bone (18,19). With increased ability to assess periacetabular bone quality and frequency of CT imaging there is a significant need for defined, repeatable measurements of CT-measured BMD.
To effectively measure peri-acetabular BMD using CT, the pelvis should be separated into ROIs. Common methods for identifying ROIs in CT include either axial slices of transverse planar CT image moving sequentially distally, circular ROIs centered at the acetabulum (avoiding the metal artefact of the acetabular component), or the use of computer software to split the acetabulum into various volumetric sections. Despite the variation within different methods, the reproducibility of CT-measured BMD has been tested across multiple formats of ROIs including quadrant and axial slice (20-22). Currently, there is no concise summary of CT-measured peri-acetabular BMD changes over time, which makes it difficult to interpret the role of various factors contributing to progressive BMD change.
Our hypothesis was twofold: first, that CT measurements of periprosthetic cancellous and cortical BMD decrease with time and, second, that the rate of decrease would be related to the proximity of the implant due to changes in stress shielding and load.
Therefore, the primary aim was to perform a meta-analysis of peri-acetabular BMD changes over time measured using quantitative CT, and the secondary aim was to investigate the influence of age or sex and fixation on the change in BMD over time.
Methods
Search, screening, and selection
A systematic literature review was performed on the January 10, 2022 in accordance with PRISMA standards. A working protocol was made prior to the start of the review (Table 1, see Supplementary data), but not registered. The search was conducted on Scopus, Embase, PubMed, and Web of Science databases using relevant mesh terms where appropriate (Table 2, see Supplementary data). The searches were exported into the Covidence systematic review software (https://www.covidence.org/) and blinded review on inclusion was performed by 2 of the authors (TR and SC). The inclusion criterion was any study that used CT to measure peri-acetabular BMD following primary THA. Studies were excluded at the full text level if either the ROI was not defined, BMD was not measured using CT, the CT was performed prior to THA, if the manuscript was a conference proceeding or in a non-English language, or if the cohort had a significant medical pathology affecting bone quality (metabolic, neoplastic, endocrine, or infectious) making them not representative of a typical arthroplasty cohort.
Data extraction
Data extraction included year of publication, sample size, sex, CT parameters, ROI, and results of density measurements by 1 reviewer (TR). In the event that only a graphical representation of data was available, attempts were made to contact corresponding authors to request full results and in the absence of a response data was extracted with WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/) (23). Similarly, when standard deviations (SD) were not reported they were calculated retrospectively from the reported range (24). If SDs were not reported, and a retrograde calculation was not possible, the measurement was allocated a mean SD, which was calculated as a mean of the reported SDs for the other studies in that region of interest (25). If a study had measurements also reported at an early timeframe for the same cohort, they were excluded and only the latest study was included. If percentage change only was reported in the absence of BMD measurements, and a reply was not obtained from the corresponding author, the study was excluded from the meta-analysis. Studies reported BMD in multiple different units, which required conversion to allow meta-analysis. All reported BMD units were converted back to ash density in mg/cm3 as previously recommended in a review of quantitative CT studies (26). Values are presented as means with 95% confidence intervals (CI).
Analysis
The ROIs were defined as ROI 1–8 starting 40 mm above the acetabular component corresponding to the most proximal axial level reported in the studies (Figure 1). Additionally, the ROIs were split into either cranial (above the level of the acetabular component) or at the level of the acetabular component. At the level of the acetabular component, ROIs were also split into ventral and dorsal. To allow comparison between studies, some assumptions were made regarding the ROIs. If the ROI was not a true axial slice and spanned consecutive axial slices, the midpoint of the ROI was taken. If the study included multiple slices measured for 1 comparison or ROI, the measurement closest to the midpoint of our suggested ROI was used. For example, if there were multiple measurements in region 3 (10–20 mm cranial to the acetabular component), the measurement closest to 15 mm was used. Early follow-up was defined as occurring between 6 and 36 months after THA and late follow-ups were studies with more than 7 years’ follow-up.
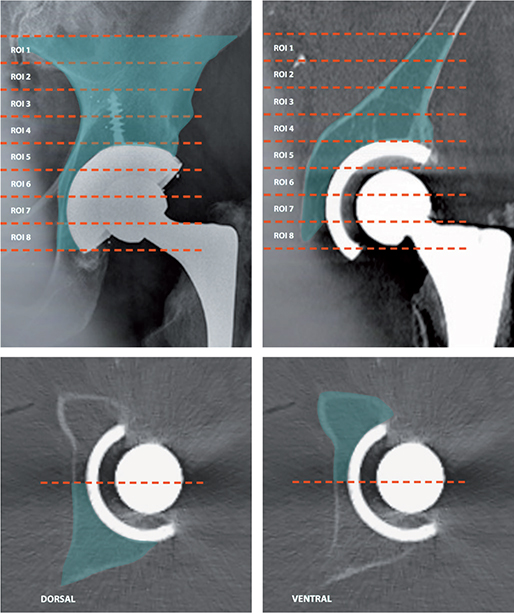
Figure 1. Proposed ROIs 1–8 on a plain radiograph (left) and coronal CT (right) and below the dorsal regions of interest (left) and ventral (right) for ROI 6.
To simplify the ROIs and allow for a larger comparison of studies for cemented and uncemented components, ROIs were simplified to either cranial to the acetabular component or, at the level of the acetabular component, split into ventral and dorsal. Specific comparisons of ROIs 1–8, rather than the 3 groups of cranial, ventral, and dorsal, had insufficient sample sizes from which to draw comparison.
A risk of bias assessment was performed by 1 reviewer using a modified version of the Newcastle–Ottawa scale. A modification of the scale was made to tailor it to the standards of CT density studies (Table 3, see Supplementary data).
The meta-analysis and forest plots were produced using the Cochrane review Review Manager (RevMan) software (https://training.cochrane.org/online-learning/core-software/revman) with a generic inverse variance approach using a random effects model for mean differences. Additionally the open source OpenMeta[Analyst] software (http://www.cebm.brown.edu/openmeta/) was used to calculate mean BMD of the sample groups.
The amount of statistical heterogeneity was assessed through inspection of the forest plots, chi2 and the I2 statistics (25). The I2 statistic estimates how much of the total variability in the effect size estimates is due to heterogeneity among the true effects (25). In the presence of heterogeneity, and if the data allowed, we performed a random-effects meta-regression on predefined factors (study-level covariates: age, percentage women, ROI, follow-up duration, and fixation). All analyses were performed using the metafor package in R statistics (R Foundation for Statistical Computing, Vienna, Austria) (27).
Results
Data collection
123 studies were screened for eligibility. Of those, 39 required full text assessment. 19 studies fulfilled the requirement of CT-measured peri-acetabular BMD after THA and were included in the systematic review (Figure 2). The characteristics of the studies including implant details, age, sex, and scan protocol are displayed in Table 4, see Supplementary data). Of those, 12 included BMD measurements at 2 time points after THA and were included in the meta-analysis investigating change in BMD over time. In addition, 13 studies had measurements at 1 timeframe and were included in the meta-analysis of mean peri-acetabular BMD, noting that duplicate cohorts from the same study group were not included at multiple time points. The remaining 6 studies did not report actual BMD values or did not separate their ROI to allow comparison. Table 5, see Supplementary data) depicts the extraction of data and conversions made for the 13 studies, which had measures available for metanalysis at 1 or more timepoints (4,11,12,20,22,28-38). The results of the modified Newcastle–Ottawa bias assessment (Table 6, see Supplementary data) showed fair quality, with all but 1 study (39) having a rating of ≥ 4 (Table 6, see Supplementary data). All studies used a similar kVp with 140 kVp being the most frequently reported, but there were variations in ROI used (Figure 3) and reporting of statistical parameters.
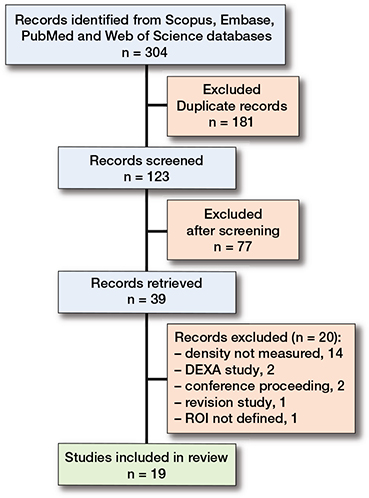
Figure 2. Prisma chart of Covidence review process.
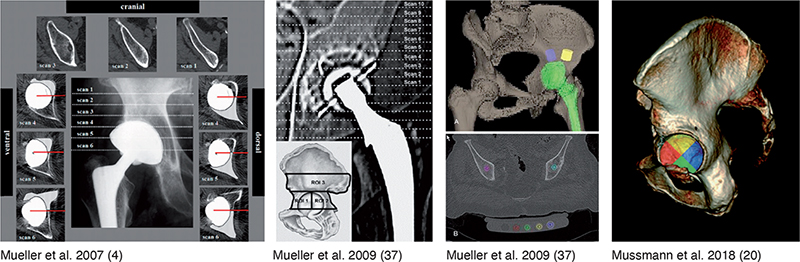
Figure 3. Examples of different regions of interest used in previous CT studies of peri-acetabular BMD following THA.
Comparison of peri-acetabular BMD change between cemented and uncemented component fixation
The meta-analysis depicts a significant change in cancellous bone density for cemented and uncemented acetabular components cranial to the level of the cup (BMD –40 mg/cm3 [CI –51 to –29], Figure 4a). This significant change is also seen for ventral cancellous bone density (Figure 4b). The heterogeneity of the results increased dorsal to the component and there are no significant changes in BMD dorsally for cemented components (Figure 4c).
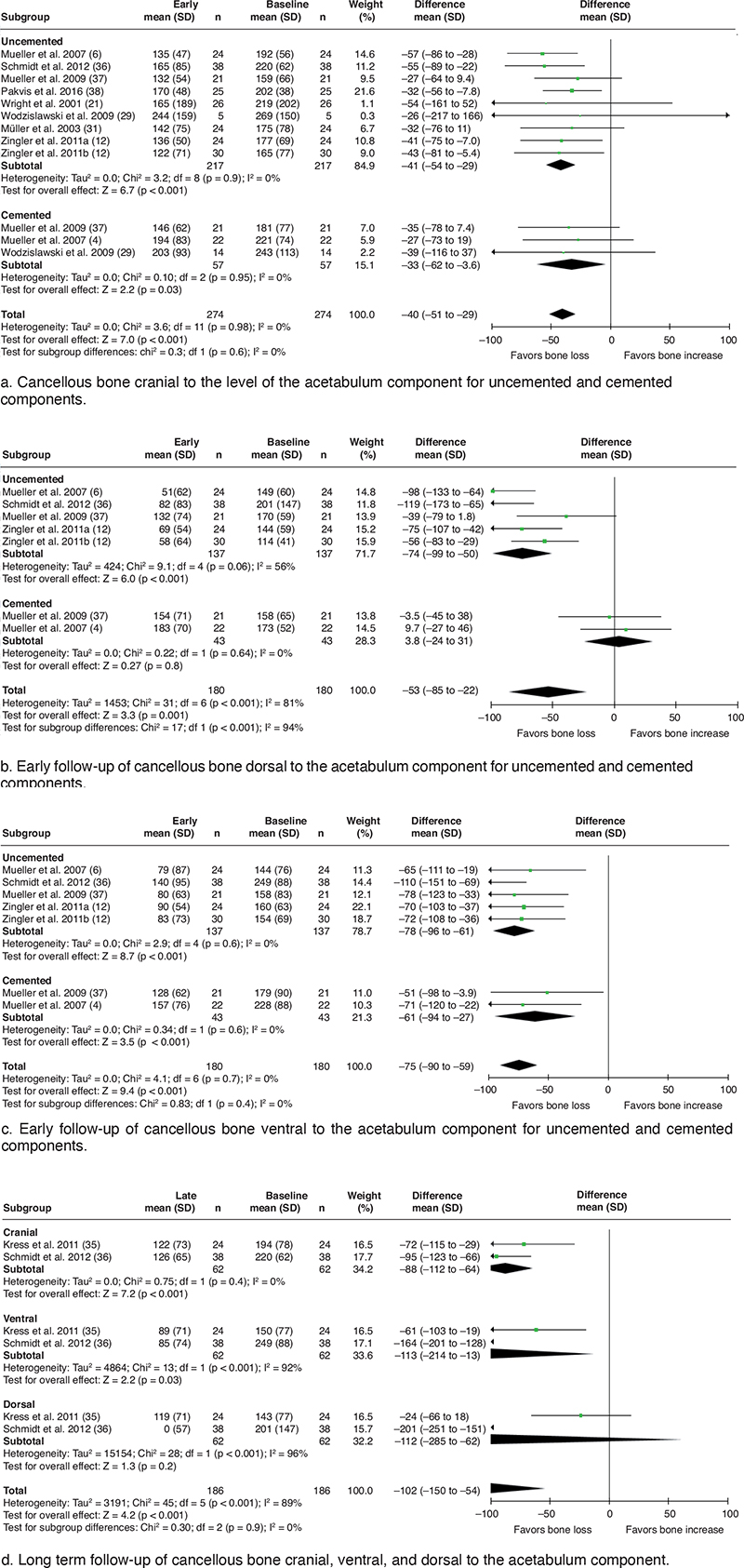
Figure 4. Forest plots meta-analysis of cortical and cancellous bone. Note all eligible studies included for each subgroup. Those not estimable have been omitted.
Mean BMD at baseline and early follow up
The meta-analysis of mean BMD at both baseline and early follow-up of cancellous bone shows the least density at baseline and short-term follow-up dorsal to the acetabular component; conversely, the bone cranial to the acetabular component is of greatest density at both early timepoints (Table 7).
Mean BMD at late follow-up
In the meta-analysis, there was a trend of increased bone loss at late follow-up in all ROIs (Figure 4d). Interestingly and potentially skewing results, in the study by Kress et al., the most central region dorsal to the component (ROI 5) shows bone loss down to 0 for cancellous bone (35).
BMD changes of cortical and cancellous bone at early follow-up
Cancellous bone
Mean patient age, follow-up duration after THA, ROIs used, and component fixation were effect modifiers on mean cancellous bone loss. For every year increase in mean age, the mean bone loss decreased by 1.6 mg/cm3 (CI 0.7–2.6, Figure 5a). For every month follow-up the mean bone loss increased by 1.8 mg/cm3 (CI 0.4–3.2, Figure 5b). For every unit increase in ROI the bone loss increased by 5.9 mg/cm3 (CI 1.0–11, Figure 5c). For uncemented implants the mean bone loss was 65 mg/cm 3 (CI 57–73) and for cemented implants it was 24 mg/cm 3 (CI 10–38). Patient sex was not an effect modifier.
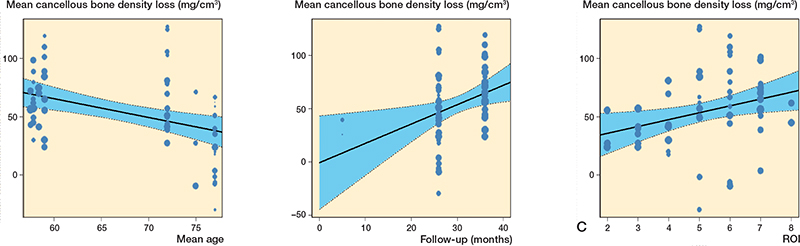
Figure 5. Changes in cancellous bone. A. Bone loss relative to age at early follow-up. B. Bone loss relative to time at early follow-up. C. Bone loss relative to region of interest.
Cortical bone
The ROI used, percentage women, and acetabular component fixation were effect modifiers on mean cortical bone loss. For every unit increase in ROI the bone loss increased by 14 mg/cm3 (CI 7.2–21, Figure 6a). For every 10% women in a study, the mean bone loss increased by 8.8 mg/cm3 (CI 2.3–15, Figure 6b). For uncemented implants the mean bone loss was 65 mg/cm3 (CI 51–80) and for cemented implants it was 35 mg/cm3 (CI 12–57). Mean patient age and follow-up after THA were not effect modifiers.
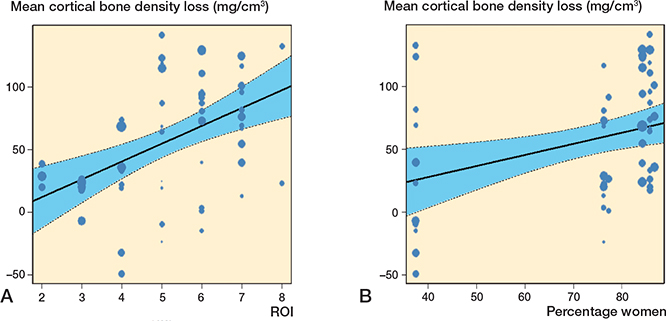
Figure 6. Changes in cortical bone. A. Bone loss relative to region of interest. B. Bone loss relative to sex by percentage of women.
Discussion
This is the first systematic review and meta-analysis performed on CT studies of peri-acetabular BMD following THA. With the increasing clinical use of CTs, and the large improvements in MAR, the authors postulate that measurements of periacetabular BMD will be used more frequently. Knowledge of the peri-acetabular BMD is important clinically for planning revision THA and in research when investigating implant and patient variables that may influence change in BMD over time. For example, improved reporting of mean BMD may be used for finite element analysis of loading patterns following THA. This implicates a necessity for standardization of measuring changes in peri-acetabular BMD. The authors propose improved standardized reporting of CT-measured BMD following THA by including CT parameters, implant specifications, patient characteristics, follow-up time points, and using standardized ROIs in analysis (Table 8).
Future CT studies of peri-acetabular BMD that use more consistent ROIs will allow investigations of correlation with early component stability and long-term loosening (40). The question is posed as to whether acetabular component migration is the due progressive loading of the component and the component migration towards the path of least resistance or reduced BMD. Additionally, a standardized way of measuring BMD in the periacetabular region allows direct comparison of implant variables that are proposed to result in increased or decreased BMD in the periacetabular region. We acknowledge that whilst adoption of our recommendations in Table 8 would be of benefit, future CT scanning protocols are expected to improve with additional MAR techniques.
Our meta-analysis demonstrates that the peri-acetabular cortical and cancellous BMD decreases significantly following THA. The differentiation in rate of cortical compared with cancellous bone moving distally from ROIs 1–8 is proposed to be due to a shift in the load to cortical bone at the level of the component. The causation of BMD loss following THA is likely multifactorial but driven largely by changes in stress loading of the bone due to implantation of the acetabular component (13). It has been observed in the native acetabulum that bone remodeling is influenced by muscle-force-induced bone stress (41). The implant orientation is known to change loading of the acetabulum following THA (15). Additional factors leading to decreased BMD include changes in postoperative use of the limb, age-related bone loss, and increasing comorbidities of the ageing patient. A comparison with the contralateral native acetabulum and loading changes was performed in 2 of the reviewed studies showing increased bone loss relative to the native side (38). Pakvis et al. particularly noted increased periacetabular cancellous bone loss with preservation of cranial and cortical bone (38). Bone loss relative to the contralateral side further contributes to the proposed mechanism of bone loss due to stress shielding and changes in postoperative limb use rather than ageing and development of comorbidities of the host.
Our meta-analysis found an early increased amount of cancellous bone loss for younger patients undergoing THA. This is likely due to younger patients having a higher initial BMD and when the loading pattern in the pelvis changes postoperatively they subsequently have a larger amount of bone loss possible. There is no known link between osteolysis and bone density changes and these are thought to be separate entities. Density changes occur due to aforementioned factors such stress shielding and load changes, whereas osteolysis occurs due to macrophages in response to the innate immune system releasing cytokines and reactive oxygen species in response to particle debris (42). Whilst separate entities, there is certainly crossover in the arthroplasty revision setting when surgeons are treating patients with a combination of osteolysis and poor BMD.
This systematic review identified the varying ROIs that have been used in clinical CT studies to date, which limits the ability to compare studies. It is also acknowledged that our review is limited by the inconsistent reporting by other studies, the conversions of units made, and the limited number of studies published in this field, particularly long-term follow-up studies. There was insufficient data to draw comparisons between different implants and liner types. Regarding measurements in close proximity to the acetabular component for cemented components, due to the irregularity of the interface it is possible a portion of the density measurements included cement (20). Of significance also is that CT density measurements of the periacetabular region are not yet validated. This would require an ex vivo cadaveric or retrieval study with elimination of metal artefacts and calibration against known density phantom measurements and is identified as an area of future work.
The ideal classification system of ROIs must be easily used by a clinician on simple radiological software assessing a patient in a busy outpatient department, as well as adequately characterizing the changes in peri-acetabular BMD. A simplified system would split the pelvis into zones of axial slices progressing towards the top of the acetabular component as several studies have done at varying intervals (11,31,38). However, a quadrant system is appealing, given the potential advantages of planning surgical fixation when the acetabular socket is split into quadrants and any screw augments are aimed towards the posterior superior zone or “safe zone.” The limitation of using quadrant ROIs is that large amounts of bone at varying distances proximal or distal to the acetabular component are pooled together. Given that distance relative to the acetabular component has been shown to be clearly related to BMD, we recommend that future investigators refer to any new description of ROI as relative to the most proximal point of the acetabular component as a subtype within that region, similar to the methods used by Barbu-McInnes et al. (39). Like the methods of the most prolific research group in this area led by Mueller and Schmidt, the regions should be split into axial slices differentiating cancellous and cortical bone (33,37). The regions would be split into 8 10-mm axial ROIs with region 1 starting 40 mm above the acetabular component and continuing 10 mm distally (Figure 1). Region 2 involves bone 30–20 mm above the component and so on until region 8 pertains to –30 to –40 mm below the top of the acetabular component. In addition, the axial ROIs 5–8 at the level of the acetabular component should be split into subgroups dorsal and ventral.
Uncemented and cemented fixation were postulated to create a difference in periacetabular BMD changes due to a difference in the potential loading of the bone and stress shielding. As outlined by Mueller et al. and others, historical finite element analysis studies predicted cemented components would transfer load to the cranial and medial or dorsal portions of periacetabular zone (4,43-45). Within our metaanalysis, cemented components had no change in cancellous bone dorsally but did have slightly smaller bone loss cranially compared with uncemented components (4,29,37). However, when corrected for age, the differences between fixation types for cancellous bone loss were not significant. The reduction in BMD for dorsal regions of cemented components at late follow-up was only noted in 1 study and more studies of cemented components at late follow-up are required. The region dorsal to the component records bone loss down to 0 for cancellous bone in ROI 5 in the Kress et al. study (35) which is thought to be an anomaly rather than taken as. It is worth noting the proximity of ROI 5 to the acetabular component and the large amount of artefact dorsally, which may have hampered measurements in this region (43). The reduction of cancellous bone density cranial to the acetabular component at late follow-up is thought to be of real significance and mean bone loss of 88 mg/cm3 compared with 41 mg/cm3 at early follow-up represents a decrease in the rate of bone loss and likely correlates with a plateau in the effect of the loading of the periacetabular region.
Conclusion
This review of CT studies identified the varying methods of measuring, stratifying, and reporting peri-acetabular BMD following primary THA. Young patients had greater cancellous bone loss and female patients were found to have greater loss of cortical bone at short-term follow-up. We recommend that future studies should report BMD in 8 ROIs relative to the top of the acetabular component and include patient, implant, and scanning details.
Supplementary data
Tables 1–6 and completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.11635
- Ries M D, Scott M L, Jani S. Relationship between gravimetric wear and particle generation in hip simulators: conventional compared with cross-linked polyethylene. J Bone Joint Surg Am 2001; 83-A(Suppl. 2, Pt 2): 116-22. doi: 10.2106/00004623-200100022-00009.
- AOANJRR. Australian Orthopaedics Association National Joint Replacement Registry Annual Report. Adelaide: AOA; 2020.
- Maier G S, Kolbow K, Lazovic D, Maus U. The importance of bone mineral density in hip arthroplasty: results of a survey asking orthopaedic surgeons about their opinions and attitudes concerning osteoporosis and hip arthroplasty. Adv Orthop 2016; 2016: 8079354. doi: 10.1155/2016/8079354.
- Mueller L A, Nowak T E, Mueller L P, Schmidt R, Ehrmann C, Pitto R P, et al. Acetabular cortical and cancellous bone density and radiolucent lines after cemented total hip arthroplasty: a prospective study using computed tomography and plain radiography. Arch Orthop Trauma Surg 2007; 127(10): 909-17. doi: 10.1007/s00402-007-0304-0.
- Smolders J M, Pakvis D F, Hendrickx B W, Verdonschot N, van Susante J L. Periacetabular bone mineral density changes after resurfacing hip arthroplasty versus conventional total hip arthroplasty: a randomized controlled DEXA study. J Arthroplasty 2013; 28(7): 1177-84. doi: 10.1016/j.arth.2012.08.025.
- Mueller L A, Voelk M, Kress A, Pitto R P, Schmidt R. An ABJS Best Paper: Progressive cancellous and cortical bone remodelling after press-fit cup fixation: a 3-year followup. Clin Orthop Relat Res 2007; 463: 213-20.
- DeLee J G, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res 1976; 121: 20-32.
- Wilkinson J M, Peel NF, Elson RA, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg Br 2001; 83(2): 283-8. doi: 10.1302/0301-620x.83b2.10562.
- Field R E, Cronin M D, Singh P J, Burtenshaw C, Rushton N. Bone remodeling around the Cambridge cup: a DEXA study of 50 hips over 2 years. Acta Orthop 2006; 77(5): 726-32. doi: 10.1080/17453670610012908.
- Laursen M B, Nielsen P T, Soballe K. Bone remodelling around HA-coated acetabular cups: a DEXA study with a 3-year follow-up in a randomised trial. Int Orthop 2007; 31(2): 199-204. doi: 10.1007/s00264-006-0148-1.
- Pitto R P, Bhargava A, Pandit S, Munro J T. Retroacetabular stress-shielding in THA. Clin Orthop Relat Res 2008; 466(2): 353-8. doi: 10.1007/s11999-007-0043-0.
- Zingler K, Haeberle L, Kress A, Holzwarth U, Forst R, Mueller L A, et al. Comparison of cortical and cancellous bone remodeling of the pelvis after press-fit cup total hip arthroplasty dependent on patient and prosthesis-specific characteristics: a computed tomography-assisted osteodensitometry study in vivo. Biomed Tech (Berl) 2011; 56(5): 267-75. doi: 10.1515/BMT.2011.105.
- Manley M T, Ong K L, Kurtz S M. The potential for bone loss in acetabular structures following THA. Clin Orthop Relat Res 2006; 453: 246-53. doi: 10.1097/01.blo.0000238855.54239.fd.
- Dickinson A S, Taylor A C, Browne M. The influence of acetabular cup material on pelvis cortex surface strains, measured using digital image correlation. J Biomech 2012; 45(4): 719-23. doi: 10.1016/j.jbio-mech.2011.11.042.
- Small S R, Berend M E, Howard L A, Tunc D, Buckley C A, Ritter M A. Acetabular cup stiffness and implant orientation change acetabular loading patterns. J Arthroplast. 2013; 28(2): 359-67. doi: 10.1016/j.arth.2012.05.026.
- Bahl J S, Millar S C, Fraysse F, Arnold J B, Taylor M, Callary S, et al. Changes in 24-hour physical activity patterns and walking gait biomechanics after primary total hip arthroplasty: a 2-year follow-upstudy. J Bone Joint Surg Am 2021; 103(13): 1166-74. doi:10.2106/JBJS.20.01679.
- Digas G, Kärrholm J, Thanner J. Different loss of BMD using uncemented press-fit and whole polyethylene cups fixed with cement: repeated DXA studies in 96 hips randomized to 3 types of fixation. Acta Orthop 2006; 77(2): 218-26. doi: 10.1080/17453670610045948.
- Kim Y J, Cha J G, Kim H, Yi J S, Kim H J. Dual-energy and iterative metal artifact reduction for reducing artifacts due to metallic hardware: a loosening hip phantom study. AJR Am J Roentgenol 2019: 1-6. doi: 10.2214/AJR.18.20413.
- Hakvoort E T, Wellenberg R H H, Streekstra G J. Quantifying near metal visibility using dual energy computed tomography and iterative metal artifact reduction in a fracture phantom. Phys Med 2020; 69: 9-18. doi: 10.1016/j.ejmp.2019.11.006.
- Mussmann B, Andersen P E, Torfing T, Overgaard S. Bone density measurements adjacent to acetabular cups in total hip arthroplasty using dual-energy CT: an in vivo reliability and agreement study. Acta Radiol Open 2018; 7(9): 2058460118796539. doi: 10.1177/2058460118796539.
- Wright J M, Pellicci P M, Salvati E A, Ghelman B, Roberts M M, Koh J L. Bone density adjacent to press-fit acetabular components: a prospective analysis with quantitative computed tomography. J Bone Joint Surg Am 2001; 83-A(4): 529-36.
- Schmidt R, Pitto R P, Kress A, Ehremann C, Nowak T E, Reulbach U, et al. Inter- and intraobserver assessment of periacetabular osteodensitometry after cemented and uncemented total hip arthroplasty using computed tomography. Arch Orthop Trauma Surg 2005; 125(5): 291-7. doi: 10.1007/s00402-005-0812-8.
- Drevon D, Fursa S R, Malcolm A L. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif 2017; 41(2): 323-39. doi: 10.1177/0145445516673998.
- Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. doi: 10.1186/1471-2288-5-13.
- Higgins J P T, Thomas J, Chandler J, Li T, Page M J, Welch V A. Cochrane handbook for systematic reviews of interventions. 6th ed. Chichester, UK, Hoboken, NJ: Wiley-Blackwell; 2019. xxi, p. 649.
- Knowles N K, Reeves J M, Ferreira L M. Quantitative computed tomography (QCT) derived bone mineral density (BMD) in finite element studies: a review of the literature. J Exp Orthop 2016; 3(1): 36. doi: 10.1186/s40634-016-0072-2.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010; 36: 1-48.
- Stepniewski A S, Egawa H, Sychterz-Terefenko C, Leung S, Engh C A, Sr. Periacetabular bone density after total hip arthroplasty: a postmortem analysis. J Arthroplasty 2008; 23(4): 593-9. doi: 10.1016/j.arth.2007.05.030.
- Wodzislawski W, Krupa S, Nowicki J, Bedzinski R, Detyna J. The reaction of the pelvis to the implantation of the acetabular component of the hip endoprosthesis: initial tests with the use of computerized tomography. Acta Bioeng Biomech 2009; 11(4): 45-54.
- Boomsma M F, Slouwerhof I, van Lingen C, Pakvis D F, van Dalen J A, Edens M A, et al. CT-based quantification of bone stock in large head metal-on-metal unilateral total hip replacements. Eur J Radiol 2016; 85(4): 760-3. doi: 10.1016/j.ejrad.2016.01.019.
- Müller L, Schmidt R, Kress A, Forst R, Pitto R. Evaluation of periacetabular bone reaction after total hip arthroplasty with alumina–alumina pairing using computed tomography. Key Engineering Materials 2003; 240-242: 863-6. doi: 10.4028/www.scientific.net/KEM.240-242.863.
- Meneghini R M, Ford K S, McCollough C H, Hanssen A D, Lewallen D G. Bone remodeling around porous metal cementless acetabular components. J Arthroplasty 2010; 25(5): 741-7. doi: 10.1016/j.arth.2009.04.025.
- Schmidt R, Muller L, Kress A, Hirschfelder H, Aplas A, Pitto R P. A computed tomography assessment of femoral and acetabular bone changes after total hip arthroplasty. Int Orthop 2002; 26(5): 299-302. doi: 10.1007/s00264-002-0377-x.
- Mueller L A, Kress A, Nowak T, Pfander D, Pitto R P, Forst R, et al. Periacetabular bone changes after uncemented total hip arthroplasty evaluated by quantitative computed tomography. Acta Orthop 2006; 77(3): 380-5. doi: 10.1080/17453670610046299.
- Kress A M, Schmidt R, Vogel T, Nowak T E, Forst R, Mueller L A. Quantitative computed tomography-assisted osteodensitometry of the pelvis after press-fit cup fixation: a prospective ten-year follow-up. J Bone Joint Surg Am 2011; 93(12): 1152-7. doi: 10.2106/JBJS.J.01097.
- Schmidt R, Kress A M, Nowak M, Forst R, Nowak T E, Mueller L A. Periacetabular cortical and cancellous bone mineral density loss after press-fit cup fixation: a prospective 7-year follow-up. J Arthroplasty 2012; 27(7): 1358-63 e1. doi: 10.1016/j.arth.2011.09.031.
- Mueller L A, Schmidt R, Ehrmann C, Nowak T E, Kress A, Forst R, et al. Modes of periacetabular load transfer to cortical and cancellous bone after cemented versus uncemented total hip arthroplasty: a prospective study using computed tomography-assisted osteodensitometry. J Orthop Res 2009; 27(2): 176-82. doi: 10.1002/jor.20742.
- Pakvis D F, Heesterbeek P J, Severens M, Spruit M. Cancellous and cortical bone mineral density around an elastic press-fit socket in total hip arthroplasty. Acta Orthop 2016; 87(6): 583-8. doi: 10.1080/17453674.2016.1237439.
- Barbu-McInnis M, Tamez-Pena J, Crilly T, Looney J, O’Keefe R, Campbell D, et al. Semi-automated CT-based analysis of regional bone-density in contralateral total hip replacement: Proceedings of SPIE 2004; 5369: 742-9. doi: 10.1117/12.535815.
- Pijls B G, Nieuwenhuijse M J, Fiocco M, Plevier J W, Middeldorp S, Nelissen R G, et al. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survival studies. Acta Orthop 2012; 83(6): 583-91. doi: 10.3109/17453674.2012.745353.
- Fernandez J, Sartori M, Lloyd D, Munro J, Shim V. Bone remodelling in the natural acetabulum is influenced by muscle force-induced bone stress. Int J Numer Method Biomed Eng 2014; 30(1): 28-41. doi: 10.1002/cnm.2586.
- Goodman S B, Gallo J. Periprosthetic osteolysis: mechanisms, prevention and treatment. J Clin Med 2019; 8(12): 2091. doi: 10.3390/jcm8122091.
- Carter D R, Vasu R, Harris W H. Stress distributions in the acetabular region, II: Effects of cement thickness and metal backing of the total hip acetabular component. J Biomech 1982; 15(3): 165-70. doi: 10.1016/0021-9290(82)90248-2.
- Carter D R, Vasu R, Harris W H. Periacetabular stress distributions after joint replacement with subchondral bone retention. Acta Orthop Scand 1983; 54(1): 29-35. doi: 10.3109/17453678308992866.
- Pedersen D R, Crowninshield R D, Brand R A, Johnston R C. An axisymmetric model of acetabular components in total hip arthroplasty. J Biomech 1982; 15(4): 305-15. doi: 10.1016/0021-9290(82)90176-2.