Poor outcome after debridement and implant retention for acute hematogenous periprosthetic joint infection: a cohort study of 43 patients
Marianne WESTBERG, Øystein Tyri FAGERBERG, and Finnur SNORRASON
Division of Orthopaedic Surgery, Oslo University Hospital Ullevål, Oslo, Norway
Background and purpose — The management of acute hematogenous periprosthetic joint infection (AHI) is challenging and the optimal treatment is not clearly defined. The aim of this study was to evaluate the treatment outcome of AHI, and secondarily to investigate potential risk factors that affect outcome.
Patients and methods — We retrospectively analyzed 43 consecutive AHIs in a total hip or knee arthroplasty between 2013 and 2020 at a single center. We used the Delphi international consensus criteria to define infection. Patients were treated by either debridement, antibiotics, and implant retention (DAIR) (n = 25), implant exchange/removal (n = 15), or suppressive antibiotics only (n = 3). AHI was defined as abrupt symptoms of infection ≥ 3 months after implantation in an otherwise well-functioning arthroplasty.
Results — AHI was most often caused by Staphylococcus aureus (16/43) and streptococcal species (13/43), but a broad spectrum of microbes were identified. 25 of 43 were treated with DAIR, with success in 10 of 25, which was significantly lower than in patients treated with removal of the implant with success in 14 of 15. S. aureus infection, knee arthroplasty, and implant age < 2 years were associated with treatment failure. The 2-year mortality rate was 8 of 43.
Conclusion — The outcome following DAIR in AHIs was poor. The majority of infections were caused by virulent microbes, and we found a high mortality rate. Removal of the implant should more often be considered.
Citation: Acta Orthopaedica 2023; 94: 115–120. DOI: https://doi.org/10.2340/17453674.2023.10312.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-05-12. Accepted: 2023-02-09. Published: 2023-03-08.
Correspondence: marianne.westberg@ous-hf.no
All authors contributed to the study design, interpretation of data and results, and the content of the final manuscript. ØTF collected the data. MW drafted the manuscript.
Handling co-editors: Eivind Witsø and Robin Christensen
Acta thanks Rob Nelissen and Christof Wagner for help with peer review of this study.
The overall incidence of prosthetic joint infection (PJI) is 1–2% and the number of PJIs is expected to increase due to the growing numbers of primary and revision procedures (1-3). According to the Tsukayama classification of PJI, acute hematogenous infection (AHI) is defined as abrupt symptoms of infection occurring more than 3 months after index surgery in an otherwise well-functioning prosthesis and is thought to be introduced by hematogenous seeding (4). The proportion of AHI is reported to be quite low among all PJIs (6–11%), but cumulative numbers may be higher, as patients are at risk of contracting an AHI during the entire lifetime of the prosthesis (4-6). An increasing population of elderly now live for decades with one or more total joint implant(s), and subsequently revision implants, which adds patients at risk. Also, more intravascular procedures are performed, and more cardiac electronic devices are implanted in the elderly population, increasing the risk of a bacteremia (7). In a recent paper by Zeller et al., 348 of 997 (35%) PJIs were classified as AHI (8). Huotari et al. reported in a registry study from Finland an increasing rate of acute late infections (9). Debridement, antibiotics, and implant retention (DAIR) is considered a suitable treatment within 4 weeks after symptoms, as it may result in lower morbidity due to being a less invasive procedure compared with exchange arthroplasty (4,10). Prior studies of DAIR treatment of AHI are limited by small numbers, and AHI is often reported either together with, or as a minority within a larger cohort of acute PJIs. Even so, increased failure rates in AHI compared with postoperative PJIs are reported (5,11-13). There are few studies on the AHI group as such, and due to the ageing of the population more patients will be at risk of hematogenous seeding of bacteria, and more knowledge is therefore warranted.
The primary aim of this study was to evaluate the treatment outcome specified as free of infection of AHI in total hip and knee replacements. The secondary aim was to study patient characteristics, plus clinical and microbiological findings.
Patients and methods
We performed a retrospective cohort review according to STROBE guidelines of all consecutive patients diagnosed with AHI following total hip or knee arthroplasty at a single tertiary center between September 2013 and February 2020. The patients were identified from prospectively collected data in an institutional quality register. We used the Delphi international consensus criteria to define PJI (14). AHI was classified according to the Tsukayama classification, as abrupt symptoms of infection more than 3 months after implantation in an otherwise well-functioning total hip or knee arthroplasty (4). Treatment success was regarded as free of infection and defined as absence of clinical and laboratory signs of infection and no signs of loosening at the latest follow-up with a minimum of 1 year. A manual chart review was performed. Patient characteristics, i.e., age, sex, body mass index (BMI), comorbidities, specific joint (hip or knee), index surgery (primary or revision), and previous PJI were registered. Clinical manifestation at the time of admission (body temperature > 37.5°C and purulence) and laboratory results (serum C-reactive protein [CRP], white blood-cell [WBC], and peripheral blood cultures) were also registered, as well as organisms cultured. Finally, type of surgical treatment and outcome were recorded.
Treatment (Figure 1)
The surgical treatment was chosen individually according to patient- and implant-specific conditions. A DAIR procedure, including exchange of modular parts, was considered when there had been a short period of symptoms (4–6 weeks) and with well-fixed implants. If these criteria were not fulfilled, revision surgery with removal of the implant, or lifelong antimicrobial suppressive therapy, was considered. 6–8 tissue samples were obtained during surgery and cultured aerobically and anaerobically for 7 days. Empiric intravenous antimicrobial therapy with vancomycin and a beta-lactam was started after the tissue samples were obtained. Upon identification of the causative microbe, antimicrobial treatment was changed according to the pattern of antibiotic susceptibility. Antimicrobial treatment was given intravenously for 14 days followed by oral treatment typically continued for an additional 4 weeks. The patients were scheduled for follow-up at 6 weeks and at 3 and 12 months after discharge. Radiographs of the joint and laboratory tests with sedimentation rate (ESR), CRP, and leucocyte count were routinely obtained.
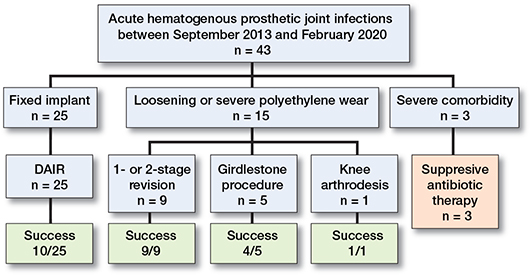
Figure 1. Flowchart of treatment and initial outcome in 43 patients with AHIs.
Outcome
The primary endpoint was free of infection at 1-year followup. Success was defined as absence of clinical and laboratory signs of infection and no signs of loosening of the implant. We used the Delphi criteria to define failure within 1-year followup (15): (i) recurrence of infection, (ii) subsequent surgical intervention, and (iii) death due to PJI.
Statistics
Descriptive statistical analyses were performed using SPSS, version 26 (IBM Corp, Armonk, NY, USA).
Ethics, data sharing, funding, and disclosures
Ethics approval from the institutional review board was obtained (20/19708). Data sharing is not possible. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.10312
Results
Demographic characteristics
We identified 43 consecutive patients with AHI during the study period. 26 of the patients were men, and median age was 75 years (range 43–92). The median ASA score was 3, and 31/43 were categorized as ASA score 3 or 4. Demographic data are presented in Table 1.
Clinical findings
28 of 43 of the AHIs were in a primary arthroplasty and the hip was the most affected joint (31/43). 15 of 43 implants had previously been revised, 5 because of infection. Infection-free period from joint replacement, either a primary arthroplasty or a revision, to treatment for AHI, was a median 6.5 years (range 0.3–23). Median duration of symptoms was 7 days (range 2–120), and 38 patients had had symptoms for less than 4 weeks. All patients had a painful joint. 22 patients presented with a fever and the median CRP was 229 mg/L (range 3–571). 32 patients had a raised WBC >10 × 109/L, median 13 (range 3.4–30). Median temperature was 37.6°C (range 35.6°– 40.3°). A blood culture was obtained preoperatively in 38 of 43 patients, with a positive culture identical to the microbe identified in biopsies from the joint in 22 of the infections. None of the patients presented with a sinus tract, but in all 40 cases operatively treated there was purulence surrounding the implant during surgery. In 15/43 patients, there was a distant infection or a procedure predisposing to bacteremia identified and considered to be the source of the AHI (Table 2). The median follow-up was 22 months (range 0.1–96). The mortality rate was 8/43 at 2 years and 12/43 at the latest follow-up. Median time to death was 10 months.
Microbiology
The AHIs were culture-positive in 41/43 cases. The most frequently isolated organisms were Staphylococcus aureus and streptococcus species, but as many as 16 different microbes were identified (Table 3). No methicillin-resistant S. aureus (MRSA) or polymicrobial infections were found.
Treatment
Surgical treatment was performed in 40/43 patients. In 3 severely ill patients, no surgery but lifelong suppressive antimicrobial treatment was chosen due to high surgical risk. 25 infections were treated with a DAIR procedure, and modular components could be exchanged in 18 of the 25 DAIR procedures. Time from start of symptoms to DAIR procedure was median 7 days (range 2–120). The patient with 120 days of symptoms was an outlier where the implant was found not to be replaceable. The success rate of a DAIR procedure was 10/25. The failure rate was not affected by exchange of modular components. In 8 patients, the implants were loose, and hence not available for a DAIR procedure. Further, in 1 patient the implant was old and with much wear, and a revision arthroplasty was also performed. 5 patients with increased comorbidity were not found fit enough to go through major revision surgery in 2 stages, and hence a resection arthroplasty (Girdlestone procedure) was performed with a successful outcome in 4/5 patients. In 1 patient, a knee arthrodesis in 2 stages was successfully performed. Median time to removal of implant was 10 days (range 4–35). Treatment success of removal of the implant as the primary treatment for AHI was 14/15, significantly better than that of a DAIR procedure (10/25). An overview of primary treatment outcome is presented in Figure 1.
15 of the patients treated with a DAIR procedure failed the initial treatment. 9 of these 15 were successfully treated in a second procedure with either a 2-stage revision (n = 5), a new DAIR (n = 1), a Girdlestone procedure (n = 1), or a femoral amputation (n = 2). In 1 patient, a 2-stage revision was performed with no success and followed by antibiotic suppression. In addition, 3 other patients were treated with suppressive antimicrobial therapy without a second surgical procedure. 2 patients were treated with a second surgical procedure, a DAIR and a resection arthroplasty, but died shortly after surgery.
While the median time from the index arthroplasty to onset of AHI was 6.5 years, the implant age was less than 2 years in 13 of the patients, of whom 12 were surgically treated. The success rate was poorer among these implants compared with the older implants, with a success rate of 4/12 versus 20/28, respectively. These young implants had also more often been previously revised for an infection, 4/13 versus 1/30. When comparing the implants successfully surgically treated with the failures, the mean implant age was likewise older in the success group, 111 months (SD 72) versus 47 months (SD 48).
Success rates following surgical treatment for AHI were lower among patients with an S. aureus infection (6/15 versus 18/25), but this was not found when analyzing the DAIRs separately. No other associations between organism and treatment outcome were found. The success rates were also lower overall in knee arthroplasties compared with hip arthroplasties (3/10 versus 21/30). An overview of clinical findings and treatment outcome is presented in Table 4.
Discussion
Our main finding was that the outcome following a DAIR procedure in patients with AHI was poor, and significantly lower than in patients treated with removal of the implant.
There are some characteristics previously described, such as older patients, more comorbidities and a more prominent clinical presentation with fever, purulence, and highly elevated inflammatory markers (12,16). This is in line with our observations. Konigsberg et al. suggest that AHI may be a marker of poor general health that predisposes to infection. They reported a high 2-year mortality rate (25%) (17). We found a mortality rate of 28% (12/43) at the latest follow-up, and 8 of 43 (19%) had died within 2 years. These rates are higher than previous reports on mortality following PJI (13.6 % at 2 years) (18), but still lower than in patients with PJI following hemiarthroplasty for an acute hip fracture (47–50 % at 1 year) (19,20).
The knee joint has previously been reported as more likely to be affected by AHI than the hip, often explained by the poorer soft-tissue envelope and larger metal surface (9,12,21,22). We found more hips than knees, which may be due to the relatively low number of total knee arthroplasties compared with hip arthroplasties that have been performed over the years in Norway (23). We did find, though, that the success rate was poorer in knees compared with hips, 3/10 versus 21/30, respectively. This may be explained by more complicated surgery around the knee, a limited soft-tissue envelope, and a larger implant surface. Half of the knee prostheses in our series had previously been revised, which may further explain the poorer treatment result. A revision arthroplasty has been reported as a risk factor for DAIR failure (12).
Our results also confirmed the different and broad pathogen spectrum causing AHI reported by others (6,8). As acute postoperative PJIs are dominated by staphylococci and the rate of polymicrobial infections may be quite high (30%), the AHIs almost exclusively are monomicrobial (3,8,22). None of the infections in this present study was polymicrobial. 13/43 (32%) of infections were caused by streptococci. This is in accordance with previous observations of streptococcal infections (33–39%) (8,12,17), all much higher than reported in acute postoperative PJIs (3). In total, 16 different organisms were identified in our series, dominated by virulent microbes, which may reflect the risk of bacteremia in patients with poor health status, and that some of the microbes, like streptococci, have an affinity for prosthetic implants.
The source of infection was identified in only 15 of the 43 patients, with skin infections as the most frequent. This is in line with previous findings, with cutaneous source reported to be a leading cause of AHI (15–26%) (8,17,22). The 2 infections with S. epidermidis were both associated with implantation of a vascular device, an observation also made by Rakow et al. (22). They suggested looking for the infection source in intravascular devices promptly when coagulase-negative staphylococci grow in blood culture. Identification of the source of infection varies between studies, and this may be caused by difficulties in identifying the source but may also reflect lack of a systematic search for the primary infection focus. Rakow et al. advocate a systematic work-up to identify the source of infection in order to avoid recurrence and to optimize the treatment (22).
A DAIR procedure is recommended as the treatment approach for postoperative PJIs and AHIs by current international guidelines (10). However, several studies have reported unsatisfactory results in treating AHIs with a DAIR, with success rates ranging between 44% and 57% (5,12,24,25). This was also confirmed in our study. The virulence of the microbes and the fact that the DAIR procedure in this situation is performed during a concomitant bacteremia and hence is prone to further bacterial seeding, may be a potential explanation for the poorer results. It could also be explained by continuous seeding of bacteria due to an unrecognized primary source of infection. Further, the health status of these patients often seems poor, which also may play a significant role. A DAIR procedure is for instance reported to be less successful in acute postoperative infections following a hemiarthroplasty in elderly patients with a hip fracture, often explained by their poor host status and frailty (20,27). Finally, it is also a possibility that the infection is an acute manifestation of a chronic PJI and misclassified as an AHI. This latter may be supported by our findings of significantly poorer treatment results in patients in whom the implant was younger than 2 years, though this is not confirmed in prior studies (21,24,25). These patients had also more often previously been revised for an infection.
While the results of a 1- or 2-stage revision are well documented, there are some concerns regarding the results when resection arthroplasty is applied as a salvage procedure following a failed DAIR procedure (27). We found that a 1- or 2-stage revision arthroplasty seemed a safe option as the initial treatment, but also satisfactory as a salvage procedure. A 2-stage procedure was performed successfully in 5 of 7 cases with a failed DAIR in our study. This finding is also supported by others (13,28). The success rate following implant removal was good (14/15) and comparable to previous reports on resection arthroplasty (29,30). Due to the lack of reports on AHI treated with implant removal, the results are somewhat difficult to compare with identical patients, but a few series are reported. Rodríguez et al. treated 9 patients with a 2-stage revision, and 7 with a resection arthroplasty in a series of 50 AHIs, with a success rate of 87% (5). Wouthuyzen-Bakker et al. reported on 20 1-stage, 78 2-stage, and 7 Girdlestone procedures as primary treatment of AHI. A significantly better outcome (75%) compared with DAIR (55%) was reported (13). Removal of the implant may hence be a safer treatment option for some patients with AHI and should probably more often be considered.
In our study, the success rate of S. aureus infections was 6/15, significantly lower than in non-staphylococcal infections at 18/25. S. aureus has in general been associated with treatment failure of PJI, especially after DAIR procedures (17,31), but in other studies this association has not been confirmed (12,16,24,32). Wouthuyzen-Bakker et al. found that staphylococcus spp. had lower treatment success in AHIs compared with acute postoperative PJIs, and they suggest that a DAIR procedure in staphylococcal AHI should be reconsidered (25). The poor results in staphylococcal infection are explained by the virulence, the biofilm production, and frequent antibiotic resistance (17). In our series, none of the infections were due to MRSA, though. S. aureus also can remain dormant in a biofilm for years, and this may cause chronic infections to be misdiagnosed as AHI and hence explain a poorer result of a DAIR procedure (33).
Strengths and limitations
There are several limitations to the study. Even though the patients were prospectively registered, the study has a retrospective design with its associated limitations. The sample size was relatively small, which limits the possibility for analysis. The diagnosis of AHI comes with uncertainties, and some infections may have been chronic infections. The types of antibiotics used were not registered. Our study also has some strengths. AHI is relatively rare, and the prospective registration of our cohort over 7 years results in one of the largest reported. We therefore believe that our cohort is representative of AHIs in general and that our findings reflect daily clinical practice.
Conclusion
AHIs treated with DAIR had lower treatment success compared with implant removal. The majority of infections were caused by virulent microbes, and the mortality rate was high. Bacterial virulence, patient frailty, and continuous bacterial seeding to the joint from a distant source could all be contributory factors to our findings. Thus, the DAIR procedure may be a viable treatment option in some AHIs, but in patients with an implant age < 2 years, and in S. aureus infections, revision surgery with implant removal should be considered.
- Dale H, Høvding P, Tveit S M, Graff J B, Lutro O, Schrama J C, et al. Increasing but levelling out risk of revision due to infection after total hip arthroplasty: a study on 108,854 primary THAs in the Norwegian Arthroplasty Register from 2005 to 2019. Acta Orthop 2021; 92(2): 208-14. doi: 10.1080/17453674.2020.1851533.
- Wolford H M, Hatfield K M, Paul P, Yi S H, Slayton R B. The projected burden of complex surgical site infections following hip and knee arthroplasties in adults in the United States, 2020 through 2030. Infect Control Hosp Epidemiol 2018; 39(10): 1189-95. doi: 10.1017/ice.2018.184.
- Tande A J, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27(2): 302-45. doi: 10.1128/CMR.00111-13.
- Tsukayama D T, Estrada R, Gustilo R B. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996; 78(4): 512-23. doi: 10.2106/00004623-199604000-00005.
- Rodríguez D, Pigrau C, Euba G, Cobo J, García-Lechuz J, Palomino J, et al.; REIPI Group (Spanish Network for Research in Infectious Disease). Acute haematogenous prosthetic joint infection: prospective evaluation of medical and surgical management. Clin Microbiol Infect 2010; 16(12): 1789-95. doi: 10.1111/j.1469-0691.2010.03157.x.
- Benito N, Franco M, Ribera A, Soriano A, Rodriguez-Pardo D, Sorlí L, et al.; REIPI (Spanish Network for Research in Infectious Disease) Group for the Study of Prosthetic Joint Infections. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect 2016; 22(8): 732.e1-8. doi: 10.1016/j.cmi.2016.05.004.
- de Kraker M E, Jarlier V, Monen J C, Heuer O E, van de Sande N, Grundmann H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 2013; 19(9): 860-8. doi: 10.1111/1469-0691.12028.
- Zeller V, Kerroumi Y, Meyssonnier V, Heym B, Metten M A, Desplaces N, et al. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J Infect 2018; 76(4): 328-34. doi: 10.1016/j.jinf.2017.12.016.
- Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop 2015; 86(3): 321-5. doi: 10.3109/17453674.2015.1035173.
- Argenson J N, Arndt M, Babis G, Battenberg A, Budhiparama N, Catani F, et al. Hip and knee section, treatment, debridement and retention of implant: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019; 34(2S): S399-S419. doi: 10.1016/j.arth.2018.09.025.
- Vilchez F, Martínez-Pastor J C, García-Ramiro S, Bori G, Tornero E, García E, et al. Efficacy of debridement in hematogenous and early post-surgical prosthetic joint infections. Int J Artif Organs 2011; 34(9): 863-9. doi: 10.5301/ijao.5000029.
- Shohat N, Goswami K, Tan T L, Fillingham Y, Parvizi J. Increased failure after irrigation and debridement for acute hematogenous periprosthetic joint infection. J Bone Joint Surg Am 2019; 101(8): 696-703. doi: 10.2106/JBJS.18.00381.
- Wouthuyzen-Bakker M, Sebillotte M, Lomas J, Kendrick B, Palomares E B, Murillo O, et al.; ESCMID Study Group for Implant-Associated Infections (ESGIAI). Timing of implant-removal in late acute periprosthetic joint infection: a multicenter observational study. J Infect 2019; 79(3): 199-205. doi: 10.1016/j.jinf.2019.07.003.
- Parvizi J, Tan T L, Goswami K, Higuera C, Della Valle C, Chen A F, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018; 33(5): 1309-1314.e2. doi: 10.1016/j.arth.2018.02.078.
- Diaz-Ledezma C, Higuera C A, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res 2013; 471(7): 2374-82. doi: 10.1007/s11999-013-2866-1.
- Sendi P, Banderet F, Graber P, Zimmerli W. Clinical comparison between exogenous and haematogenous periprosthetic joint infections caused by Staphylococcus aureus. Clin Microbiol Infect 2011; 17(7): 1098-100. doi: 10.1111/j.1469-0691.2011.03510.x.
- Konigsberg B S, Della Valle C J, Ting N T, Qiu F, Sporer S M. Acute hematogenous infection following total hip and knee arthroplasty. J Arthroplasty 2014; 29(3): 469-72. doi: 10.1016/j.arth.2013.07.021.
- Zmistowski B, Karam J A, Durinka J B, Casper D S, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am 2013; 95(24): 2177-84. doi: 10.2106/JBJS.L.00789.
- Edwards C, Counsell A, Boulton C, Moran C G. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br 2008; 90(6): 770-7. doi: 10.1302/0301-620X.90B6.20194.
- Westberg M, Snorrason F, Frihagen F. Preoperative waiting time increased the risk of periprosthetic infection in patients with femoral neck fracture. Acta Orthop 2013; 84(2): 124-9. doi: 10.3109/17453674.2013.775044.
- Tande A J, Palraj B R, Osmon D R, Berbari E F, Baddour L M, Lohse C M, et al. Clinical presentation, risk factors, and outcomes of hematogenous prosthetic joint infection in patients with Staphylococcus aureus bacteremia. Am J Med 2016; 129(2): 221.e11-20. doi: 10.1016/j.amjmed.2015.09.006.
- Rakow A, Perka C, Trampuz A, Renz N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin Microbiol Infect 2019; 25(7): 845-50. doi: 10.1016/j.cmi.2018.10.010.
- Furnes O, Hallan G, Gjertsen J E, Visnes H, Gundersen T, Fenstad A M, et al. The Norwegian Arthroplasty Register, annual report. Norwegian Arthroplasty Register; 2021. Available from http://nrlweb.ihelse.net/eng/Rapporter.
- Fink B, Schuster P, Schwenninger C, Frommelt L, Oremek D. A standardized regimen for the treatment of acute postoperative infections and acute hematogenous infections associated with hip and knee arthroplasties. J Arthroplasty 2017; 32(4): 1255-61. doi: 10.1016/j.arth.2016.10.011.
- Wouthuyzen-Bakker M, Sebillotte M, Huotari K, Escudero Sánchez R, Benavent E, Parvizi J, et al.; ESCMID Study Group for Implant-Associated Infections (ESGIAI). Lower success rate of debridement and implant retention in late acute versus early acute periprosthetic joint infection caused by Staphylococcus spp.: results from a matched cohort study. Clin Orthop Relat Res 2020; 478(6): 1348-1355. doi: 10.1097/CORR.0000000000001171.
- del Toro M D, Nieto I, Guerrero F, Corzo J, del Arco A, Palomino J, et al.; PJIG-SAEI/REIPI group. Are hip hemiarthroplasty and total hip arthroplasty infections different entities? The importance of hip fractures. Eur J Clin Microbiol Infect Dis 2014; 33(8): 1439-48. doi: 10.1007/s10096-014-2091-1.
- Sherrell J C, Fehring T K, Odum S, Hansen E, Zmistowski B, Dennos A, et al.; Periprosthetic Infection Consortium. The Chitranjan Ranawat Award: Fate of two-stage reimplantation after failed irrigation and debridement for periprosthetic knee infection. Clin Orthop Relat Res 2011; 469(1): 18-25. doi: 10.1007/s11999-010-1434-1.
- Nodzo S R, Boyle K K, Nocon A A, Henry M W, Mayman D J, Westrich G H. The influence of a failed irrigation and debridement on the outcomes of a subsequent 2-stage revision knee arthroplasty. J Arthroplasty 2017; 32(8): 2508-12. doi: 10.1016/j.arth.2017.03.026.
- Rowan F E, Donaldson M J, Pietrzak J R, Haddad F S. The role of one-stage exchange for prosthetic joint infection. Curr Rev Musculoskelet Med 2018; 11(3): 370-9. doi: 10.1007/s12178-018-9499-7.
- Akgün D, Müller M, Perka C, Winkler T. High cure rate of periprosthetic hip joint infection with multidisciplinary team approach using standardized two-stage exchange. J Orthop Surg Res 2019; 14(1): 78. doi: 10.1186/s13018-019-1122-0.
- Lora-Tamayo J, Murillo O, Iribarren J A, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu J M, et al.; REIPI Group for the Study of Prosthetic Infection. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56(2): 182-94. doi: 10.1093/cid/cis746.
- Westberg M, Grøgaard B, Snorrason F. Early prosthetic joint infections treated with debridement and implant retention: 38 primary hip arthroplasties prospectively recorded and followed for median 4 years. Acta Orthop 2012; 83(3): 227-32. doi: 10.3109/17453674.2012.678801.
- Trouillet-Assant S, Lelièvre L, Martins-Simões P, Gonzaga L, Tasse J, Valour F, et al. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol 2016; 18(10): 1405-14. doi: 10.1111/cmi.12582.