Dislocation rate after hip arthroplasty due to metastatic bone disease: a retrospective cohort study evaluating the postoperative dislocation risk across different articulating solutions
Afrim ILJAZI 1, Michala Skovlund SØRENSEN 1, Thea Hovgaard LADEGAARD 1, Søren OVERGAARD 2,3, and Michael Mørk PETERSEN 1,3
1 Musculoskeletal Tumor Section, Department of Orthopedic Surgery, Copenhagen University Hospital—Rigshospitalet; 2 Department of Orthopedic Surgery and Traumatology, Copenhagen University Hospital—Bispebjerg; 3 Department of Clinical Medicine, Faculty of Health Science, University of Copenhagen, Denmark
Background and purpose — Joint stability after hip replacement (HR) in patients with metastatic bone disease (MBD) is of special importance. Dislocation is the second leading cause of implant revision in HR, while survival after MBD surgery is poor with an expected 1-year survival of around 40%. As few studies have investigated the dislocation risk across different articulation solutions in MBD, we conducted a retrospective study on primary HR for patients with MBD treated in our department.
Patients and methods — The primary outcome is the 1-year cumulative incidence of dislocation. We included patients with MBD who received HR at our department in 2003–2019. We excluded patients with partial pelvic reconstruction, total femoral replacement, and revision surgery. We assessed the incidence of dislocation with competing risk analysis with death and implant removal as competing risks.
Results — We included 471 patients. Median followup was 6.5 months. The patients received 248 regular total hip arthroplasties (THAs), 117 hemiarthroplasties, 70 constrained liners, and 36 dual mobility liners. Major bone resection (MBR), defined as resection below the lesser trochanter, was performed in 63%. The overall 1-year cumulative incidence of dislocation was 6.2% (95% CI 4.0–8.3). Dislocation stratified by articulating surface was 6.9% (CI 3.7–10) for regular THA, 6.8% (CI 2.3–11) for hemiarthroplasty, 2.9% (CI 0.0–6.8) for constrained liner, and 5.6% (CI 0.0–13) for dual mobility liners. There was no significant difference between patients with and without MBR (p = 0.5).
Conclusion — The 1-year cumulative incidence of dislocation is 6.2% in patients with MBD. Further studies are needed to determine any real benefits of specific articulations on the risk of postoperative dislocation in patients with MBD.
| CL = constrained liner. |
| DM = dual mobility liner. |
| HA = hemiarthroplasty. |
| HR = hip replacement. |
| MBD = metastatic bone disease of the hip. |
| MBR = major bone resection. |
| THA = total hip arthroplasty. |
Citation: Acta Orthopaedica 2023; 94: 107–114. DOI: https://doi.org/10.2340/17453674.2023.10311.
Copyright: © 2023 The Author(s). This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for non-commercial purposes, provided proper attribution to the original work.
Submitted: 2022-11-24. Accepted: 2023-02-16. Published: 2023-03-06.
Correspondence: Afrim.iljazi.04@regionh.dk
AI contributed with data collection, data analysis, and interpretation, writing the first draft of the manuscript and critical revisions of the manuscript. MSS contributed with the conception of the study, data collection, data analysis and interpretation, and critical revisions of the manuscript. THL contributed with data collection and critical revisions of the manuscript. SO contributed data interpretation and critical revisions of the manuscript. MMP contributed with the conception of the study, data interpretation, and critical revisions of the manuscript.
Handling co-editors: Bart A. Swierstra and Philippe Wagner
Acta thanks Michelle Ghert and Marc Nijhof for help with peer review of this study.
Joint stability following hip arthroplasty represents a special challenge in patients with metastatic bone disease of the hip (MBD). Dislocation is one of the most common causes for revision surgery in the first postoperative year after total hip arthroplasty (THA) (1,2). While patients with MBD also benefit from hip replacement (HR), these patients are prone to a higher frequency of complications such as dislocation (3-6). Major bone resection (MBR), defined as bone resection below the lesser trochanter, is often required to remove osteolytic bone of poor quality. This, in turn, detaches the hip abductor muscles from their bony anchorage and adversely affects the postoperative joint stability (3). Further, patients with MBD have a poor survival prognosis with a 1-year survival at approximately 40% (7,8). It is therefore crucial to choose a prosthesis that mitigates the risks of instability and minimizes the need for hospital readmissions or revision surgeries in the remaining lifespan. There are only a few recent studies reporting the postoperative dislocation risk in patients with HR for MBD (3,4,6). Hence, we conducted a retrospective record review of all patients who received a primary HR due to MBD at our department in 2003–2019 to evaluate the dislocation risk and survival in these patients, and to investigate whether these vary between patients that have received different types of articulating surfaces.
Patients and methods
Design
This is a retrospective cohort study conducted at the Musculoskeletal Tumor Section at the Department of Orthopedic Surgery, Rigshospitalet, Denmark. The study is reported according to the STROBE/RECORD guidelines. Our department at Rigshospitalet is a highly specialized tertiary referral center, with an intake area that covers all of eastern and southern Denmark. We identified all patients who received a HR due to a pathologic fracture or an impending pathologic fracture at our department in 2003–2019.
Study population
We included patients who met the following criteria:
Inclusion criteria:
– Age ≥ 18 at the time of the surgery.
– Pathologic fracture or impending pathologic fracture of the proximal femur and/or acetabulum due to cancer dissemination or local hematologic malignancy.
– Primary HR in the period January 1, 2003 to December 31, 2019 with either regular THA, hemiarthroplasty (HA), constrained liner (CL), or dual mobility liner (DM).
Exclusion criteria:
– Revision surgery of an existing endoprosthesis in the ipsilateral hip.
– Partial pelvic reconstruction of the ipsilateral hip.
– Total femoral placement of the ipsilateral femur.
Data sources and data collection
We identified patients by searching our institutional surgery scheduling system for all HRs due to MBD. We then collected information on patient demographics, cancer history, the surgical procedure, the implant, and study outcomes from the electronic patient record (EPIC) for patients from the Capital Region and Zealand Region, and the digital hospital records (in Danish, Sundhedsjournalen) for patients from the rest of Denmark. We also retrieved data from paper-based patient records for patients with hospital visits pre-dating any electronic patient records. Finally, we searched the Danish National Imaging Archive for hip imaging of our cohort to identify any dislocations that might have been missing from the patient records. Data was extracted and entered into prespecified data extraction sheets. Data for patients receiving surgery between 2003 and 2013 was extracted by MSS. Data for patients from the Capital Region of Copenhagen who received surgery between 2014 and 2019 was extracted by THL, while data on patients from the Zealand Region and the Southern Region of Denmark who received surgery between 2014 and 2019 was extracted by AI.
Outcome measures
We followed the patients from the day of surgery and until the first dislocation, death, revision of a bone-anchored component, or April 16, 2022, whichever came first. The primary outcome was the cumulative incidence of dislocation 1-year following surgery for the overall cohort. Secondary outcomes included: (i) the cumulative incidence of dislocation within 1 month, 3 months, 1 year, and 5 years both overall and stratified by type of articulation and MBR; (ii) patient survival within 1 month, 3 months, 1 years, and 5 years overall and stratified by type of articulation and MBR, and (iii) the influence of age, sex, Karnofsky Performance Status (9), ASA score, type of articulation, and MBR on the cumulative influence of dislocation or competing events. Competing events were defined as death or revision of a bone-anchored component.
Statistics
We calculated the cumulative incidence of dislocation using the Aalen–Johnson Estimator (competing risk analysis) with death and implant removal as competing risks. Results were reported as the point estimate with the 95% confidence interval (CI). We defined dislocation as a displacement of the femoral head from the joint socket. We included only the first dislocation when calculating the cumulative incidence of dislocation, after which we censored the patients. The cumulative incidence of dislocation was calculated for the entire cohort and stratified by type of articulating surface (regular THA, HA, CL, and DM) and by MBR. We restricted the analysis to the first surgery in the observation period for patients with bilateral surgery to avoid dependency issues (10). The difference between groups was assessed with Gray’s test. We analyzed the influence of age, sex, ASA score (1–2 vs. 3–4), Karnofsky Performance Status score (≥ 70 vs. < 70), MBR, and type of articulation on the risk of dislocation using cause-specific Cox proportional hazards regression. We checked the proportional hazard assumption by evaluating the Schoenfeld residuals for each variable and found that the proportional hazard assumption was not violated. We calculated the overall survival and survival stratified by articulation type and MBR using the Kaplan–Meier estimate for cumulative survival. The difference between the survival curves stratified by articulation type and MBR was evaluated with the log-rank test. R version 4.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis.
Ethics, registration, data sharing plan, funding, and disclosures
The Danish Patient Safety Authority (R-21041715) and the Data Protection Agency of the Capital Region of Copenhagen (P-2021-578) have approved this study. Data can be shared upon reasonable request. The study is funded from Rigshospitalet’s Forskningspulje, which has provided a grant covering the salary for 1 PhD student (AI). The authors declare no conflicts of interest. Completed disclosure forms for this article following the ICMJE template are available on the article page, doi: 10.2340/17453674.2023.10311
Results
Patients
We identified 471 patients who received 489 primary HRs from January 1, 2003 until December 31, 2019 (Figure 1). Patient characteristics for the overall cohort and stratified by type of articulating surface are presented in Table 1 (see Appendix). We identified 223 (47%) males and 248 (53%) females with an average age of 66 years (SD 11) at the time of surgery. More than 50% of the surgeries were performed in 2014–2019 while ≥ 75% were performed after 2009. The most frequent cancer types were breast cancer (27%), lung cancer (19%), and prostate cancer (16%). A majority of patients had disseminated disease, with 68% having both axial and appendicular metastases and 46% with visceral metastases besides their bone metastases. The reason for surgery was a complete fracture for 75% of patients and an impending fracture for 25% of the patients. 97% of surgeries were due to metastatic lesions in the proximal femur, while 2% were treated for metastatic lesions in the acetabulum and 1% for combined lesions in both the acetabulum and the proximal femur. All surgeries were performed with a posterolateral approach and all implants were cemented. The choice of articulation stratified by year of surgery is presented in Figure 2. The patients were treated as follows:
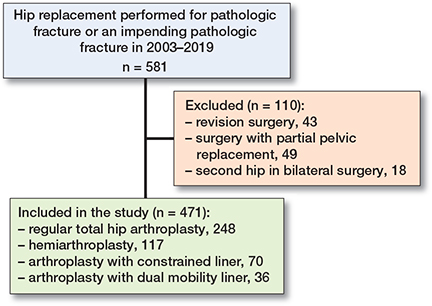
Figure 1. Flowchart of the study inclusion process.
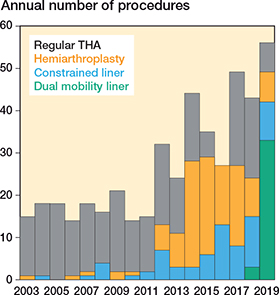
Figure 2. Articulation type by year of surgery.
- Regular THA: 248 (53%) with predominantly a Lubinus cup (LINK; Link Orthopaedics UK, Edinburgh, Scotland) (n = 245, 99%) and either a 32-mm (n = 228, 92%) or a 28-mm head (n = 20, 8%);
- HA: 117 (25%) with a MultiPolar/Bipolar liner (Zimmer Biomet, Warsaw, IN, USA) and a 28-mm head (100%);
- CL: 70 (15%). 47(67%) received a Lubinus cup with a safety ring (LINK), 21 (30%) received a Freedom cup (Zimmer Biomet), 1 received a Trident cup (Stryker, Kalamazoo, MI, USA), and 1 received a Trilogy cup (Zimmer Biomet);
- DM: 36 (7%) received a dual mobility liner with an Avantage cup (Zimmer Biomet).
Major bone resection was performed in 296 (63%) patients, with an average resection length of 13 cm (range 6–27).
Dislocation (Figure 3)
A detailed overview of the cumulative incidence of dislocation is presented in Table 2. 32 out of 471 patients experienced a dislocation at any time point with a median time to dislocation of 42 days (range 0–3.8 years). The overall 1-year cumulative incidence of dislocation was estimated to 6.2% (CI 4.0–8.3). When stratified by type of articulation, the 1-year incidence was 6.9% (CI 3.7–10) for regular THA, 6.8% (CI 2.3–11) for HA, 2.9% (CI 0.0–6.8) for CL, and 5.6% (CI 0.0–13) for DM. The difference between groups was not statistically significant for dislocation (p = 0.5), while there was a statistically significant difference between groups for the incidence of competing events, i.e., the occurrence of death or revision of a bone-anchored component prior to a dislocation (p = 0.04). The cumulative incidence was 5.7% (CI 2.3–9.2) for patients without MBR and 6.4% (CI 3.6–9.2) for patients with MBR. The difference between groups was not statistically significant either for dislocation (p = 0.5) or for competing events (p = 0.07).
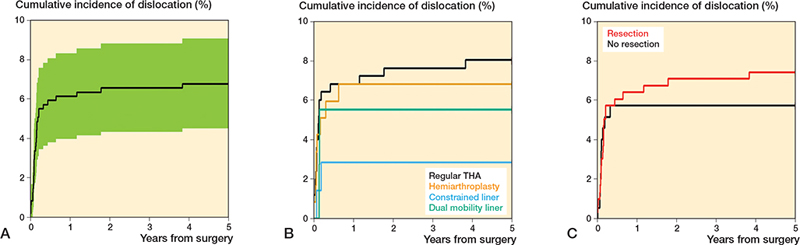
Figure 3. A. Overall cumulative incidence of dislocation. B. Cumulative incidence of dislocation-stratified articulation. C. Cumulative incidence of dislocation stratified by major bone resection.
We observed a steady, but statistically insignificant increase in the incidence of dislocation over time for patients who received a regular THA, from a 3-month incidence at 6.5% (CI 3.4–9.5) to a 5-year incidence at 8.1% (CI 4.7–12). In comparison, none of the patients with an HA who survived beyond 12 months dislocated, while none of the patients with CL or DM who survived beyond 3 months dislocated. Thus, the 5-year cumulative incidence for HA and CL/DM equaled the 1-year and 3-month incidences of dislocation respectively (Table 2).
Cause-specific Cox regression
Articulation type did not have a statistically significant influence on the incidence of dislocation in either a univariate or a multivariate cause-specific Cox regression (Table 3). In the multivariate analysis, male sex (HR 2.1, CI 1.0–4.4) and ASA Score 3–4 (HR 2.5, CI 1.1–5.6) had an adverse influence on the risk of dislocation. The univariate causespecific Cox regression showed that patients with HA had an increased risk of death or implant removal (HR 1.4, CI 1.1–1.8). However, this finding did not remain significant after adjusting for covariates in the multivariate analysis (HR 1.3, CI 0.99–1.6).
Patient survival (Figure 4)
40 out of 471 patients were alive at the end of the study. The median postoperative survival was 6.5 months (range 0–196) (Table 1, see Appendix). A detailed overview of postoperative survival stratified by type of articulation and MBR is presented in Table 4. The 1-year survival probability for the entire cohort was 37% (CI 33–42). The 1-year survival probability stratified by articulation type was 43% (CI 37–49) for patients receiving a regular THA, 27% (CI 19–35) for patients receiving HA, 30% (CI 19–41) for patients receiving CL, and 42% (CI 26–58) for patients receiving DM. The log-rank test revealed a significant difference between groups for the 1-year survival probability (χ2 = 13.9, p = 0.003). When stratifying by MBR, 1-year survival probability was 40% (CI 34–45) for patients with MBR and 33% (CI 26–40) for patients without MBR (χ2 = 4.4, p = 0.04).
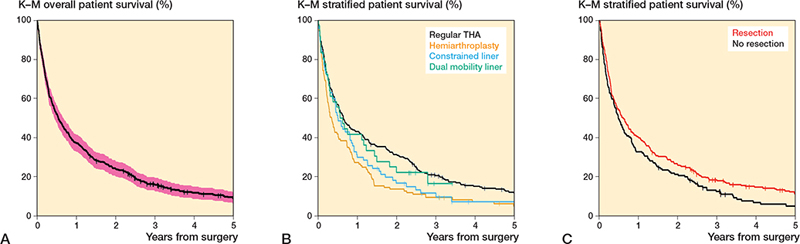
Figure 4. A. Overall survival. B. Survival stratified by articulation. C. Survival stratified by major bone resection.
Discussion
We investigated the postoperative cumulative incidence of dislocation after hip arthroplasty in 471 patients with MBD of the hip. Due to exclusion criteria, none of the patients had acetabular defects that required pelvic reconstruction or received surgery for a failed endoprosthesis, which are known risk factors for dislocation (11,12). We found a 1-year cumulative incidence of dislocation of 6.2% and a 5-year incidence of 6.8%. Our study showed equal results between HA and regular THA regarding the dislocation risk.
The results from our study fall in line with previous results on incidence of dislocation following HR for primary or secondary neoplastic disorders. Previous studies have reported dislocation rates ranging from 0% to 20% for HA (4-6,11,13-18) and 3.5% to 22% for THA (13-17,19-21). Most studies in this population report data on patients who have received HA. Studies that have included patients with both HA and THA have previously reported that THA conveys an increased risk of dislocation compared with HA (13-17). In contrast to previous studies, we did not find a difference in the incidence of dislocation between HA and THA. The discrepancy between our and previous results could be related to historic differences and the choice of THA. Previous studies comparing THA with HA included patients who received surgery in the period 1988–2003 (13-17), while all surgeries in our study were performed from 2003 onwards. It is known that smaller diameter femoral heads of 22/28 mm were more common in the 1990s and that these are associated with an increased risk of dislocation compared with larger diameter femoral heads (22,23). In our study, 92% of the femoral heads for regular THA were 32 mm. Likewise, we almost exclusively used the Lubinus cup, which has been shown to be more stable (24). Patients who received HA in our institution had a higher preoperative morbidity, as discussed in the next section. Selection bias can therefore not be ruled out, as it is uncertain whether THA would have provided equivalent results in these patients.
The univariate cause-specific Cox regression showed that patients receiving HA had an increased risk of competing events (death or revision). However, this finding is most likely explained by selection bias towards HA for patients with a higher preoperative morbidity. This is substantiated by the multivariate cause-specific Cox regression, which showed that an ASA score of 3–4 and a poorer preoperative performance status (Karnofsky score < 70) both significantly increased the risk of competing events occurring prior to dislocation, while the effect of HA no longer remained statistically significant after adjusting for covariates.
Our study shows that CL and DM perform well in patients with MBD regarding the postoperative dislocation risk. We found a 5-year dislocation risk of 2.9% and 5.6% for CL and DM respectively, with no dislocations occurring in patients surviving beyond 3 months. Our findings are in accordance with previous literature on the use of CL and DM in oncologic patients. 1 study with 38 patients who received DM due to MBD found a 5.2% dislocation risk (3), while another study with 126 patients with periacetabular metastases treated with DM found a 2% dislocation risk (25). Similarly for CL, 1 study with 33 oncologic patients undergoing HR with subtrochanteric femoral resection (26) and another study of 47 patients with MBD and periacetabular destruction (27) both demonstrated a 0% dislocation risk. Although these results are encouraging, we suggest caution in a large-scale implementation in clinical practice. Compared with other surgical indications, there is a scarcity of data on the use of CL and DM in patients with MBD, with a general lack of prospective, randomized trials. Although our point estimate for the incidence of dislocation compares favorably for CL and DM, it is important to mention that the confidence intervals for the risk estimates are overlapping with those for THA and HA (Table 2). Our retrospective study is thus underpowered to draw any definite conclusions on the superiority or non-inferiority of specific articulations for the prevention of dislocation in this population.
The incidence of dislocation in patients with MBD treated with THA is on a level with that of patients with femoral neck fractures (FNF) treated with HA when the surgical approach is taken into consideration. All patients in our study were treated using a posterolateral approach. In FNF patients treated with HA using the posterolateral approach, the incidence of dislocation is reported at 6.1–11.7% (28,29). Both populations have a high risk of dislocation and resemble each other in that FNF patients often have lower functional status and higher morbidity compared with osteoarthritic patients (30). Patients with MBD furthermore often undergo reconstruction with MBR, which theoretically should put these patients at even higher risk than FNF patients. Despite this, our findings show that the dislocation risks are comparable between these populations, which indicates that the functional status and overall frailty of the patients are more important factors.
Strengths and limitations
The main strength of this study is the large and homogeneous sample. The majority of HRs in this study were performed in the past decade, which reflects recent improvements in implant design and the efficacy of current solutions in preventing dislocations compared with previous studies. We thoroughly searched electronic patient records, physical patient records, and the national imaging database for dislocations in our cohort, which is why we are confident that we have identified all cases. However, this study also has several limitations. First, this study is an observational study and includes all the limitations attributed to this study design. Second, treatment allocation was non-random and at the discretion of the operating surgeon, which introduces bias by indication. A majority of the implants until 2013 were regular THAs for our cohort, while this proportion dropped below 50% thereafter. This is consistent with a divergence toward HA, CL, and DM for patients with a higher preoperative morbidity or who were considered at a higher risk of instability a priori. This is supported by higher ASA scores and lower Karnofsky Performance Status scores, and lower survival for HA and CL. This discrepancy likely would have been larger if the analysis was restricted to patients receiving surgery after 2012. Finally, interpretation of our results is limited by the sample size of our subgroups. Despite a large total sample size, our analysis stratified by prosthetic concept was underpowered to detect whether the difference between point estimates amounted to any real difference between groups. Further studies with larger sample sizes are needed to address this limitation.
Conclusion and future directions
The 1-year cumulative incidence of dislocation following HR due to MBD was 6.2% while the 1-year survival was 37%. Our study was underpowered to detect a statistically significant difference in the incidence of dislocation among different types of articulating surfaces. Further studies are needed to determine any real benefits of specific articulations on the risk of postoperative dislocation in patients with MBD.
- Van Steenbergen L N, Mäkelä K T, Kärrholm J, Rolfson O, Overgaard S, Furnes O, et al. Total hip arthroplasties in the Dutch Arthroplasty Register (LROI) and the Nordic Arthroplasty Register Association (NARA): comparison of patient and procedure characteristics in 475,685 cases. Acta Orthop 2021; 92(1): 15-22. doi: 10.1080/17453674.2020.1843875.
- Danish Hip Arthroplasty Register. National annual report 2021. Available from: http://danskhoftealloplastikregister.dk/wp-content/uploads/2021/08/DHR-aarsrapport-2021_Offentliggoerelse.pdf.
- Philippeau J M, Durand J M, Carret J P, Leclercq S, Waast D, Gouin F. Dual mobility design use in preventing total hip replacement dislocation following tumor resection. Orthop Traumatol Surg Res 2010; 96(1): 2-8. doi: 10.1016/j.rcot.2009.12.011.
- Gusho C A, Clayton B, Mehta N, Colman M W, Gitelis S, Blank A T. Survival and outcomes of modular endoprosthetic reconstruction of the proximal femur for primary and non-primary bone tumors: single institutional results. J Orthop 2021; 25:145-50. doi: 10.1016/j.jor.2021.05.008.
- Chandrasekar C R, Grimer R J, Carter S R, Tillman R M, Abudu A, Buckley L. Modular endoprosthetic replacement for tumours of the proximal femur. J Bone Joint Surg Br 2009; 91(1): 108-12. doi: 10.1302/0301-620X.91B1.20448.
- Harvey N, Ahlmann E R, Allison D C, Wang L, Menendez L R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin Orthop Relat Res 2012; 470(3): 684-91. doi: 10.1007/s11999-011-2038-0.
- Hovgaard T B, Horstmann P F, Petersen M M, Sørensen M S. Patient survival following joint replacement due to metastatic bone disease: comparison of overall patient and prostheses survival between cohorts treated in two different time-periods. Acta Oncol 2018; 57(6): 839-48. doi: 10.1080/0284186X.2017.1420910.
- Skovlund Sørensen M, Hindsø K, Frederik Horstmann P, Troelsen A, Dalsgaard S, Fog T, et al. Incidence of surgical interventions for metastatic bone disease in the extremities: a population-based cohort study. Acta Oncol 2019; 58(4): 456-62. doi: 10.1080/0284186X.2018.1549368.
- Karnofsky D A, Abelmann W H, Craver L F, Burchenal J H. The use of the nitrogen mustards in the palliative treatment of carcinoma: with particular reference to bronchogenic carcinoma. Cancer 1948; 1(4): 634-56. doi: 10.1002/1097-0142(194811)1:4%3C634::AIDCNCR2820010410%3E3.0.CO;2-L.
- Ranstam J, Robertsson O. Statistical analysis of arthroplasty register data. Acta Orthop 2010; 81(1): 10-14. doi: 10.3109/17453671003587168.
- Puchner S E, Funovics P T, Hipfl C, Dominkus M, Windhager R, Hofstaetter J G. Incidence and management of hip dislocation in tumour patients with a modular prosthesis of the proximal femur. Int Orthop 2014; 38(8): 1677-84. doi: 10.1007/s00264-014-2376-0.
- Klemt C, Chen W, Bounajem G, Tirumala V, Xiong L, Kwon Y M. Outcome and risk factors of failures associated with revision total hip arthroplasty for recurrent dislocation. Arch Orthop Trauma Surg 2022; 142(8): 1801-7. doi: 10.1007/s00402-021-03814-2.
- Wedin R, Bauer H C F. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg Br 2005; 87(12): 1653-7. doi: 10.1302/0301-620X.87B12.16629.
- Menendez L R, Ahlmann E R, Kermani C, Gotha H. Endoprosthetic reconstruction for neoplasms of the proximal femur. Clin Orthop Relat Res 2006; 450: 46-51. doi: 10.1097/01.blo.0000229332.91158.05.
- Ahlmann E R, Menendez L R, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br 2006; 88(6): 790-5. Doi: 10.1302/0301-620X.88B6.17519.
- Bischel O E, Böhm P M. The use of a femoral revision stem in the treatment of primary or secondary bone tumours of the proximal femur: a prospective study of 31 cases. J Bone Joint Surg Br 2010; 92(10): 1435-41. doi: 10.1302/0301-620X.92B10.24024.
- Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res 2006; 450:164-71. doi: 10.1097/01.blo.0000223978.36831.39.
- Ilyas I, Pant R, Kurar A, Moreau P G, Younge D A. Modular megaprosthesis for proximal femoral tumors. Int Orthop 2002; 26(3): 170-3. doi: 10.1007/s00264-002-0335-7.
- Sokolovski V A, Voloshin V P, Aliev M D, Zubikov V S, Saravanan S A, Martynenko D V, et al. Total hip replacement for proximal femoral tumours: our midterm results. Int Orthop 2006; 30(5): 399-402. doi: 10.1007/s00264-006-0124-9.
- Quinn R H, Drenga J. Perioperative morbidity and mortality after reconstruction for metastatic tumors of the proximal femur and acetabulum. J Arthroplasty 2006; 21(2): 227-32. doi: 10.1016/j.arth.2005.04.032.
- Nilsson J, Gustafson P. Surgery for metastatic lesions of the femur: good outcome after 245 operations in 216 patients. Injury 2008; 39(4): 404-10. doi: 10.1016/j.injury.2007.07.006.
- Hailer N P, Weiss R J, Stark A, Kärrholm J. The risk of revision due to dislocation after total hip arthroplasty depends on surgical approach, femoral head size, sex, and primary diagnosis: an analysis of 78,098 operations in the Swedish Hip Arthroplasty Register. Acta Orthop 2012; 83(5): 442-8. doi: 10.3109/17453674.2012.733919.
- Byström S, Espehaug B, Furnes O, Havelin L I. Femoral head size is a risk factor for total hip luxation: a study of 42,987 primary hip arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop Scand 2003; 74(5): 514-24. doi: 10.1080/00016470310017893.
- Lindgren V, Garellick G, Kärrholm J, Wretenberg P. The type of surgical approach influences the risk of revision in total hip arthroplasty: a study from the Swedish Hip Arthroplasty Register of 90,662 total hip replacements with 3 different cemented prostheses. Acta Orthop 2012; 83(6): 559. doi: 10.3109/17453674.2012.742394.
- Wegrzyn J, Malatray M, Al-Qahtani T, Pibarot V, Confavreux C, Freyer G. Total hip arthroplasty for periacetabular metastatic disease: an original technique of reconstruction according to the Harrington classification. J Arthroplasty 2018; 33(8): 2546-55. doi: 10.1016/j.arth.2018.02.096.
- Jawad M U, Brien E W. Proximal femoral reconstruction with a constrained acetabulum in oncologic patients. Orthopedics 2014; 37(2). doi: 10.3928/01477447-20140124-24.
- Bagsby D T, Wurtz L D. Effectiveness of constrained liner use during Harrington hip reconstruction in oncology patient. J Arthroplasty 2017; 32(4): 1250-4. doi: 10.1016/j.arth.2016.11.038.
- Bue M, Jakobsen S S, Barckman J, Tábori-Jensen S. Dislocation rate, revisions and other complications of primary cemented hemiarthroplasty for displaced femoral neck fractures: a single-center cohort study of 743 unselected hips with a mean 2.7-year follow-up. Arch Orthop Trauma Surg 2022; 142(12). doi: 10.1007/s00402-021-04252-w.
- Enocson A, Tidermark J, Törnkvist H, Lapidus L J. Dislocation of hemiarthroplasty after femoral neck fracture: better outcome after the anterolateral approach in a prospective cohort study on 739 consecutive hips. Acta Orthop 2009; 79(2): 211-17. doi: 10.1080/17453670710014996.
- Charette R S, Sloan M, Lee G C. Not all hip arthroplasties are created equal: increased complications and re-admissions after total hip arthroplasty for femoral neck fractures compared with osteoarthritis. Bone Joint J 2019; 101-B(6_Supple_B): 84-90. doi: 10.1302/0301-620X.101B6.BJJ-2018-1427.R1.
Appendix
| Factor | Overall | Regular THA | Hemi-arthroplasty | Constrained liner | Dual mobility liner |
| No. of patients | 471 | 248 | 117 | 70 | 36 |
| No. of primary surgeries | 489 | 260 | 121 | 72 | 36 |
| Unilateral surgery | 453 | 236 | 113 | 68 | 36 |
| Bilateral surgery | 18 | 12 | 4 | 2 | 0 |
| Follow-up, median (range), months | 7 (0–196) | 8 (0–196) | 4 (0–76) | 6 (0–78) | 7 (0–41) |
| Female sex | 248 (53) | 140 (56) | 61 (52) | 28 (40) | 19 (53) |
| Age at surgery, mean (SD) | 66 (11) | 65 (11) | 68 (11) | 68 (11) | 68 (12) |
| Karnofsky score, median (range) | 70 (10–100) | 70 (10–100) | 70 (20–100) | 70 (30–100) | 80 (30–100) |
| Score ≥ 70, n (%) | 309 (66) | 153 (62) | 79 (68) | 50 (71) | 27 (75) |
| Missing data | 1 | 1 | 0 | 0 | 0 |
| Most frequent cancers, % | |||||
| Breast | 27 | 30 | 22 | 21 | 28 |
| Lung | 19 | 16 | 23 | 24 | 17 |
| Prostate | 16 | 15 | 18 | 16 | 17 |
| Renal | 10 | 11 | 12 | 0 | 0 |
| Head/neck | 0 | 0 | 0 | 7 | 0 |
| Unknown | 0 | 0 | 5 | 0 | 8 |
| Multiple myeloma | 6 | 6 | 0 | 7 | 8 |
| Other | 22 | 22 | 20 | 254 | 22 |
| Appendicular and axial bone metastases, yes | 322 (68) | 169 (68) | 84 (72) | 45 (64) | 24 (67) |
| No | 147 (31) | 78 (31) | 33 (28) | 24 (34) | 12 (33) |
| Unknown | 2 (1) | 1 (1) | 0 (0) | 1 (2) | 0 (0) |
| Visceral metastases, yes | 218 (46) | 114 (46) | 58 (50) | 24 (34) | 22 (61) |
| No | 237 (50) | 130 (52) | 55 (47) | 40 (57) | 12 (33) |
| Unknown | 16 (4) | 4 (2) | 4 (3) | 6 (9) | 2 (6) |
| Pathologic fracture first sign of disease | 115 (24) | 70 (28) | 25 (21) | 13 (19) | 7 (20) |
| Years from diagnosis to surgery a, median (range) | 2.7 (0.1–38) | 2.7 (0.1–38) | 2.7 (0.1–22) | 2.1 (0.1–20) | 2.7 (0.1–34) |
| Fracture | |||||
| Complete | 353 (75) | 179 (72) | 96 (82) | 54 (77) | 24 (67) |
| Impending | 118 (25) | 69 (28) | 21 (18) | 16 (23) | 12 (33) |
| Tumor location | |||||
| Left | 239 (51) | 128 (52) | 55 (47) | 39 (56) | 17 (47) |
| Right | 232 (49) | 120 (48) | 62 (53) | 31 (44) | 19 (53) |
| Proximal femur | 458 (97) | 241 (97) | 117 (100) | 64 (91) | 36 (100) |
| Pelvis/acetabulum | 10 (2) | 6 (2) | 0 (0) | 4 (6) | 0 (0) |
| Both proximal femur and pelvis/acetabulum | 3 (1) | 1 (1) | 0 (0) | 2 (3) | 0 (0) |
| ASA score | |||||
| 1–2 | 177 (38) | 116 (47) | 35 (31) | 22 (31) | 4 (11) |
| 3–4 | 286 (62) | 129 (53) | 77 (69) | 48 (69) | 32 (89) |
| Missing data | 8 | 3 | 5 | 0 | 0 |
| Perioperative blood loss, L, mean (SD) | 0.93 (0.78) | 1.1 (0.92) | 0.55 (0.42) | 0.98 (0.52) | 0.95 (0.61) |
| Missing data | 9 | 4 | 4 | 1 | 0 |
| Major bone resection | 296 (63) | 166 (67) | 62 (53) | 42 (60) | 26 (72) |
| Length (cm), mean (SD) | 13.3 (6.9) | 13.1 (6.7) | 13.0 (6.7) | 13.8 (7.6) | 14.3 (6.9) |
| a Only patients where pathologic fracture was not the first sign of disease. | |||||